INTRODUCTION
Breast cancer (BC) and non-melanoma skin cancer are the most common types of cancers in women (Bray et al., 2018; Kumar et al., 2016). The incidence of BC is rapidly increasing and in the year 2020, around 2.3 million women were diagnosed with BC and there were 685,000 BC-associated deaths worldwide (Breast Cancer, 2021). BC affects women of any age group, and the prevalence is more amongst elderly women. BC represents multiple subcategories comprising of discrete cellular compositions, characteristic molecular perturbations, and specific clinical presentations. The prognosis and response to therapy depend upon multiple factors like size of the tumor, histological characteristics, metastasis, presence of hormone receptors—estrogen receptor (ER) and progesterone receptor (PR), and presence of human epidermal growth factor receptor 2 (EGFR-2 or ErbB2) (Eliyatkin et al., 2015).
The BC patients were conventionally categorized as non-specific ductal carcinoma and other specific types dependent upon the morphology, histological findings, etc. (Eliyatkin et al., 2015). The emerging details on the molecular and genetic characteristics are also being used for patient categorization. The ERα and ErbB2 (previously Her-2 or Her-2/neu) are the principal molecular targets expressed on the BC cells (Figs. 1 and 2). Progesterone, another steroid hormone, is closely related to ERα signaling and acts through the PRs (Joshi and Press, 2018). About 70% of the patients with invasive BC express either ER or PR receptors in >1% cancer tissue cells (Hammond et al., 2010; Joshi and Press, 2018). Similarly, ErbB2 is also expressed on the BC cells. Triple-negative BC (TNBC) lacks the said molecular target (ER, PR, and EGFR-2) and roughly constitute 15% of the cases (Denkert et al., 2017). TNBC has a high prevalence of relapse during the first and third years after diagnosis and the average life expectancy after diagnosis of TNBC is 5 years (Sporikova et al., 2018). Also, germline mutation in BC gene 1 (BRCA1) and BRCA2 genes (responsible for the repair of DNA single-strand break) is associated with approximately 5% of BC cases (Robson et al., 2017).
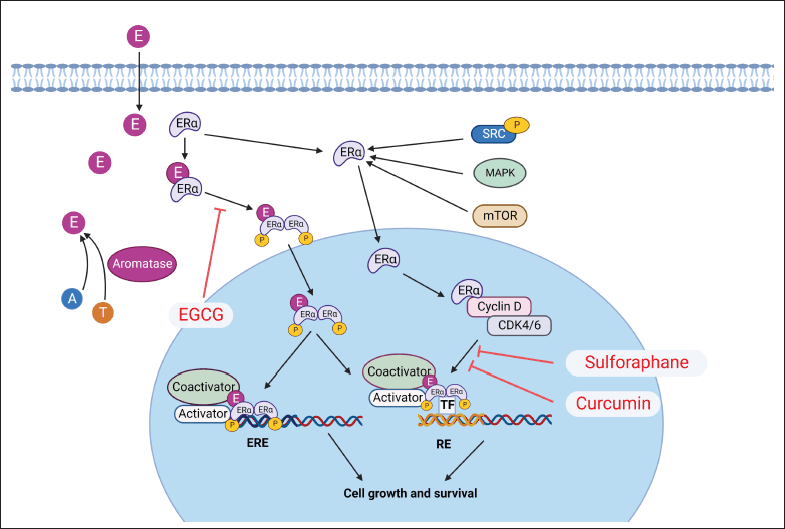 | Figure 1. ER signaling pathway. CDK: cyclin-dependent kinase; E: estrogen; ERα: estrogen receptor alpha; ERE: estrogen response element; MAPK: mitogen-activated protein kinase; mTOR: mammalian target of Rapamycin; RE: response element; SRC: Proto-oncogene tyrosine-protein kinase Src; TF: transcription factor. [Click here to view] |
ErbB2 is a receptor tyrosine kinase that activates several signaling pathways involving the Ras/c-Raf/MAPK-ERK kinase (MEK)/extracellular signal-regulated kinase (ERK), signal transducer, and activator of transcription (STAT), Phosphoinositide-3-Kinase (PI3K)/ protein kinase B (Akt)/mammalian target of Rapamycin (mTOR), Phospholipase C (PLC)-γ1, and c-Src pathways (Wang et al., 2018). Nearly 20% of BCs overexpress the ErbB2 gene (classified as ErbB2+/Her2+) and have a poor prognosis if lacking systemic therapy (Piccart-Gebhart et al., 2005; Wolff et al., 2013).
The foremost objectives of therapy for non-metastatic BC are eliminating tumors from the breast and neighboring lymph nodes and suppressing metastatic relapse. For non-metastatic BC, local therapy consists of surgical removal of breast tumor and/or axillary lymph nodes, with post-surgery radiation therapy. Systemic treatment could be adjuvant (post-surgery), neoadjuvant (pre-surgery), or both. BC subtype influences the standard systemic treatment provided, consisting of hormone therapy for all HR+ tumors (plus chemotherapy in certain cases), ErbB2-targeted therapy along with chemotherapy for all ErbB2+ tumors (plus hormone therapy in case of HR+ tumors), and only chemotherapy for TNBC (Waks and Winer, 2019).
In metastatic BC, therapy objectives are extending life and palliative care as metastatic BC is presently terminal in almost all cases. However, similar elementary sets of adjuvant/neoadjuvant systemic treatments are practiced in metastatic BC. Local therapy procedures (surgery and radiation) are utilized only for palliative purposes. Also, targeted drugs like abemaciclib, palbociclib, and ribociclib (inhibitors of Cyclin-dependent kinases 4 and 6) to target cancer cells’ cell cycle received approval for adjuvant therapy in the management of metastatic BCs (Waks and Winer, 2019).
Notwithstanding the benefits of the available systemic therapies in different BC patients, these therapies have several issues including adverse effects (Goss et al., 2016), recurrence of the disease (Waks and Winer, 2019), limited effect (von Minckwitz et al., 2017), cost, and no significant improvement in disease-free survival (Francis et al., 2018).
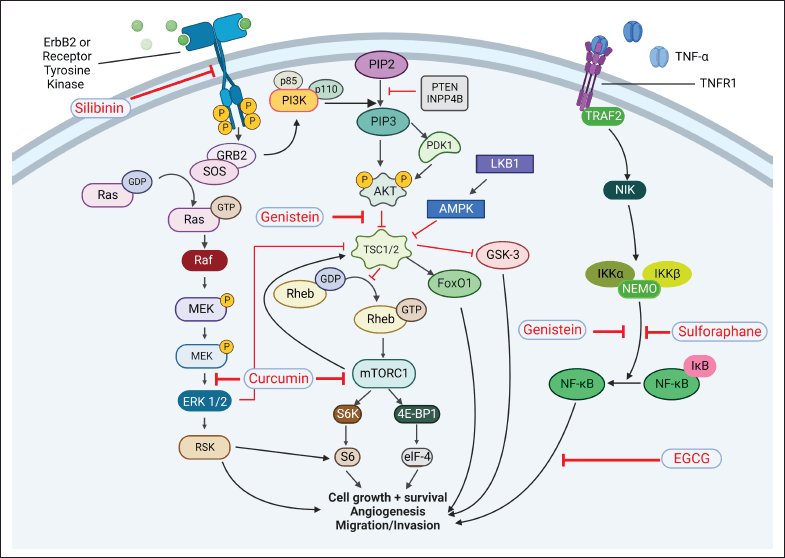 | Figure 2. Role of receptor tyrosine kinase and TNF-α signaling pathways in BC. ErrB2: receptor tyrosine kinase belongs to the EGFR family and activates PI3K/Akt and Ras/Raf pathways. PI3K: phosphatidylinositol-3 kinase; PTEN: phosphatase and tensin homolog deleted on chromosome 10; INPPB4: inositol polyphosphate 4-phosphatase type II; PIP2: phosphatidylinositol-4, 5-bisphosphate; PIP3: phosphatidylinositol-3, 4, 5-triphosphate; PDK1: phosphoinositide-dependent kinase-1; AKT: protein kinase B; TSC1/2: Tuberous sclerosis proteins 1/2; GSK-3: glycogen synthase kinase-3; Fox O1: forkhead box O1 transcription factor; Rheb: Ras homolog enriched in brain protein; mTORC1: mammalian target of rapamycin complex 1 play important role in the initiation of translation; Eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) is a translation repressor protein that interacts with and inhibits eukaryotic translation initiation factor 4E (eIF4E); S6K: ribosomal protein S6 kinase activates the ribosomal protein S6 for carcinogenesis. GRB2: growth factor receptor-bound protein 2; SOS: son of sevenless (guanine nucleotide exchange factor); Ras: rat sarcoma GTPase; Raf: rapidly accelerated fibrosarcoma kinase; MEK: mitogen-activated protein kinases (MAPKs) kinase; ERK1/2: extra-cellular signal receptor kinases; RSK: ribosomal S6 kinase. TNFR1: tumor necrosis factor receptor 1; TRAF-2: TNF receptor-associated factor-2; NIK: NF-κB inducing kinase; NEMO: NF-κB essential modulator; IKKα/β: IκB kinase; IκB: inhibitor of NF-κB; NF-κB: nuclear factor kappa B transcription factor. [Click here to view] |
Therefore, new treatment approaches are needed with reduced adverse effects specifically during longer therapies, especially for patients with TNBC. Also, novel agents are required to reduce the number of chemotherapeutic agents needed for low-risk patients and novel agents that are effective in high-risk BC patients like trastuzumab in ErbB2+ BC (Waks and Winer, 2019).
Diet with high-fiber, low-fat, fruits, and vegetables is linked to a lower risk of cancer, according to studies (Soerjomataram et al., 2010). Evidence is available to back up plant-derived bioactive compounds’ chemopreventive and therapeutic potential in BC (Hussain et al., 2022). Furthermore, some of these bioactive compounds are well tolerated by normal cells and are generally less toxic (Khan et al., 2019). In this review, we will discuss certain secondary metabolites of plant origin which are promising in BC treatment and have reached the clinical phase of the investigation.
GROWTH FACTORS AND THEIR RECEPTORS
Insulin-like growth factor (IGF)
A study was conducted to analyze the effect of green tea extract (GTE) containing 832 mg of Epigallocatechin Gallate (EGCG) on mammographic density (MD) in healthy postmenopausal women. After 12 months of oral administration, no significant change was observed in the percent or absolute MD of treated women. However, a significant reduction in the percent MD was observed in women aged 50–55 years. The GTE administration also significantly increased circulating estradiol in healthy postmenopausal women, but no significant change was observed in the plasma levels of IGF-1 and IGF binding protein (IGFBP)-3 (Samavat et al., 2017). The study did not assess the ER expression which increases in benign breast epithelium with age and postmenopause and positively correlated with BC risk. Other studies have reported mixed results on the effect of EGCG on BC risk and its association with the Catechol-O-methyltransferase (COMT) gene SNP rs4680, mainly in Asian-American women (Lazzeroni et al., 2017; Samavat et al., 2017).
Fibroblast growth factor receptor 2 (FGFR2)
FGFR2 is one of four receptors that control signaling from fibroblast growth factors (FGFs), a family of genes that play a role in development, cell growth, and death. FGFR2 signaling pathways are divided into two groups, one that relies on FGFR substrate 2α (FRS2α) and one that does not. These pathways include RAS-MAPK, PLCγ, PI3K, and Janus kinase (JAK)/STAT (Lei and Deng, 2017). Genetic changes in FGFR2 have been found in BC, leading to the activation of downstream FGFR2 signaling pathways, like the FGFR2 rs2981582T/C variant linked to BC in the Saudi population (AlRaddadi et al., 2021).
Soy intake for 7–30 days resulted in significant overexpression of the FGFR2 gene, protumorigenic growth factor receptor, in breast tumor, and no significant change was observed in Ki67 or caspase 3 levels (Shike et al., 2014). However, the study has not evaluated the clinical impact of increased expression of the FGFR2 gene and the short period of intervention might not be enough to produce significant changes in phenotypes. Also, heterogeneity in breast tumor and menopause status of subjects might have influenced the outcome of the study.
Genistein is a type of isoflavonoid found in plants such as soybeans, clovers, and lupins (Garbiec et al., 2022). Epidemiological studies have shown that a diet rich in soy is associated with cancer prevention in Asian populations (Ahn-Jarvis et al., 2015; Dong and Qin, 2011). Meta-analysis studies have also found that consuming soy isoflavones can reduce the risk of BC before and after menopause (Boutas et al., 2022; Chen et al., 2014). The incidence of BC also increases in Asian women after they migrate to the United States, suggesting that lifestyle, diet, and environmental factors play a role in the development of these cancers (Shimizu et al., 1991). A study in Japan found that consuming miso soup and isoflavones was inversely associated with BC risk (Fujimaki et al., 2003). It is believed that the isoflavones in soy-containing foods are responsible for their chemopreventive effects.
The ambiguity about the estrogenic effects of genistein continued in clinical studies as well. On one side studies have reported the safety of genistein on breast tissue (Atteritano et al., 2008; Marini et al., 2008) whereas, on the other side, soy isoflavones stimulated breast proliferation in premenopausal women (Hargreaves et al., 1999; Khan et al., 2012; McMichael-Phillips et al., 1998). It is a possibility that genistein might have behaved differently in premenopausal and menopausal women. Interestingly, a phase II clinical study namely “Gemcitabine Hydrochloride and Genistein in Treating Women with Stage IV Breast Cancer,” has reported the closure of the study because of the lack of efficacy (Gemcitabine hydrochloride and genistein in treating women with stage IV breast cancer—study results, n.d.). Therefore, further studies with a large sample size are needed to ascertain the potential of genistein in BC and to clarify the mechanism of opposite effects in women before and after menopause.
OXIDATIVE STRESS AND INNATE ANTIOXIDANTS
Nuclear factor erythroid 2 (NF-E2-p45)- related factor 2 (Nrf2)/antioxidant response element (ARE) signaling pathway
Transducer KEAP1 controls the equilibrium between apoptosis and autophagy in addition to regulating the oxidative stress response through the downstream Nrf2-dependent signaling (Sajadimajd and Khazaei, 2018; St?pkowski and Kruszewski, 2011). In BC, dipeptidyl-peptidase 3 is substantially overexpressed and interacts with KEAP1 to increase Nrf2 expression (Lu et al., 2017). p62, an autophagy adapter protein, and KEAP1, an adaptor for the Cul3 E3 ubiquitin ligase for Nrf2, directly interact to regulate autophagy in large part (Jiang et al., 2015b). Sulforaphane prevents KEAP1 from promoting Nrf2 proteasome-mediated breakdown, however, KEAP1 can still network with LC3 and p62 under duress by employing p62 as a link between autophagosomes and ubiquitin aggregates (Fan et al., 2010). Therefore, KEAP1 is nearly entirely involved in directing cells into autophagy.
A study of eight healthy women showed that consuming broccoli sprouts increased sulforaphane in breast tissue (Cornblatt et al., 2007). Several trials found a significant decrease in Ki-67 protein expression in women with ductal carcinoma in situ (DCIS) after eating cruciferous vegetables or sprout extracts (Study to evaluate the effect of sulforaphane in broccoli sprout extract on breast tissue—study results, n.d.; Zhang et al., 2016). Short-term sulforaphane supplementation reduced Ki-67 and HDAC3 in benign tissue, but more research is needed to determine the effects on DCIS and invasive ductal carcinoma (Atwell et al., 2015). An open-label study is evaluating the efficacy of sulforaphane in combination with tamoxifen and fulvestrant (SFX-01 in the treatment and evaluation of metastatic breast cancer, n.d.). A current double-blind trial is examining the protective effects of sulforaphane on doxorubicin-related heart damage in BC patients and its impact on Nrf2- and SIRT1 expression (Protective effects of the nutritional supplement sulforaphane on doxorubicin-associated cardiac dysfunction—no study results posted, n.d.).
Hypoxia inducible factor (HIF)-1α pathway
Compared to normal cells, cancer cells are more hypoxic and subject to oxidative stress. Hypoxia and oxygen radicals work together to induce tumor angiogenesis (Brown and Bicknell, 2001) HIF-1, activated by hypoxia and encourages the production of vascular endothelial growth factor (VEGF) for angiogenesis regulation in BC (Ferrara et al., 2003). Oxygen radicals also elevate HIF-1 levels. Oxygen radicals also trigger the NF-κB activation, which boosts the production of VEGF. To accelerate the development of BC, VEGF also promotes the development and migration of cancer cells (Yang et al., 2011). By lowering VEGF and HIF-1expression and reducing NF-κB activation, EGCG considerably reduces breast tumor angiogenesis and prevents the growth and metastasis of cultured mice and human BC cells without influencing healthy tissues like the heart and skeletal muscles (Braicu et al., 2013; Gu et al., 2013; Wei et al., 2018).
CELL PROLIFERATION AND APOPTOSIS PATHWAYS
p53 Pathway
The tumor suppressor protein i.e., p53 is often altered in a variety of human cancers and regulates a diverse range of pathways that include cell proliferation, DNA repair, and apoptosis (Kandoth et al., 2013; Muller and Vousden, 2014). Apoptosis regulator proteins of the Bcl-2 family influence p53 which activates Bax and leads to cell death through mitochondrial rupture and release of cytochrome c (Marzo et al., 1998; Oltval et al., 1993). This activates caspase-3 and, poly (ADP-ribose) polymerase (PARP), two major mediators of apoptosis (Kluck et al., 1997; Reed, 1995). EGCG increases p53 expression and decreases Bcl-2, leading to a higher Bax/Bcl-2 ratio and increased apoptosis (Huang et al., 2017; Roy et al., 2005). Silibinin boosts proapoptotic gene expression such as IFN-stimulated gene (ISG)15, p53, CASP9, and BAX, while curcumin reduces BC growth through mechanisms such as cell cycle arrest and p53-mediated apoptosis, modulation of signaling pathways, Hedgehog/Glioma-associated oncogene homolog-1 (Gli1) signaling pathway, down-regulation of transcription factors, and halting angiogenesis (Hossainzadeh et al., 2019; Khan et al., 2022; Song et al., 2019; Zhang et al., 2022a).
The p53 gene is involved in regulating cell growth and apoptosis in BC, and mutations in the gene are observed in 20% of cases (Hallman et al., 2017). P53 promotes the expression of p21, which arrests cells in the G1 phase for DNA repair, and upregulates apoptotic target genes such as PUMA (p53 upregulated modulator of apoptosis) and Bax if DNA repair fails (Takaoka et al., 2003). EGCG triggers apoptosis through mitochondrial dysfunction and stimulates autophagy by increasing ATG5 (autophagy-related protein 5), Beclin1, and autophagy marker light chain 3-B expression (Baliga et al., 2005; Wei et al., 2018). These proteins are major autophagy markers and play a role in the formation of autophagosome membrane and interaction with lysosomes (Mizushima and Yoshimori, 2007; Tanida et al., 2005; Wirth et al., 2013).
Wnt/β-catenin pathway
Wnt/β-catenin pathway is known to play a crucial role in the self-renewal of cancer stem cells (CSCs) in BC (Zhang and Wang, 2020). The pathway is activated by β-catenin through T cell factor/lymphoid enhancer factor to regulate the expression of downstream genes such as c-Myc and stem cell markers (Zhang and Hao, 2015). Glycogen synthase kinase 3 (GSK3) promotes the degradation of β-catenin via ubiquitin-mediated degradation and modulates the Wnt pathway (Takahashi-Yanaga, 2013). Curcumin therapy has been shown to inhibit the Wnt/β-catenin pathway by reducing GSK3, β-catenin, and c-Myc (Li et al., 2018). Sulforaphane also inhibits the Wnt/β-catenin pathway by activating GSK3 and promoting the degradation of β-catenin, potentially making it effective against breast CSCs (Li et al., 2010).
The Wnt/-catenin pathway is essential for the growth and differentiation of mammary glands, angiogenesis, and hormone signaling, all of which are significant risk factors for BC (Amado et al., 2011). This pathway is activated by the interaction of Wnt proteins with the lipoprotein receptor-related protein5/6 and Frizzled receptors and inhibited by the tumor suppressor adenomatous polyposis coli-Axin-GSK3β- casein kinase 1 complex in the absence of Wnt (Barker and Clevers, 2006; MacDonald et al., 2009; Polakis, 2007). In BC cells, substances like curcumin, sulforaphane, EGCG, and silibinin have been shown to inhibit the Wnt/β-catenin pathway and its downstream gene c-Myc, leading to reduced cancer cell growth and self-renewal (Hong et al., 2017; Loh et al., 2013; Lu et al., 2012).
ErbB2/ErbB3/ PI3K/Akt/mTOR pathway
The PI3K/AKT/mTOR pathway is a common signaling route in human cancer, triggered by the loss of phosphatase and tensin homolog (PTEN) activity, and stimulation of growth factor receptors (IGF-1R and ErbB2) (Liu et al., 2009). EGCG, an adenosine triphosphate (ATP)-competitive inhibitor, has been shown to inhibit PI3K and mTOR in BC cells and reduce levels of EGFR, ERK, and phospho-ERK p42/44, as well as cell migration and extracellular matrix (ECM) metalloproteinase inducer expression (Farabegoli et al., 2011). Curcumin has been found to block TGF-β and PI3K/AKT pathways and improve the inhibitory effect of doxorubicin in TNBC cells (Guan et al., 2016; Kunihiro et al., 2022; Mao et al., 2019). ErbB2 activates several pathways, including the PI3K/Akt/mTOR pathway, and is overexpressed in 20% of BCs (Piccart-Gebhart et al., 2005). Integrins are transmembrane receptors that link the ECM to intracellular actin and activate focal adhesion kinase, which leads to the activation of downstream proteins including Ras, Rac, and Cdc42 (Burbelo et al., 2004; Dovas and Couchman, 2005; Hood and Cheresh, 2002; Machacek et al., 2009). Silibinin has been shown to affect the β1-integrin signaling pathway and the Ras-Raf-MEK-ERK pathway (Dastpeyman et al., 2012; Kolch et al., 2002; Lee et al., 2007; Lin et al., 2009).
ERK1/2-MAPK pathway
Raf, a serine/threonine protein kinase, directly phosphorylates or enhances protein phosphorylation by stimulating MEK/ERK signaling pathway (Chang et al., 2003; Roberts and Der, 2007). The Raf/MEK/ERK cascade regulates the growth and progression of several cancers, including BC (Li et al., 2016; De Luca et al., 2012). ARAF, BRAF, and CRAF are examples of Raf family members. Recent research has demonstrated that ARAF dimerization increases MAPK pathway activation and cell metastasis (Mooz et al., 2014). Sulforaphane hindered MEK and ERK phosphorylation via interacting with Raf family members such as ARAF, BRAF, and CRAF and reduced BC cell metastasis by preventing actin stress fiber production (Zhang et al., 2022b).
BRCA1 pathway
The BRCA1 gene is a pertinent DNA repair gene that is crucial for DNA repair, transcription, and regulation of the cell cycle. The cases of hereditary BCs are significantly associated with germline mutation of the BRCA1 gene and sporadic incidences of BC are linked with aberrant methylation of the promoter region of the BRCA1 gene that renders the gene nonfunctioning (Al-Moghrabi et al., 2015; Gupta et al., 2014; Iwamoto et al., 2011; Wong et al., 2011). Further, the breast cancer-specific gene 1 (BCSG1) also called the SNCG gene expresses the γ synuclein protein of the synuclein family (Ji et al., 1997). The SNCG gene, a proto-oncogene, is related to metastasis and decreased duration of disease-free survival in stages III and IV of breast ductal carcinomas (Wu et al., 2007). The demethylation of Exon 1 of SNCG is incidental to an errant expression of SNCG in BC (Lu et al., 2001). Curcumin induced the expression of the BRCA1 gene by decreasing methylation in DNA promoter methylation in ER-/PR- and TNBC cells. Further, curcumin-induced methylation in the promoter region of the SNCG gene in the ER+/PR+ BC cell line (T47D cells) (Al-Yousef et al., 2020).
Apoptosis
miRNAs are small noncoding RNAs that control cellular processes by inhibiting messenger RNA (mRNA) translation or assisting mRNA degradation (Curtale, 2018; O’Bryan et al., 2017; Zadeh et al., 2016). miRNAs can have either tumor-suppressive or oncogenic impacts on cells (Garzon et al., 2010; Paranjape et al., 2009). Silibinin has been shown to inhibit the production of carcinogenic miRNAs such as miR-125b and miR182 and cause autophagic cell death by increasing the expression of Bcl-2 adenovirus E1B 19-kDa-interacting protein 3 (BNIP3), a pro-apoptotic protein, and elevating reactive oxygen species formation (Hossainzadeh et al., 2019; Jiang et al., 2015a). Inhibiting matrix metalloproteinases (MMPs) has been shown to protect against tumor advancement due to its effect on cyclooxygenase-2 (COX-2), an enzyme involved in inflammation and wound healing that can also promote carcinogenesis (Chun and Surh, 2004; Surh et al., 2001). Silibinin has been shown to suppress 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced COX-2 expression and MMP-9 expression in BC cells (Kim et al., 2009).
HORMONAL PATHWAY AND METABOLIC PATHWAY
Estrogen and progesterone
ER-α levels are higher in ER-positive cancers, and hyperplastic lesions, and linked to an increased likelihood of invasive breast disease (Frech et al., 2005; Poola et al., 2005). Estrogen-regulated genes (insulin receptor substrate 1, pS2, & cyclin D1) are suppressed when PR-β expression is high in BC cells, indicating PR/PR-B affects ER-α gene transcription (De Amicis et al., 2013; Khan et al., 1994).
EGCG has been found to have co-estrogenic effects at lower concentrations and anti-estrogenic effects at higher concentrations (Belguise et al., 2007; Farabegoli et al., 2007). It may regulate the nuclear translocation of PR and the binding of PR-B onto the ER-alpha promoter, leading to transcriptional repression of genomic and non-genomic activity of ER-alpha, slowing the development of ER+ PR+ BC cells (De Amicis et al., 2013). Studies have shown that higher levels of EGCG are achieved in BC tissues with phosphatidylcholine formulation of EGCG (Lazzeroni et al., 2017). However, more research is needed to confirm the potential of EGCG in reducing BC risk, especially in the Asian population with the less active COMT genotype.
Early exposure to genistein in immature rats may promote the differentiation of mammary gland cells and reduce BC susceptibility (Lamartiniere et al., 1998). However, genistein-containing foods may be harmful to ER+ BC in postmenopausal women (Ju et al., 2006). Low doses of genistein promote cell proliferation and overexpression of estrogen response element (ERE) in ER+ BC cells and can act like an estrogen molecule (Hsieh et al., 2006; Seo et al., 2006). It can also inhibit the tumor preventive and antiproliferative effects of tamoxifen and hydroxytamoxifen (Liu et al., 2005; Seo et al., 2006).
Silibinin stimulates apoptosis in MCF-7 cells by downregulating ERα and upregulating ERβ, and by promoting nuclear translocation of AIF. The apoptosis is reversed by AIF knockdown using siRNA (Liu et al., 2020; Zheng et al., 2015, 2016).
Lipid metabolism
Deregulation of the metabolism is thought to be a characteristic of cancer (Nicot et al., 2004; Pizer et al., 1996). Fatty acid synthase (FAS) inhibition has little effect on non-cancerous cells, but a recent study showed that FAS is expressed in TNBC patient samples and that FAS inhibition is beneficial for TNBC preclinical models (Pizer et al., 2000). EGCG has been shown to have antiproliferative effects on MDA-MB-231 TNBC cells and has been reported to inhibit FAS, trigger apoptosis, and reduce tumor growth without concurrent carnitine palmitoyltransferase-1 stimulation or weight reduction (Chisholm et al., 2004; Puig et al., 2008b, 2008a; Roy et al., 2005).
Leptin, a hormone produced by adipose tissue, has been shown to promote proliferation, transformation, and migration in healthy and cancerous mammary epithelial cells through the leptin receptor i.e., ObR (Dieudonne et al., 2002; Laud et al., 2002). Studies have shown that leptin stimulates the proliferation and transformation of T47D BC cells and various BC cell types express both leptin and its receptor (Hu, 2002). Silibinin has been found to suppress the development of T47D cells by reducing leptin expression through ERβ activation (Nejati-Koshki et al., 2012; Zheng et al., 2016).
Glutamine metabolism
Ferroptosis, or iron-dependent programmed cell death, plays a critical role in cancer-related immune evasion and drug resistance (Friedmann Angeli et al., 2019; Shin et al., 2018; Sun et al., 2016). Glutamine metabolism can promote ferroptosis by increasing the accumulation of oxidizable lipids (Gao et al., 2015; Xu et al., 2019). Solute carrier family 1 member 5 (SLC1A5), a glutamine uptake transporter, has been linked to tumor progression. Curcumin may inhibit tumorigenesis by increasing SLC1A5 expression in BC (Cao et al., 2022; Jiang et al., 2020).
Glycolysis
Glycolysis is a metabolic pathway used by cancer cells to produce anabolic substrates such as nicotinamide adenine dinucleotide phosphate and ATP from glucose, allowing them to adapt to fluctuating oxygen supply (Jose et al., 2011). Myc, a cancer metabolism regulator, suppresses the Thioredoxin-interacting protein (TXNIP) to increase glycolysis, essential for tumor development (Lim et al., 2016; Lunt and Vander Heiden, 2011). Silibinin has been found to lower glucose consumption and inhibit critical metabolic processes in TNBC, resulting in decreased cell growth, biomass, and stem cell features and improved anti-TNBC effectiveness when combined with paclitaxel. It also reverses the results of ectopic EGFR, Myc silencing, and TXNIP overexpression in TNBC (Iqbal et al., 2021; Lim et al., 2016).
INFLAMMATORY PATHWAY
C-X-C Chemokine receptor type 4 (CXCR4) signaling pathway
CXCR4 overexpression is linked to an increased risk of lung and bone metastasis in metastatic BC (Felix et al., 2010; Furusato et al., 2010). Silibinin at a concentration of 80 µM can prevent C-X-C Motif Chemokine Ligand 12-induced migration in MDA-MB-231 cells and CXCR4 stimulation by suppressing CXCR4 signaling, potentially explaining its anti-tumor activity (Wang et al., 2014).
Akt and nuclear factor–kappa B (NF-κB) pathway
The nuclear NF-κB pathway controls cell survival, inflammation, differentiation, and proliferation. Tumor necrosis factor-alpha (TNF-α) activates inhibitor of kappa B kinase (IKK), leading to phosphorylation and destruction of inhibitors of NF-κB (IκB), resulting in the transcription of downstream targets genes that control pro-inflammatory and anti-apoptotic processes (Park and Hong, 2016; Poma et al., 2017; Yu et al., 2009). EGFR and Akt also activate NF-κB. Genistein has been found to induce apoptosis and inhibit growth in BC cell lines by inhibiting Akt and NF-kB pathways when combined with other anticancer agents (Liu et al., 2005; Ma et al., 2022).
Intra-ductal administration of curcumin and its nano-formulation in the mammary duct of SD rats, treated with N-methyl-N-nitrosourea to induce mammary carcinoma, significantly lowers the incidence of mammary tumors by decreasing activation of NF-κB (Chun et al., 2012). In T47D BC cells, curcumin therapy inhibits growth hormone-triggered invasion-metastasis, and epithelial mesenchymal transition (EMT) activation by blocking the NF-κB pathway and promoting apoptotic cell death by modifying the Bcl-2 family of proteins (Coker-Gurkan et al., 2018; Zhang et al., 2022a).
Different kinases such as NIK, NAK, MAPK, and Akt control the IKK-NF-κB signaling in different ways (Hwang et al., 2006). IKK enzyme is composed of two catalytic subunits (IKKα and IKKβ) and a regulatory component (IKKγ) (Li et al., 2001). Sulforaphane suppresses TPA-induced NF-κB activation and COX-2 expression in MCF-10A cells by inhibiting the ERK1/2-IKKα pathway and NAK-IKKβ signaling (Kim et al., 2014a; Taoqi Zhou et al., 2022).
Inflammation can aid in the development of malignant cells by suppressing the immune system (O’Callaghan et al., 2010). Reduced accumulation of Myeloid-derived suppressor cells (MDSCs) may enhance immune surveillance and antitumor immunity (Cuenca et al., 2011). In mice, Silibinin increases tumor-infiltrating T cells, and reduces tumor development and the number of MDSCs (Forghani et al., 2014).
STAT3 pathway
The transcription factor STAT3 transmits signals to the nucleus and its constitutive activation is linked to tumor growth and poor prognosis in BC (Turkson and Jove, 2000; Zhong et al., 1994). Studies show that STAT3 activation is a key factor in tumor invasion and activation of BC stem cells (Azare et al., 2011; Dauer et al., 2005). EGCG modifies STAT3-NF-κB signaling pathways to decrease the number of cancer stem-like clusters of differentiation 44-positive cells (Chung and Vadgama, 2015).
The STAT3 and STAT5b members of the STAT family are oncogenes involved in the development, growth, and spread of malignant BCs (Garcia et al., 2001, 1997; Siveen et al., 2014). These proteins are activated by JAK and phosphorylation, resulting in the transcription of target genes (Darnell, 1997; Imada and Leonard, 2000). Silibinin at a 200 M concentration reduces Jak2 expression and phosphorylation and prevents Jak2 from phosphorylating STAT3, preventing TNBC cells from proliferating, migrating, and invading by inhibiting MMP2 gene-specific transcriptional activation (Byun et al., 2017).
Fibronectin (FN) is a glycoprotein in the ECM that aids in cell adhesion, proliferation, migration, differentiation, and neoplastic transformation (Fernandez-Garcia et al., 2014). FN expression is higher in TNBC instances and is linked with a worse clinical outcome in BC patients (Bae et al., 2013; Balanis et al., 2013) Additionally, FN can prevent apoptosis from conventional chemotherapy drugs. Silibinin has been found to suppress EGF-induced FN expression in MDA-MB468 BC cells by suppressing STAT3 activity (Kim et al., 2014b).
Transforming growth factor-beta (TGF-β), a class of polypeptides, induces EMT, resistance to chemotherapy and cell death, and escape from immune surveillance (Thiery et al., 2009; Xu et al., 2009). Silibinin can inhibit the metastasis of TNBC cells in vivo and reduce FN and MMP-2 expression levels, which are induced by TGF-β (Kim et al., 2016).
LONG NONCODING RNAS (LNCRNAS)
lncRNAs have been demonstrated to act as major transcriptional regulators in a variety of biological activities and disease processes, including cancer (Peng et al., 2017). Also, lncRNA H19 plays a significant role in BC growth, metastasis, chemoresistance, and endocrine resistance (Peng et al., 2017; Zhou et al., 2017). According to a recent study, H19 is significantly elevated in tamoxifen?resistant (TAMR) BC and is also linked to tamoxifen resistance (Basak et al., 2018). Several investigations have shown that H19 dysregulation promotes tumor metastasis by influencing tumor cell EMT (Wu et al., 2019; Ye et al., 2020; Zhou et al., 2017).
H19 upregulation promoted EMT, invasion, and migration in MCF7/TAMR cells through upregulating Snail. Curcumin reduced the expression of H19 in a dose-dependent manner. Curcumin significantly reduced the expression of H19-induced epithelial marker E-cadherin while significantly enhancing the expression of the mesenchymal marker N-cadherin in MCF7/TAMR cells (Cai et al., 2021).
CONCLUSION
The modern paradigm of drug discovery has provided promising therapies for the treatment of BC but with some drawbacks like severe toxicities, dose limitation, and cost of therapy. The phytoconstituents which are reported to exert anticancer effects provide certain advantages like multi-targeted actions, lesser toxicity, and a unique bioactivity profile that warrants further systematic clinical investigation of these phytoconstituents. Notwithstanding the advantages, the phytoconstituents have their own sets of limitations such as low bioavailability, varied effects in different populations, and mechanistic interference of available systemic therapies. The bioavailability can be improved with the help of novel delivery systems and chemical derivatization. The variable effects and mechanistic interference can be overcome with the meticulous designing of therapeutic regimens. Further investigations in these directions will add promising adjuvants to the existing chemotherapy for BC.
LIMITATIONS
Although several phytoconstituents with various underlying processes have been studied in preclinical settings, only a small number of them have been studied in clinical settings to see whether they may be used to treat BC. In this study, only those phytoconstituents were reviewed which have reached the clinical phases of the investigation. The author has consulted the PubMed database and the clinical trial registry of the US for this study.
AUTHOR CONTRIBUTIONS
SK, CRP conceptualized the review; SK, CRP, SB, RJJ prepared the first draft; Himangini, MM, VJ, SY prepared the artwork, tabulated data and critically evaluated the manuscript. All authors critically revised the draft. SK, CRP and SB approved the final version.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahn-Jarvis JH, Clinton SK, Grainger EM, Riedl KM, Schwartz SJ, Lee MLT, Cruz-Cano R, Young GS, Lesinski GB, Vodovotz Y. Isoflavone pharmacokinetics and metabolism after consumption of a standardized soy and soy-almond bread in men with asymptomatic prostate cancer. Cancer Prev Res, 2015; 8:1045–54; doi:10.1158/1940-6207.CAPR-14-0465. CrossRef
Al-Moghrabi N, Nofel A, Al-Yousef N, Madkhali S, Bin Amer SM, Alaiya A, Shinwari Z, Al-Tweigeri T, Karakas B, Tulbah A, Aboussekhra A. The molecular significance of methylated BRCA1 promoter in white blood cells of cancer-free females. BMC Cancer, 2014; 14:830.
AlRaddadi RIR, Alamri RJN, Shebli WTY, Fallatah EIY, Alhujaily AS, Mohamed HS, Alotibi MK. Fibroblast growth factor receptor 2 gene (FGFR2) rs2981582T/C polymorphism and susceptibility to breast cancer in Saudi women. Saudi J Biol Sci, 2021; 28:6112–5; doi:10.1016/j.sjbs.2021.07.005. CrossRef
Al-Yousef N, Shinwari Z, Al-Shahrani B, Al-Showimi M, Al-Moghrabi N. Curcumin induces re-expression of BRCA1 and suppression of γ synuclein by modulating DNA promoter methylation in breast cancer cell lines. Oncol Rep, 2020; 43:827–38; doi:10.3892/or.2020.7473. CrossRef
Amado NG, Fonseca BF, Cerqueira DM, Neto VM, Abreu JG. Flavonoids: potential Wnt/beta-catenin signaling modulators in cancer. Life Sci, 2011; 89:545–54; doi:10.1016/j.lfs.2011.05.003. CrossRef
Atteritano M, Pernice F, Mazzaferro S, Mantuano S, Frisina A, D’Anna R, Cannata ML, Bitto A, Squadrito F, Frisina N, Buemi M. Effects of phytoestrogen genistein on cytogenetic biomarkers in postmenopausal women: 1 year randomized, placebo-controlled study. Eur J Pharmacol, 2008; 589:22–6; doi:10.1016/j.ejphar.2008.04.049. CrossRef
Atwell LL, Zhang Z, Mori M, Farris PE, Vetto JT, Naik AM, Oh KY, Thuillier P, Ho E, Shannon J. Sulforaphane bioavailability and chemopreventive activity in women scheduled for breast biopsy. Cancer Prev Res, 2015; 8:1184–91; doi:10.1158/1940-6207.CAPR-15-0119. CrossRef
Azare J, Doane A, Leslie K, Chang Q, Berishaj M, Nnoli J, Mark K, Al-Ahmadie H, Gerald W, Hassimi M, Viale A, Stracke M, Lyden D, Bromberg J. Stat3 mediates expression of autotaxin in breast cancer. PLoS One, 2011; 6:e27851; doi:10.1371/journal.pone.0027851. CrossRef
Bae YK, Kim A, Kim MK, Choi JE, Kang SH, Lee SJ. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum Pathol, 2013; 44:2028–37; doi:10.1016/j.humpath.2013.03.006. CrossRef
Balanis N, Wendt MK, Schiemann BJ, Wang Z, Schiemann WP, Carlin CR. Epithelial to mesenchymal transition promotes breast cancer progression via a fibronectin-dependent STAT3 signaling pathway. J Biol Chem, 2013; 288:17954–67; doi:10.1074/jbc.M113.475277. CrossRef
Baliga MS, Meleth S, Katiyar SK. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clin Cancer Res, 2005; 11:1918–27; doi:10.1158/1078-0432.CCR-04-1976. CrossRef
Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov, 2006; 5:997–1014; doi:10.1038/nrd2154. CrossRef
Basak P, Chatterjee S, Bhat V, Su A, Jin H, Lee-Wing V, Liu Q, Hu P, Murphy LC, Raouf A. Long non-coding RNA H19 acts as an estrogen receptor modulator that is required for endocrine therapy resistance in ER+ breast cancer cells. Cell Physiol Biochem, 2018; 51:1518–32; doi:10.1159/000495643. CrossRef
Belguise K, Guo S, Sonenshein GE. Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor α expression reversing invasive phenotype of breast cancer cells. Cancer Res, 2007; 67:5763–70; doi:10.1158/0008-5472.CAN-06-4327. CrossRef
Boutas I, Kontogeorgi A, Dimitrakakis C, Kalantaridou SN. Soy isoflavones and breast cancer risk: a meta-analysis. In Vivo (Brooklyn), 2022; 36:556–62; doi:10.21873/invivo.12737. CrossRef
Braicu C, Gherman CD, Irimie A, Berindan-Neagoe I. Epigallocatechin-3-gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. J Nanosci Nanotechnol, 2013; 13:632–7; doi:10.1166/jnn.2013.6882. CrossRef
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018; 68:394–424; doi:10.3322/caac.21492. CrossRef
Breast cancer. 2021. Available via https://www.who.int/news-room/fact-sheets/detail/breast-cancer (Accessed 27 January 2023).
Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer oxidative stress—its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res, 2001; 3:323; doi:10.1186/bcr315. CrossRef
Burbelo P, Wellstein A, Pestell RG. Altered Rho GTPase signaling pathways in breast cancer cells. Breast Cancer Res Treat, 2004; 84:43–8; doi:10.1023/B:BREA.0000018422.02237.f9. CrossRef
Byun HJ, Darvin P, Kang DY, Sp N, Joung YH, Park JH, Kim SJ, Yang YM. Silibinin downregulates MMP2 expression via Jak2/STAT3 pathway and inhibits the migration and invasive potential in MDA-MB-231 cells. Oncol Rep, 2017; 37:3270–8; doi:10.3892/or.2017.5588. CrossRef
Cai J, Sun H, Zheng B, Xie M, Xu C, Zhang G, Huang X, Zhuang J. Curcumin attenuates lncRNA H19?induced epithelial?mesenchymal transition in tamoxifen?resistant breast cancer cells. Mol Med Rep, 2021; 23:1–9; doi:10.3892/mmr.2020.11651. CrossRef
Cao X, Li Y, Wang Y, Yu T, Zhu C, Zhang X, Guan J. Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS One, 2022; 17:e0261370; doi:10.1371/journal.pone.0261370. CrossRef
Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia, 2003; 17:1263–93; doi:10.1038/sj.leu.2402945. CrossRef
Chen M, Rao Y, Zheng Y, Wei S, Li Y, Guo T, Yin P. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS One, 2014; 9; doi:10.1371/journal.pone.0089288. CrossRef
Chisholm K, Bray BJ, Rosengren RJ. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells. Anticancer Drugs, 2004; 15:889–97; doi:10.1097/00001813-200410000-00010. CrossRef
Chun K-S, Surh Y-J. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol, 2004; 68:1089–100; doi:10.1016/j.bcp.2004.05.031. CrossRef
Chun YS, Bisht S, Chenna V, Pramanik D, Yoshida T, Hong SM, de Wilde RF, Zhang Z, Huso DL, Zhao M, Rudek MA. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: Implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis, 2012; 33:2242–9; doi:10.1093/carcin/bgs248. CrossRef
Chung SS, Vadgama J V. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFκB signaling. Anticancer Res, 2015; 35:39–46.
Coker-Gurkan A, Celik M, Ugur M, Arisan ED, Obakan-Yerlikaya P, Durdu ZB, Palavan-Unsal N. Curcumin inhibits autocrine growth hormone-mediated invasion and metastasis by targeting NF-κB signaling and polyamine metabolism in breast cancer cells. Amino Acids, 2018; 50:1045–69; doi:10.1007/s00726-018-2581-z. CrossRef
Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis, 2007; 28:1485–90; doi:10.1093/carcin/bgm049. CrossRef
Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, LaFace DM, Heyworth PG, Efron PA, Moldawer LL. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med, 2011; 17:281–92; doi:10.2119/molmed.2010.00178. CrossRef
Curtale G. MiRNAs at the crossroads between innate immunity and cancer: focus on macrophages. Cells, 2018; 7:12; doi:10.3390/cells7020012. CrossRef
Darnell JE. STATs and gene regulation. Science, 1997; 277:1630–5; doi:10.1126/science.277.5332.1630. CrossRef
Dastpeyman M, Motamed N, Azadmanesh K, Mostafavi E, Kia V, Jahanian-Najafabadi A, Shokrgozar MA. Inhibition of silibinin on migration and adhesion capacity of human highly metastatic breast cancer cell line, MDA-MB-231, by evaluation of β1-integrin and downstream molecules, Cdc42, Raf-1 and D4GDI. Med Oncol, 2012; 29:2512–8; doi:10.1007/s12032-011-0113-8. CrossRef
Dauer DJ, Ferraro B, Song L, Yu B, Mora L, Buettner R, Enkemann S, Jove R, Haura EB. Stat3 regulates genes common to both wound healing and cancer. Oncogene, 2005; 24:3397–408; doi:10.1038/sj.onc.1208469. CrossRef
De Amicis F, Russo A, Avena P, Santoro M, Vivacqua A, Bonofiglio D, Mauro L, Aquila S, Tramontano D, Fuqua SA, Andò S. In vitro mechanism for downregulation of ER-α expression by epigallocatechin gallate in ER+/PR+ human breast cancer cells. Mol Nutr Food Res, 2013; 57:840–53; doi:10.1002/mnfr.201200560. CrossRef
De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets, 2012; 16:S17–27; doi:10.1517/14728222.2011.639361. CrossRef
Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer—the road to new treatment strategies. Lancet, 2017; 389:2430–42; doi:10.1016/S0140-6736(16)32454-0. CrossRef
Dieudonne M-N, Machinal-Quelin F, Serazin-Leroy V, Leneveu M-C, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun, 2002; 293:622–8; doi:10.1016/S0006-291X(02)00205-X. CrossRef
Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat, 2011; 125:315–23; doi:10.1007/s10549-010-1270-8. CrossRef
Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J, 2005; 390:1–9; doi:10.1042/BJ20050104. CrossRef
Eliyatkin N, Yalcin E, Zengel B, Akta? S, Vardar E. Molecular classification of breast carcinoma: from traditional, old-fashioned way to a new age, and a new way. J Breast Heal, 2015; 11:59–66; doi:10.5152/tjbh.2015.1669. CrossRef
Fan W, Tang Z, Chen D, Moughon D, Ding X, Chen S, Zhu M, Zhong Q. Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy, 2010; 6:614–21; doi:10.4161/auto.6.5.12189. CrossRef
Farabegoli F, Barbi C, Lambertini E, Piva R. (−)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect Prev, 2007; 31:499–504; doi:10.1016/j.cdp.2007.10.018. CrossRef
Farabegoli F, Papi A, Orlandi M. (-)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9 and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci Rep, 2011; 31:99–108; doi:10.1042/BSR20090143. CrossRef
Felix AS, Edwards R, Bowser R, Linkov F. Chemokines and cancer progression: a qualitative review on the role of stromal cell-derived factor 1-alpha and CXCR4 in endometrial cancer. Cancer Microenviron, 2010; 3:49–56; doi:10.1007/s12307-010-0042-7. CrossRef
Fernandez-Garcia B, Eiró N, Marín L, González-Reyes S, González LO, Lamelas ML, Vizoso FJ. Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology, 2014; 64:512–22; doi:10.1111/his.12300. CrossRef
Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med, 2003; 9:669–76; doi:10.1038/nm0603-669. CrossRef
Forghani P, Khorramizadeh MR, Waller EK. Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer. Cancer Med, 2014; 3:215–24; doi:10.1002/cam4.186. CrossRef
Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, Gómez HL, Tondini C, Ciruelos E, Burstein HJ, Bonnefoi HR. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med, 2018; 379:122–37; doi:10.1056/nejmoa1803164. CrossRef
Frech MS, Halama ED, Tilli MT, Singh B, Gunther EJ, Chodosh LA, Flaws JA, Furth PA. Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res, 2005; 65:681–5.
Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer, 2019; 19:405–14; doi:10.1038/s41568-019-0149-1. CrossRef
Fujimaki S, Hayashi K, Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S, Japan Public Health Center-Based Prospective Study on Cancer Cardiovascular Diseases Group. Re: Soy, isoflavones, and breast cancer risk in Japan (multiple letters). J Natl Cancer Inst, 2003; 95:1881–2; doi:10.1093/jnci/djg131. CrossRef
Furusato B, Mohamed A, Uhlén M, Rhim JS. CXCR4 and cancer. Pathol Int, 2010; 60:497–505; doi:10.1111/j.1440-1827.2010.02548.x. CrossRef
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell, 2015; 59:298–308; doi:10.1016/j.molcel.2015.06.011. CrossRef
Garbiec E, Cielecka-Piontek J, Kowalówka M, Ho?ubiec M, Zalewski P. Genistein—opportunities related to an interesting molecule of natural origin. Molecules, 2022; 27:815; doi:10.3390/molecules27030815. CrossRef
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene, 2001; 20:2499–513; doi:10.1038/sj.onc.1204349. CrossRef
Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, Fujita DJ, Ethier SP, Jove R. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ, 1997; 8:1267–76.
Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov, 2010; 9:775–89; doi:10.1038/nrd3179. CrossRef
Gemcitabine hydrochloride and genistein in treating women with stage IV breast cancer—study results. ClinicalTrials, n.d. Available via https://clinicaltrials.gov/ct2/ show/results/ NCT00244933?cond = genistein + and + breast + cancer &draw = 2 & rank = 1 (Accessed 12 July 2021).
Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S, Sturtz K, Wolff AC, Winer E, Hudis C, Stopeck A, Beck JT, Kaur JS, Whelan K, Tu D, Parulekar WR. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med, 2016; 375:209–19; doi:10.1056/nejmoa1604700. CrossRef
Gu JW, Makey KL, Tucker KB, Chinchar E, Mao X, Pei I, Thomas EY, Miele L. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc Cell, 2013; 5:1–10; doi:10.1186/2045-824X-5-9. CrossRef
Guan F, Ding Y, Zhang Y, Zhou Y, Li M, Wang C. Curcumin suppresses proliferation and migration of MDA-MB-231 breast cancer cells through autophagy-dependent Akt degradation. PLoS One, 2016; 11:1–18; doi:10.1371/journal.pone.0146553. CrossRef
Gupta S, Jaworska-Bieniek K, Narod SA, Lubinski J, Wojdacz TK, Jakubowska A. Methylation of the BRCA1 promoter in peripheral blood DNA is associated with triple-negative and medullary breast cancer. Breast Cancer Res Treat, 2014; 148:615–22; doi:10.1007/s10549-014-3179-0. CrossRef
Hallman K, Aleck K, Dwyer B, Lloyd V, Quigley M, Sitto N, Siebert AE, Dinda S. The effects of turmeric (Curcumin) on tumor suppressor protein (p53) and estrogen receptor (ERα) in breast cancer cells. Breast Cancer Targets Ther, 2017; 9:153–61; doi:10.2147/BCTT.S125783. CrossRef
Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol, 2010; 28:2784–95; doi:10.1200/JCO.2009.25.6529. CrossRef
Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, Howell A, Bundred NJ. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab, 1999; 84:4017–24; doi:10.1210/jc.84.11.4017. CrossRef
Hong OY, Noh EM, Jang HY, Lee YR, Lee BK, Jung SH, Kim JS, Youn HJ. Epigallocatechin gallate inhibits the growth of MDA-MB-231 breast cancer cells via inactivation of the β-Catenin signaling pathway. Oncol Lett, 2017; 14:441–6; doi:10.3892/ol.2017.6108. CrossRef
Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer, 2002; 2:91–100; doi:10.1038/nrc727. CrossRef
Hossainzadeh S, Ranji N, Naderi Sohi A, Najafi F. Silibinin encapsulation in polymersome: a promising anticancer nanoparticle for inducing apoptosis and decreasing the expression level of miR-125b/miR-182 in human breast cancer cells. J Cell Physiol, 2019; 234:22285–98; doi:10.1002/jcp.28795. CrossRef
Hsieh RW, Rajan SS, Sharma SK, Guo Y, DeSombre ER, Mrksich M, Greene GL. Identification of ligands with bicyclic scaffolds provides insights into mechanisms of estrogen receptor subtype selectivity. J Biol Chem, 2006; 281:17909–19; doi:10.1074/jbc.M513684200. CrossRef
Hu X. Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. CancerSpectrum Knowl Environ, 2002; 94:1704–11; doi:10.1093/jnci/94.22.1704. CrossRef
Huang CY, Han Z, Li X, Xie HH, Zhu SS. Mechanism of egcg promoting apoptosis of MCF–7 cell line in human breast cancer. Oncol Lett, 2017; 14:3623–7; doi:10.3892/ol.2017.6641. CrossRef
Hussain A, Bourguet-Kondracki M-L, Hussain F, Rauf A, Ibrahim M, Khalid M, Hussain H, Hussain J, Ali I, Khalil AA, Alhumaydhi FA, Khan M, Hussain R, Rengasamy KRR. The potential role of dietary plant ingredients against mammary cancer: a comprehensive review. Crit Rev Food Sci Nutr, 2022; 62:2580–605; doi:10.1080/10408398.2020.1855413. CrossRef
Hwang D-M, Kundu JK, Shin J-W, Lee J-C, Lee HJ, Surh Y-J. cis-9,trans-11-Conjugated linoleic acid down-regulates phorbol ester-induced NF- B activation and subsequent COX-2 expression in hairless mouse skin by targeting I B kinase and PI3K-Akt. Carcinogenesis, 2006; 28:363–71; doi:10.1093/carcin/bgl151. CrossRef
Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol, 2000; 37:1–11; doi:10.1016/S0161-5890(00)00018-3. CrossRef
Iqbal MA, Chattopadhyay S, Siddiqui FA, Ur Rehman A, Siddiqui S, Prakasam G, Khan A, Sultana S, Bamezai RN. Silibinin induces metabolic crisis in triple-negative breast cancer cells by modulating EGFR-MYC-TXNIP axis: potential therapeutic implications. FEBS J, 2021; 288:471–85; doi:10.1111/febs.15353. CrossRef
Iwamoto T, Yamamoto N, Taguchi T, Tamaki Y, Noguchi S. BRCA1 promoter methylation in peripheral blood cells is associated with increased risk of breast cancer with BRCA1 promoter methylation. Breast Cancer Res Treat, 2011; 129:69–77; doi:10.1007/s10549-010-1188-1. CrossRef
Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao G, Joseph BK, Rosen C, Shi YE. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res, 1997; 57:759–64.
Jiang H, Zhang N, Tang T, Feng F, Sun H, Qu W. Target the human alanine/serine/cysteine transporter 2(ASCT2): achievement and future for novel cancer therapy. Pharmacol Res, 2020; 158:104844; doi:10.1016/j.phrs.2020.104844. CrossRef
Jiang K, Wang W, Jin X, Wang Z, Ji Z, Meng G. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol Rep, 2015a; 33:2711–8; doi:10.3892/or.2015.3915. CrossRef
Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med, 2015b; 88:199–204; doi:10.1016/j.freeradbiomed.2015.06.014. CrossRef
Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta Bioenerg, 2011; 1807:552–61; doi:10.1016/j.bbabio.2010.10.012. CrossRef
Joshi H, Press MF. Molecular oncology of breast cancer. 5th edition, Elsevier, 2018; doi:10.1016/B978-0-323-35955-9.00022-2. CrossRef
Ju YH, Allred KF, Allred CD, Helferich WG. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis, 2006; 27:1292–9; doi:10.1093/carcin/bgi370. CrossRef
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, Leiserson MDM, Miller CA, Welch JS, Walter MJ, Wendl MC, Ley TJ, Wilson RK, Raphael BJ, Ding L. Mutational landscape and significance across 12 major cancer types. Nature, 2013; 502:333–9; doi:10.1038/nature12634. CrossRef
Khan H, Ullah H, Nabavi SM. Mechanistic insights of hepatoprotective effects of curcumin: therapeutic updates and future prospects. Food Chem Toxicol, 2019; 124:182–91; doi:10.1016/j.fct.2018.12.002. CrossRef
Khan M, Nur S, Abdulaal W. A study on DNA methylation modifying natural compounds identified EGCG for induction of IFI16 gene expression related to the innate immune response in cancer cells. Oncol Lett, 2022; 24:218; doi:10.3892/ol.2022.13339. CrossRef
Khan SA, Chatterton RT, Michel N, Bryk M, Lee O, Ivancic D, Heinz R, Zalles CM, Helenowski IB, Jovanovic BD, Franke AA, Bosland MC, Wang J, Hansen NM, Bethke KP, Dew A, Coomes M, Bergan RC. Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase ii trial. Cancer Prev Res, 2012; 5:309–19; doi:10.1158/1940-6207.CAPR-11-0251. CrossRef
Khan SA, Rogers MA, Obando JA, Tamsen A. Estrogen receptor expression of benign breast epithelium and its association with breast cancer. Cancer Res, 1994; 54:993–7.
Kim HN, Kim DH, Kim EH, Lee MH, Kundu JK, Na HK, Cha YN, Surh YJ. Sulforaphane inhibits phorbol ester-stimulated IKK-NF-κB signaling and COX-2 expression in human mammary epithelial cells by targeting NF-κB activating kinase and ERK. Cancer Lett, 2014a; 351:41–9; doi:10.1016/j.canlet.2014.03.037. CrossRef
Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, Kim JH, Choe JH, Yang JH, Nam SJ, Lee JE. Silibinin prevents TPA-induced MMP-9 expression and VEGF secretion by inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast cancer cells. Phytomedicine, 2009; 16:573–80; doi:10.1016/j.phymed.2008.11.006. CrossRef
Kim S, Han J, Jeon M, You D, Lee J, Kim HJ, Bae S, Nam SJ, Lee JE. Silibinin inhibits triple negative breast cancer cell motility by suppressing TGF-β2 expression. Tumor Biol, 2016; 37:11397–407; doi:10.1007/s13277-016-5000-7. CrossRef
Kim S, Jeon M, Lee J, Han J, Oh SJ, Jung T, Nam SJ, Kil WH, Lee JE. Induction of fibronectin in response to epidermal growth factor is suppressed by silibinin through the inhibition of STAT3 in triple negative breast cancer cells. Oncol Rep, 2014b; 32:2230–6; doi:10.3892/or.2014.3450. CrossRef
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science, 1997; 275:1132–6; doi:10.1126/science.275.5303.1132. CrossRef
Kolch W, Kotwaliwale A, Vass K, Janosch P. The role of Raf kinases in malignant transformation. Expert Rev Mol Med, 2002; 4:1–18; doi:10.1017/S1462399402004386. CrossRef
Kumar S, Bajaj S, Bodla R. Preclinical screening methods in cancer. Indian J Pharmacol, 2016; 48:481; doi:10.4103/0253-7613.190716. CrossRef
Kunihiro AG, Brickey JA, Frye JB, Cheng JN, Luis PB, Schneider C, Funk JL. Curcumin inhibition of TGFβ signaling in bone metastatic breast cancer cells and the possible role of oxidative metabolites. J Nutr Biochem, 2022; 99:108842; doi:10.1016/j.jnutbio.2021.108842. CrossRef
Lamartiniere CA, Zhang JX, Cotroneo MS. Genistein studies in rats: potential for breast cancer prevention and reproductive and developmental toxicity. Am J Clin Nutr, 1998; 68:1400S–5S; doi:10.1093/ajcn/68.6.1400S. CrossRef
Laud K, Gourdou I, Pessemesse L, Peyrat JP, Djiane J. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol Cell Endocrinol, 2002; 188:219–26; doi:10.1016/S0303-7207(01)00678-5. CrossRef
Lazzeroni M, Guerrieri-Gonzaga A, Gandini S, Johansson H, Serrano D, Cazzaniga M, Aristarco V, Macis D, Mora S, Caldarella P, Pagani G. A presurgical study of lecithin formulation of green tea extract in women with early breast cancer. Cancer Prev Res, 2017; 10:363–9; doi:10.1158/1940-6207.CAPR-16-0298. CrossRef
Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC, Lee IS. Silibinin suppresses PMA-induced MMP-9 expression by blocking the AP-1 activation via MAPK signaling pathways in MCF-7 human breast carcinoma cells. Biochem Biophys Res Commun, 2007; 354:165–71; doi:10.1016/j.bbrc.2006.12.181. CrossRef
Lei H, Deng C-X. Fibroblast growth factor receptor 2 signaling in breast cancer. Int J Biol Sci, 2017; 13:1163–71; doi:10.7150/ijbs.20792. CrossRef
Li X-H, Fang X, Gaynor RB. Role of IKKγ/NEMO in assembly of the IκB kinase complex. J Biol Chem, 2001; 276:4494–500; doi:10.1074/jbc.M008353200. CrossRef
Li X, Wang X, Xie C, Zhu J, Meng Y, Chen Y, Li Y, Jiang Y, Yang X, Wang S, Chen J. Sonic hedgehog and Wnt/β-catenin pathways mediate Curcumin inhibition of breast cancer stem cells. Anticancer Drugs, 2018; 29:208–15; doi:10.1097/CAD.0000000000000584. CrossRef
Li Y, Buckhaults P, Cui X, Tollefsbol TO. Combinatorial epigenetic mechanisms and efficacy of early breast cancer inhibition by nutritive botanicals. Epigenomics, 2016; 8:1019–37; doi:10.2217/epi-2016-0024. CrossRef
Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res, 2010; 16:2580–90; doi:10.1158/1078-0432.CCR-09-2937. CrossRef
Lim S-O, Li C-W, Xia W, Lee H-H, Chang S-S, Shen J, Hsu JL, Raftery D, Djukovic D, Gu H, Chang WC. EGFR signaling enhances aerobic glycolysis in triple-negative breast cancer cells to promote tumor growth and immune escape. Cancer Res, 2016; 76:1284–96; doi:10.1158/0008-5472.CAN-15-2478. CrossRef
Lin CJ, Sukarieh R, Pelletier J. Silibinin inhibits translation initiation: implications for anticancer therapy. Mol Cancer Ther, 2009; 8:1606–12; doi:10.1158/1535-7163.MCT-08-1152. CrossRef
Liu B, Edgerton S, Yang X, Kim A, Ordonez-Ercan D, Mason T, Alvarez K, McKimmey C, Liu N, Thor A. Low-dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumor prevention. Cancer Res, 2005; 65:879–86.
Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov, 2009; 8:627–44; doi:10.1038/nrd2926. CrossRef
Liu W, Ji Y, Sun Y, Si L, Fu J, Hayashi T, Onodera S, Ikejima T. Estrogen receptors participate in silibinin-caused nuclear translocation of apoptosis-inducing factor in human breast cancer MCF-7 cells. Arch Biochem Biophys, 2020; 689:108458; doi:10.1016/j.abb.2020.108458. CrossRef
Loh YN, Hedditch EL, Baker LA, Jary E, Ward RL, Ford CE. The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer, 2013; 13:174; doi:10.1186/1471-2407-13-174. CrossRef
Lu AP, Gupta A, Li C, Ahlborn TE, Ma YS, Shi EY, Liu J. Molecular mechanisms for aberrant expression of the human breast cancer specific gene 1 in breast cancer cells: control of transcription by DNA thylation and intronic sequences. Oncogene, 2001; 20:5173–85; doi:10.1038/sj.onc.1204668. CrossRef
Lu K, Alcivar AL, Ma J, Foo TK, Zywea S, Mahdi A, Huo Y, Kensler TW, Gatza ML, Xia B. NRF2 induction supporting breast cancer cell survival is enabled by oxidative stress–induced DPP3–KEAP1 interaction. Cancer Res, 2017; 77:2881–92; doi:10.1158/0008-5472.CAN-16-2204. CrossRef
Lu W, Lin C, King TD, Chen H, Reynolds RC, Li Y. Silibinin inhibits Wnt/β-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells. Cell Signal, 2012; 24:2291–6; doi:10.1016/j.cellsig.2012.07.009. CrossRef
Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol, 2011; 27:441–64; doi:10.1146/annurev-cellbio-092910-154237. CrossRef
Ma X, Yu X, Min J, Chen X, Liu R, Cui X, Cheng J, Xie M, Diel P, Hu X. Genistein interferes with antitumor effects of cisplatin in an ovariectomized breast cancer xenograft tumor model. Toxicol Lett, 2022; 355:106–15; doi:10.1016/j.toxlet.2021.11.013. CrossRef
MacDonald BT, Tamai K, He X. Wnt/β-Catenin signaling: components, mechanisms, and diseases. Dev Cell, 2009; 17:9–26; doi:10.1016/j.devcel.2009.06.016. CrossRef
Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature, 2009; 461:99–103; doi:10.1038/nature08242. CrossRef
Mao M, Chen Y, Jia Y, Yang J, Wei Q, Li Z, Chen L, Chen C, Wang L. PLCA8 suppresses breast cancer apoptosis by activating the PI3k/AKT/NF-κB pathway. J Cell Mol Med, 2019; 23:6930–41; doi:10.1111/jcmm.14578. CrossRef
Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Di Stefano V, Minutoli L, Atteritano M, Levy RM, D’Anna R, Frisina N. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab, 2008; 93:4787–96; doi:10.1210/jc.2008-1087. CrossRef
Marzo I, Brenner C, Zamzami N, Ju?rgensmeier JM, Susin SA, Vieira HLA, Prévost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science, 1998; 281:2027–31; doi:10.1126/science.281.5385.2027. CrossRef
McMichael-Phillips DF, Harding C, Morton M, Roberts SA, Howell A, Potten CS, Bundred NJ. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr, 1998; 68; doi:10.1093/ajcn/68.6.1431S. CrossRef
Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy, 2007; 3:542–5; doi:10.4161/auto.4600. CrossRef
Mooz J, Oberoi-Khanuja TK, Harms GS, Wang W, Jaiswal BS, Seshagiri S, Tikkanen R, Rajalingam K. Dimerization of the kinase ARAF promotes MAPK pathway activation and cell migration. Sci Signal, 2014; 7; doi:10.1126/scisignal.2005484. CrossRef
Muller PAJ, Vousden KH. Mutant p53 in Cancer: new functions and therapeutic opportunities. Cancer Cell, 2014; 25:304–17; doi:10.1016/j.ccr.2014.01.021. CrossRef
Nejati-Koshki K, Zarghami N, Pourhassan-Moghaddam M, Rahmati-Yamchi M, Mollazade M, Nasiri M, Esfahlan RJ, Barkhordari A, Tayefi-Nasrabadi H. Inhibition of leptin gene expression and secretion by silibinin: possible role of estrogen receptors. Cytotechnology, 2012; 64:719–26; doi:10.1007/s10616-012-9452-3. CrossRef
Nicot C, Napal L, Relat J, González S, Llebaria A, Woldegiorgis G, Marrero PF, Haro D. C75 activates malonyl-CoA sensitive and insensitive components of the CPT system. Biochem Biophys Res Commun, 2004; 325:660–4; doi:10.1016/j.bbrc.2004.10.085. CrossRef
O’Bryan S, Dong S, Mathis JM, Alahari SK. The roles of oncogenic miRNAs and their therapeutic importance in breast cancer. Eur J Cancer, 2017; 72:1–11; doi:10.1016/j.ejca.2016.11.004. CrossRef
O’Callaghan DS, O’Donnell D, O’Connell F, O’Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol, 2010; 5:2024–36; doi:10.1097/jto.0b013e3181f387e4. CrossRef
Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell, 1993; 74:609–19; doi:10.1016/0092-8674(93)90509-O. CrossRef
Paranjape T, Slack FJ, Weidhaas JB. MicroRNAs: tools for cancer diagnostics. Gut, 2009; 58:1546–54; doi:10.1136/gut.2009.179531. CrossRef
Park M, Hong J. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells, 2016; 5:15; doi:10.3390/cells5020015. CrossRef
Peng W-X, Koirala P, Mo Y-Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene, 2017; 36:5661–7; doi:10.1038/onc.2017.184. CrossRef
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med, 2005; 353:1659–72; doi:10.1056/NEJMoa052306. CrossRef
Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res, 1996; 56:2745–7.
Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res, 2000; 60:213–8.
Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev, 2007; 17:45–51; doi:10.1016/j.gde.2006.12.007. CrossRef
Poma P, Labbozzetta M, D’Alessandro N, Notarbartolo M. NF-κB is a potential molecular drug target in triple-negative breast cancers. Omi A J Integr Biol, 2017; 21:225–31; doi:10.1089/omi.2017.0020. CrossRef
Poola I, Abraham J, Baldwin K, Saunders A, Bhatnagar R. Estrogen receptors beta4 and beta5 are full length functionally distinct ERβ isoforms: cloning from human ovary and functional characterization. Endocrine, 2005; 27:227–38; doi:10.1385/ENDO:27:3:227. CrossRef
Protective effects of the nutritional supplement sulforaphane on doxorubicin-associated cardiac dysfunction—no study results posted. ClinicalTrials, n.d. Available via https://clinicaltrials.gov/ct2/show/results/NCT03934905?cond=NCT03934905&draw=2&rank=1 (Accessed 12 July 2021).
Puig T, Relat J, Marrero PF, Haro D, Brunet J, Colomer R. Green tea catechin inhibits fatty acid synthase without stimulating carnitine palmitoyltransferase-1 or inducing weight loss in experimental animals. Anticancer Res, 2008a; 28:3671–6.
Puig T, Vázquez-Martín A, Relat J, Pétriz J, Menéndez JA, Porta R, Casals G, Marrero PF, Haro D, Brunet J, Colomer R. Fatty acid metabolism in breast cancer cells: differential inhibitory effects of epigallocatechin gallate (EGCG) and C75. Breast Cancer Res Treat, 2008b; 109:471–9; doi:10.1007/s10549-007-9678-5. CrossRef
Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol, 1995; 7:541–6; doi:10.1097/00001622-199511000-00012. CrossRef
Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene, 2007; 26:3291–310; doi:10.1038/sj.onc.1210422. CrossRef
Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med, 2017; 377:523–33; doi:10.1056/nejmoa1706450. CrossRef
Roy AM, Baliga MS, Katiyar SK. Epigallocatechin-3-gallate induces apoptosis in estrogen receptor-negative human breast carcinoma cells via modulation in protein expression of p53 and Bax and caspase-3 activation. Mol Cancer Ther, 2005; 4:81–90.
Sajadimajd S, Khazaei M. Oxidative stress and cancer: the role of Nrf2. Curr Cancer Drug Targets, 2018; 18:538–57; doi:10.2174/1568009617666171002144228. CrossRef
Samavat H, Ursin G, Emory TH, Lee E, Wang R, Torkelson CJ, Dostal AM, Swenson K, Le CT, Yang CS, Yu MC, Yee D, Wu AH, Yuan JM, Kurzer MS. A randomized controlled trial of green tea extract supplementation and mammographic density in postmenopausal women at increased risk of breast cancer. Cancer Prev Res, 2017; 10:710–8; doi:10.1158/1940-6207.CAPR-17-0187. CrossRef
Seo HS, DeNardo DG, Jacquot Y, Laïos I, Vidal DS, Zambrana CR, Leclercq G, Brown PH. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat, 2006; 99:121–34; doi:10.1007/s10549-006-9191-2. CrossRef
SFX-01 in the treatment and evaluation of metastatic breast cancer. ClinicalTrials, n.d. Available via https://clinicaltrials.gov/ct2/show/NCT02970682?cond=NCT02970682&draw=2&rank=1 (Accessed 12 July 2021).
Shike M, Doane AS, Russo L, Cabal R, Reis-Filho JS, Gerald W, Cody H, Khanin R, Bromberg J, Norton L. The effects of soy supplementation on gene expression in breast cancer: a randomized placebo-controlled study. J Natl Cancer Inst, 2014; 106; doi:10.1093/jnci/dju189. CrossRef
Shimizu H, Ross RK, Bernstein L, Henderson BE, Mack TM, Yatani R. Cancers of the prostate and breast among japanese and white immigrants in los angeles county. Br J Cancer, 1991; 63:963–6; doi:10.1038/bjc.1991.210. CrossRef
Shin D, Kim EH, Lee J, Roh J-L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med, 2018; 129:454–62; doi:10.1016/j.freeradbiomed.2018.10.426. CrossRef
Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BK, Sethi G, Bishayee A. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta Rev Cancer, 2014; 1845:136–54; doi:10.1016/j.bbcan.2013.12.005. CrossRef
Soerjomataram I, Oomen D, Lemmens V, Oenema A, Benetou V, Trichopoulou A, Coebergh JW, Barendregt J, de Vries E. Increased consumption of fruit and vegetables and future cancer incidence in selected European countries. Eur J Cancer, 2010; 46:2563–80; doi:10.1016/j.ejca.2010.07.026. CrossRef
Song X, Zhang M, Dai E, Luo Y. Molecular targets of curcumin in breast cancer (Review). Mol Med Rep, 2019; 19:23–9; doi:10.3892/mmr.2018.9665. CrossRef
Sporikova Z, Koudelakova V, Trojanec R, Hajduch M. Genetic markers in triple-negative breast cancer. Clin Breast Cancer, 2018; 18:e841–50; doi:10.1016/j.clbc.2018.07.023. CrossRef
St?pkowski TM, Kruszewski MK. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic Biol Med, 2011; 50:1186–95; doi:10.1016/j.freeradbiomed.2011.01.033. CrossRef
Study to evaluate the effect of sulforaphane in broccoli sprout extract on breast tissue—study results. ClinicalTrials, n.d. Available via https://clinicaltrials.gov/ct2/show/results/NCT00982319?cond=NCT00982319&draw=2&rank=1 (Accessed 12 July 2021).
Sun X, Niu X, Chen R, He W, Chen D, Kang R, Tang D. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology, 2016; 64:488–500; doi:10.1002/hep.28574. CrossRef
Surh Y-J, Chun K-S, Cha H-H, Han SS, Keum Y-S, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res Mol Mech Mutagen, 2001; 480–481:243–68; doi:10.1016/S0027-5107(01)00183-X. CrossRef
Takahashi-Yanaga F. Activator or inhibitor? GSK-3 as a new drug target. Biochem Pharmacol, 2013; 86:191–9; doi:10.1016/j.bcp.2013.04.022. CrossRef
Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, Taniguchi T. Integration of interferon-α/β signalling to p53 responses in tumour suppression and antiviral defence. Nature, 2003; 424:516–23; doi:10.1038/nature01850. CrossRef
Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy, 2005; 1:84–91; doi:10.4161/auto.1.2.1697. CrossRef
Taoqi Zhou, Minming Zhou, Chaochao Tong, Mali Zhuo. Cauliflower bioactive compound sulforaphane inhibits breast cancer development by suppressing NF-κB /MMP-9 signaling pathway expression. Cell Mol Biol, 2022; 68:134–43; doi:10.14715/cmb/2022.68.4.17. CrossRef
Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell, 2009; 139:871–90; doi:10.1016/j.cell.2009.11.007. CrossRef
Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene, 2000; 19:6613–26; doi:10.1038/sj.onc.1204086. CrossRef
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med, 2017; 377:122–31; doi:10.1056/nejmoa1703643. CrossRef
Waks AG, Winer EP. Breast cancer treatment: a review. J Am Med Assoc, 2019; 321:288–300; doi:10.1001/jama.2018.19323. CrossRef
Wang D, Liu C, Wang J, Jia Y, Hu X, Jiang H, Shao ZM, Zeng YA. Protein C receptor stimulates multiple signaling pathways in breast cancer cells. J Biol Chem, 2018; 293(4):1413–24; doi: 10.1074/jbc.M117.814046. CrossRef
Wang Y, Liang WC, Pan WL, Law WK, Hu JS, Ip DTM, Waye MM, Ng TB, Wan DC. Silibinin, a novel chemokine receptor type 4 antagonist, inhibits chemokine ligand 12-induced migration in breast cancer cells. Phytomedicine, 2014; 21:1310–7; doi:10.1016/j.phymed.2014.06.018. CrossRef
Wei R, Mao L, Xu P, Zheng X, Hackman RM, MacKenzie GG, Wang Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct, 2018; 9:5682–96; doi:10.1039/c8fo01397g. CrossRef
Wirth M, Joachim J, Tooze SA. Autophagosome formation-the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin Cancer Biol, 2013; 23:301–9; doi:10.1016/j.semcancer.2013.05.007. CrossRef
Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology, College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast. J Clin Oncol, 2013; 31:3997–4013; doi:10.1200/JCO.2013.50.9984. CrossRef
Wong EM, Southey MC, Fox SB, Brown MA, Dowty JG, Jenkins MA, Giles GG, Hopper JL, Dobrovic A. Constitutional methylation of the BRCA1 promoter is specifically associated with BRCA1 mutation-associated pathology in early-onset breast cancer. Cancer Prev Res, 2011; 4:23–33; doi:10.1158/1940-6207.CAPR-10-0212. CrossRef
Wu K, Quan Z, Weng Z, Li F, Zhang Y, Yao X, Chen Y, Budman D, Goldberg ID, Shi YE. Expression of neuronal protein synuclein gamma gene as a novel marker for breast cancer prognosis. Breast Cancer Res Treat, 2007; 101:259–67; doi:10.1007/s10549-006-9296-7. CrossRef
Wu Y, Zhou Y, He J, Sun H, Jin Z. Long non-coding RNA H19 mediates ovarian cancer cell cisplatin-resistance and migration during EMT. Int J Clin Exp Pathol, 2019; 12:2506–15.
Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res, 2009; 19:156–72; doi:10.1038/cr.2009.5. CrossRef
Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W, Wang J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med, 2019; 23:4900–12; doi:10.1111/jcmm.14511. CrossRef
Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol Res, 2011; 64:113–22; doi:10.1016/j.phrs.2011.03.001. CrossRef
Ye Y, Guo J, Xiao P, Ning J, Zhang R, Liu P, Yu W, Xu L, Zhao Y, Yu J. Macrophages-induced long noncoding RNA H19 up-regulation triggers and activates the miR-193b/MAPK1 axis and promotes cell aggressiveness in hepatocellular carcinoma. Cancer Lett, 2020; 469:310–22; doi:10.1016/j.canlet.2019.11.001. CrossRef
Yu Y, Wan Y, Huang C. The biological functions of NF-κB1 (p ) and its potential as an anti-cancer target. Curr Cancer Drug Targets, 2009; 9:566–71; doi:10.2174/156800909788486759. CrossRef
Zadeh MM, Motamed N, Ranji N, Majidi M, Falahi F. Silibinin-induced apoptosis and downregulation of microRNA-21 and MicroRNA-155 in MCF-7 human breast cancer cells. J Breast Cancer, 2016; 19:45–52; doi:10.4048/jbc.2016.19.1.45. CrossRef
Zhang J, Zheng J, Chen H, Li X, Ye C, Zhang F, Zhang Z, Yao Q, Guo Y. Curcumin targeting NF-κB/ubiquitin-proteasome-system axis ameliorates muscle atrophy in triple-negative breast cancer cachexia mice. Mediators Inflamm, 2022a; 2022:1–18; doi:10.1155/2022/2567150. CrossRef
Zhang X, Hao J. Development of anticancer agents targeting the Wnt/β-catenin signaling. Am J Cancer Res, 2015; 5:2344–60.
Zhang Y, Lu Q, Li N, Xu M, Miyamoto T, Liu J. Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway. NPJ Breast Cancer, 2022b; 8:40; doi:10.1038/s41523-022-00402-4. CrossRef
Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol, 2020; 13:165; doi:10.1186/s13045-020-00990-3. CrossRef
Zhang Z, Atwell LL, Farris PE, Ho E, Shannon J. Associations between cruciferous vegetable intake and selected biomarkers among women scheduled for breast biopsies. Public Health Nutr, 2016; 19:1288–95; doi:10.1017/S136898001500244X. CrossRef
Zheng N, Liu L, Liu W, Zhang P, Huang H, Zang L, Hayashi T, Tashiro S, Onodera S, Xia M, Ikejima T. ERβ up-regulation was involved in silibinin-induced growth inhibition of human breast cancer MCF-7 cells. Arch Biochem Biophys, 2016a; 591:141–9; doi:10.1016/j.abb.2016.01.002. CrossRef
Zheng N, Zhang P, Huang H, Liu W, Hayashi T, Zang L, Zhang Y, Liu L, Xia M, Tashiro S, Onodera S, Ikejima T. ERα down-regulation plays a key role in silibinin-induced autophagy and apoptosis in human breast cancer MCF-7 cells. J Pharmacol Sci, 2015; 128:97–107; doi:10.1016/j.jphs.2015.05.001. CrossRef
Zhong Z, Wen Z, Darnell JE. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science, 1994; 264:95–8; doi:10.1126/science.8140422. CrossRef
Zhou W, Ye X, Xu J, Cao M-G, Fang Z-Y, Li L-Y, Guan GH, Liu Q, Qian YH, Xie D. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR-200b/c and let-7b. Sci Signal, 2017; 10; doi:10.1126/scisignal.aak9557. CrossRef