INTRODUCTION
In people with type 2 diabetes, Teneligliptin, a dipeptidyl peptidase-4 inhibitor is used in association with proper diet and exercise to control blood sugar levels (Kishimoto, 2013; Sharma et al., 2016). Teneligliptin increases the level of natural substances such as incretins that help to control blood sugar by releasing insulin, especially after a meal. Drug substances are generally obtained by a series of manufacturing steps and as a consequence, either from the raw materials or by the process (intermediate stages and/or degradation products) the impurities are incorporated with the drug substances. In addition, the residual solvents and catalysts are also present in the pharmaceuticals as impurities, which are subject to evaluation and in fact to be controlled as much as possible because they do not contribute to the therapeutic activity but are most probably harmful (United States Pharmacopoeia USP, 2022). In reality, the process impurities are neither completely controlled nor removed by practical manufacturing techniques (International Conference on Harmonization ICH, 2011). Here the use of tetra-n-butylammonium bromide (TBAB) as a phase transfer catalyst in Teneligliptin synthesis leads to the presence of TBAB as a residual impurity in the final drug. Even though many attempts were made during the synthetic process to remove the potential organic ionic impurity (TBAB), hitherto it is not been completely eradicated from the final drug according to the existing reports.
As per the governing authorities’ guidelines on drugs, it is essentially important to control impurities in the formulations of drug substances and drug products below the threshold, based on dosage. But to our best, there are no considerable numbers of reports on the estimation of TBAB in drugs either by GC or HPLC in the literature. Generally, the estimation of amines is difficult due to their interaction with the stationary phase of both GC and LC. Particularly, the low molecular weight amines make more interaction with the stationary phase’s silanol groups (causing retention time and peak shape problems), leading to column aging and retention differences. In addition, the lack of chromophores in low molecular weight amines makes them difficult to be detected. However, there are few reports available with low molecular weight amines using HPLC associated with the derivatization techniques (Hao et al., 2004; Herraez-Hernandez et al., 2006; Meseguer-Lloret et al., 2004). As we know that the derivatization techniques are laborious and less sensitive towards pharmaceutical products it is no longer recommended for the trace level estimation. On the other hand, a recent report on the direct estimation of TBAB in Daclatasvir dihydrochloride API by LC-MS/MS seems to be specific but the LOQ (310 ppm) was high (Reddy et al., 2019).
In GC, mostly the derivatization reports end up with the sorption on stationary phase along with the other disruptions due to the basic, volatile, and polar nature of the amines. With a view of overcome the above issues, derivatization techniques were reported for the estimation of some aliphatic amines but they were neither sensitive nor performed on pharmaceuticals, tested on effluents (Chang et al., 2005; Jerome et al., 2008; Zhao et al., 2003). The earlier pyrolysis method of TBAB (Lopez et al., 1988) to produce n-butyl bromide and tert-butylamine for the estimation of TBAB by GC was also laborious, and not sensitive for measuring TBAB in trace level according to the impurity control strategy (Lopez et al.’s (1988) quantification with linearity basis was in the range of 0.0485–0.2416 g/ml).
Alternatively, the low molecular weight aliphatic amines such as amyl amine, trimethylamine, tert-butylamine, and piperazine were separated from the pharmaceuticals (Hajos et al., 2002; Hall et al., 1995; Jagota et al., 1996; Tan et al., 1995) and certain low molecular weight amines and ammonium derivatives, hydroxylamine and ethanolamine were estimated from the effluents (Christian et al., 2020; Fernando et al., 2022), saline water (Fernanda et al., 2017) and natural gas (Kadnar, 1999; Maryam et al., 2021) using ion chromatography (IC) on appropriate column through either suppressed or non-suppressed conductivity detection (Krol, 1992; Kumagai et al., 1996). There are considerable numbers of reports on the estimation of low molecular weight amines using IC including the sensitive direct estimation of TBAB in Levetiracetam (Subramanian et al., 2009), whereas, to our knowledge best, neither GC nor LC methods are available for the direct estimation of trace level TBAB in pharmaceuticals (except the one with tandem mass LC with low sensitivity). Keeping the above facts in mind, we were involved in the development of a suitable, selective, and sensitive direct method to determine the TBAB in the Teneligliptin drug.
MATERIALS AND METHODS
Materials
Analytical grade reagents and solvents were used for the research work. TBAB was procured from Sigma-Aldrich Chemicals Pvt. Ltd., India.
Optimized GC-MS conditions
To ensure the GC system’s suitability in providing a valid peak shape and acceptable recovery, a range of chromatographic parameters were optimized during the method development process. The optimization parameters include the column temperature (80°C –150°C), flow rate (1.0–1.5 ml with the constant flow), injector temperature (initial temperature 200°C–250°C), and capillary GC columns (HP-5, DB-1701, and DB-1 with varying film thickness). In terms of retention time at lower temperatures, the HP-5 (30 m × 0.32 mm × 1 µm) and the DB-1701 (30 m × 0.32 mm × 1 µm) columns performed well. The stationary phases, however, showed greater bleed and less uniform baseline across the temperature range tested. On the DB-1 capillary column (60 m × 0.32 mm × 0.25 µm) with helium as a carrier gas, we achieved an acceptable level of selectivity, sensitivity, resolution, and chromatographic separation with a stable baseline. Hence, Agilent 5977B GC/MSD, USA was used for the analysis (GC paired with a quadrupole mass spectrometer) using DB-1. The injection volume was chosen to be 1 µl with a split inlet of 10:1. GC oven temperatures were set at 120°C and held for 1 minute before ramped to 280°C @ 15°C/minute. It took 10.7 minutes to reach the final temperature. GC-MS interface, ion source, and injection temperatures were 260°C, 250°C, and 230°C, respectively. Helium (carrier gas) was used at a flow rate of 2 ml/minute and 70 eV was used to ionize the gas. We collected three mass peaks at m/z: 100, 142, and 185 using selective ion monitoring (SIM) mode. The analyses were performed using GC-MS solution software, Version 2.50. Based on the mass spectral library of the National Institute of Standards and Technology, the compounds were identified.
Standard preparation
The standard stock solution was prepared by diluting 250 mg of TBAB in 100 ml acetonitrile (CH3CN). Further, 1 ml of the above solution was diluted to 50 ml.
Sample preparation
A 500 mg of sample was diluted to 10 ml using CH3CN.
RESULTS AND DISCUSSION
Method development
The attempt to separate TBAB using a DB-5 column (5% phenyl: 95% dimethylpolysiloxane) was unsuccessful due to the irregular peak shape, whereas, the replacement of DB-5 by DB-1 (100% dimethylpolysiloxane) resulted in sharp peaks. By injecting 1 µl solution, the effects on separation and determination of injection volume ratios were studied. The split ratio was fixed at 10:1 according to the detector response. The separation of TBAB was investigated as a function of column temperature (120°C was preferred as the initial column temperature) and the injections were performed as follows: blank (one injection), standard solution (six injections), blank (one injection), sample solution (two injections), and standard solution-bracketing (one injection).
Method validation
Method validation was executed according to the validation of analytical methods outlined in the ICH guidelines. To determine the suitability of GC-MS, a standard solution of TBAB of 1,000 ppm was injected and the data is provided in Table 1.
For the six preparations of standard solution, there should not be a difference of more than 15% in the relative standard deviation (RSD) area of the TBAB peak. The TBAB content in Teneligliptin was estimated using the system precision and the % RSD was found to be in the acceptance criteria.
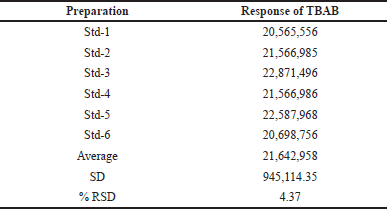 | Table 1. System suitability data for standard samples. [Click here to view] |
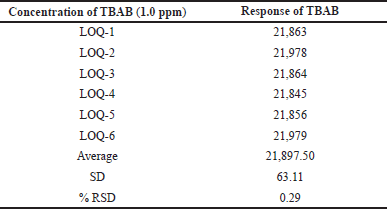 | Table 2. Precision at LOQ level. [Click here to view] |
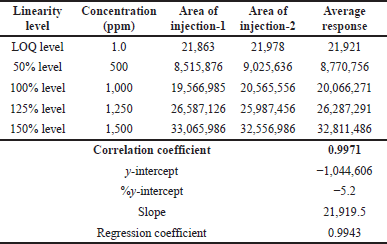 | Table 3. Linearity of TBAB. [Click here to view] |
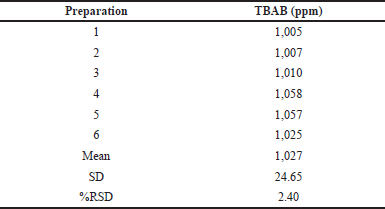 | Table 4. System precision analysis. [Click here to view] |
 | Table 5. Accuracy of TBAB. [Click here to view] |
Specificity
Methanol, ethanol, isopropyl chloride, thionyl chloride, and octane sulfonic acid were used in the Teneligliptin manufacturing process. Hence, the solvents used to manufacture Teneligliptin were injected as part of the specificity study but no interference was found with TBAB (the ICH listed solvents were prepared according to the level stated accordingly but the other solvents were prepared at 0.1% level). In this method, none of the process solvents exhibited mass fragments of the selected ion, and thus, indicate the absence of interference with the concerned TBAB analyte.
Limit of detection (LOD) and limit of quantification (LOQ)
Calibration curves were used according to the ICH guidelines for the determination of LOD and LOQ values and are outlined in Table 2 for better precision. TBAB was found to possess LOD and LOQ values of 0.3 and 1.0 ppm, respectively. Based on the six LOQ preparations, the RSD peak area of TBAB was found to be 0.29.
Linearity
To achieve a stable baseline, the system and column were conditioned. The injections were performed as specified and the observations are summarized in Table 3. The linearity correlations coefficient was 0.9971 and linearity was tested between LOQ and 150%.
The results indicate that the proposed method meets the acceptance criteria as evidenced by the correlation coefficient (>0.99).
Precision and accuracy
Six replicate preparations were injected with the standard solution containing 1,000 ppm of TBAB to determine the method’s precision. TBAB showed an RSD of 2.4 on six replicates within the permissible limits as indicated in Table 4. Analysis of the peak areas of the analyte confirms the precision of this method with low RSD. The TBAB sample was spiked at QL levels, 100% and 150% for accuracy testing, and the data are reproduced in Table 5. All accuracy levels of TBAB should have a recovery rate between 80 and 120 according to the acceptance criteria. The average recovery percentage was well within the permissible range.
Robustness
Method robustness was assessed by studying the impact of small variations in oven temperature, injector temperature, and flow rate on the peak area of TBAB at a 1,000 ppm concentration level. The results showed that the %RSD found in the solution using each of the modified and optimized GC conditions is well within the acceptance criterion. Thus, the observed data (Table 6) demonstrated the robustness of the suggested method.(Acceptance criteria: The RSD of peak areas should be ≤15% for six injections).
As an outcome of the successful method validation, a few of the significant recommended parameters are shown in Table 7 as standard test procedures for the estimation of TBAB content by GC-MS.
 | Table 6. Robustness of TBAB. [Click here to view] |
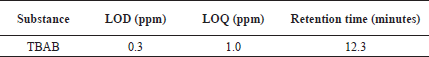 | Table 7. Method recommendations for TBAB. [Click here to view] |
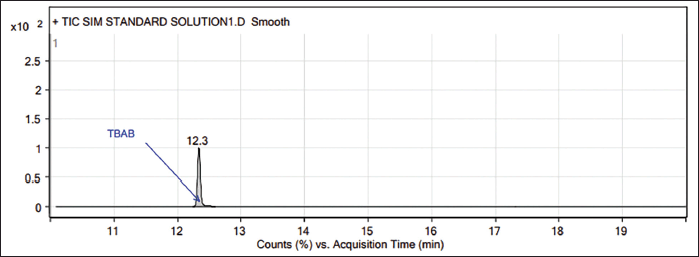 | Figure 1. GC-MS chromatogram of TBAB standard (1,000 ppm). [Click here to view] |
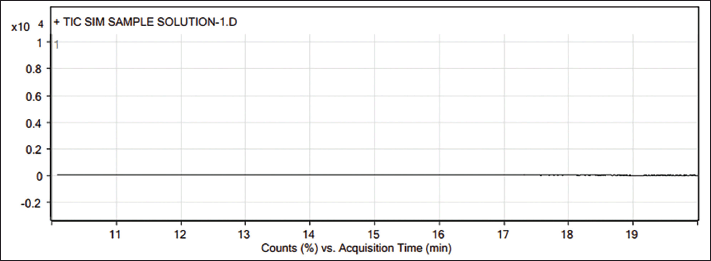 | Figure 2. GC-MS Sample chromatogram of Teneligliptin. [Click here to view] |
Mass spectral analysis
In GC-MS, the TBAB appears at 12.35 minutes (Figs. 1–3), and the presence of TBAB (C16H36NBr, m/z 322.4) is confirmed by its mass spectrum through the major fragments of TBAB at m/z 185, 142, 100 and 57 (Spectrum and fragments are reported in the Supplementary material). In general, diluent interference is critical in trace-level quantitation in other detectors such as FID, TCD, etc., and or other tools, particularly with high boiling diluents. This method is SIM mode with the selection of fragments m/z 57, 100, 142, and 185 (these ions only pass the ion filter and reach the detector while the rest go to the vent). Acetonitrile is the diluent (molecular weight: 41) and other related impurities of less than 50 m/z are filtered through SIM mode, hence, there is no diluent interference with this method.
Batch analysis report
Three batches of Teneligliptin drug substances were analyzed according to the above method to find practical applications in industries. As an outcome of the batch analyses, the results were found to be lower than the detection limit, which ensures the absence of TBAB in the tested batches. The complete batch analysis reports of the three different batches are systematically summarized with appropriate chromatograms and provided as Supplementary Material.
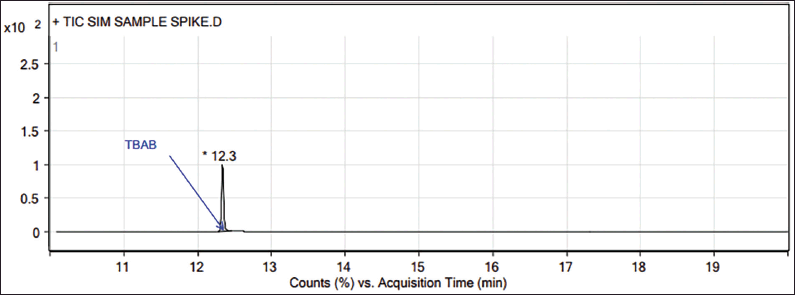 | Figure 3. GC-MS chromatogram of Teneligliptin spiked with TBAB. [Click here to view] |
CONCLUSIONS
The challenges of determining low molecular weight amines in GC are well-known due to the basic nature of the analyte including that makes strong binding with free silanol groups in the GC columns. However, we have developed a method for the quantitative determination of TBAB in Teneligliptin, and it appears to be simple, specific, rapid, robust, linear, precise, and accurate. The developed method has been validated according to ICH guidelines, which is an essential step to ensure the reliability and reproducibility of the results. It is also noteworthy that the developed method does not require any derivatizing agents, making it easier to implement in pharmaceutical analytical labs. Further, the potential applicability of the GC-MS method for determining the presence of TBAB in a range of gliptins and other drug substances is possible but the specificity and sensitivity of the method may vary depending on the specific analyte and matrix. Overall, our findings are valuable for the pharmaceutical industry, and our method may contribute to the development of safer and more effective drugs.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
There is no funding to report.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Chang, JS, Abu-Orf M, Dentel, SK. Alkylamine odors from degradation of flocculant polymers in sludges. Water Res. 2005;39:3369–75. CrossRef
Christian B, Johannes R, Detlef J. Simultaneous determination of tetrabutylammonium and tributylamine in wastewater byIC/LC-MS. ThermoFischer Scientific Technical Report, 2020; DOI: 10.13140/RG.2.2.29215.10402
European Medicines Agency ICH Topic Q 2 (R1). Note for guidance on validation of analytical procedures text and methodology. 1995;CPMP/ICH/381/95.
Fernanda NF, Julio CA, Fernanda VMP, Manuel CC, Arnaldo AN, Rainério EJ, Maria ICM, Ultrasound-assisted purge-and-trap extraction for simultaneous determination of low-molecular weight amines and ammonium in high salinity waters by ion chromatography. Microchem J, 2017;133:658–62. CrossRef
Fernando PN, Egwu IN, Hussain MS. Ion chromatographic determination of trace hydroxylamine in waste streams generated by a pharmaceutical reaction process. J Chromatogr A. 2002;956:149–55. CrossRef
Hajos P, Horvath K, Conca R, Sarzanini C. Histidine as a dipolar eluent in ion chromatography of aliphatic amines. Chromatographia, 2002;56:S103–6. CrossRef
Hall RE, Havner GD, Good R, Dunn DL. Ion chromatographic method for rapid and quantitative determination of trimethylamine. J Chromatogr A, 1995;718:305–8. CrossRef
Hao F, Lwin T, Bruckard WJ, Woodcock JT. Determination of aliphatic amines in mineral flotation liquors and reagents by high-performance liquid chromatography after derivatisation with 4-chloro-7-nitrobenzofurazan. J Chromatogr A. 2004; 1055:77–85. CrossRef
Herraez-Hernandez R, Chafer-Pericas C, Verdu-Andres J, Campins-Falco P. An evaluation of solid phase microextraction for aliphatic amines using derivatization with 9-fluorenylmethyl chloroformate and liquid chromatography. J Chromatogr A, 2006; 1104:40–6. CrossRef
ICH Q3C (R5): Technical requirements for registration of pharmaceuticals for human use impurities guideline for residual solvents. In: international Conference on Harmonization, 2011. https://www.pmda.go.jp/files/000156308.pdf
Jagota NK, Chetram AJ, Nair JB. Ion chromatography of amylamine and tert-butylamine in pharmaceuticals. J Chromatogr A, 1996;739:343–9. CrossRef
Jerome V, Hermann M, Hilbrig F, Freitag, R. A fast method for the quantification of methylamine in fermentation broths by gas chromatography. J Chromatogr B, 2008;861:88–94. CrossRef
Kadnar R. Determination of amines used in the oil and gas industry (upstream section) by ion chromatography. J Chromatogr A, 1999;850:289–95. CrossRef
Kishimoto M. Teneligliptin: a DPP-4 inhibitor for the treatment of type 2 diabetes. Diabetes Metab Syndr Obes: targets Therapy, 2013;6:187–95. CrossRef
Krol J, Alden PG, Morawski J, Jackson PE. Ion chromatography of alkylamines and alkanolamines using conductivity detection. J Chromatogr A, 1992;626:165–70. CrossRef
Kumagai H, Shimizu N, Shimomura Y, Sakai T, Inoue Y. Retention behavior of methylamines ethylenediamine and N-methylsubstituted ethylenediamines on a cation-exchange resin having a polycarboxylic acid as the functional group. J Chromatogr A. 1996;739:327–31. CrossRef
Lopez AF, de Ariza MTP, Orio OA. Rapid method for quantitative determination of tetrabutylammonium bromide in aqueous solution by gas chromatography. J High Resolut Chrom, 1988;12:503–4. CrossRef
Maryam A, Bahman F, Mehdi V, Akbar Z, Application of cation-exchange chromatography for quantification of some ethanolamine degradation products in the natural gas sweetening solution. J Taiwan Inst Chem Eng, 2021;127:69–78. CrossRef
Meseguer-Lloret S, Molins-Legua C, Verdu-Andres J, Campins-Falco P. Sensitive determination of aliphatic amines in water by high-performance liquid chromatography with chemiluminescence detection. J Chromatogr A. 2004;1035:75–82. CrossRef
Reddy, YS. Analytical method validation for the determination of tetrabutlylammonium bromide content in Daclatasvir dichloride by LC/MS/MS. J Global Pharma Tech. 2019; 11(1):15–21.
Sharma SK, Panneerselvam A, Singh KP, Parmar G, Gadge P, Swami O. Teneligliptin in management of type 2 diabetes mellitus. Diabetes Metab Syndr Obes: targets Therapy. 2016;9:251–6. CrossRef
Subramanian NH, Manigandan P, Ganesh JR, Radhakrishnan G. Ion chromatographic determination of residual phase transfer catalyst in active pharmaceutical ingredient. J Chromatogr Sci. 2009;47:540–4. CrossRef
Tan HSI, Xu J, Zheng Y. Cation-exchange high-performance liquid chromatographic assay of piperazine in some pharmaceutical formulations. J Chromatogr A. 1995;693:307–14. CrossRef
The European Agency for the Evaluation of Medicinal Products ICH topic Q 2 B. Note for Guidance on Validation of Analytical Procedures Text and Methodology. 1997;CPMP/ICH/281/95.
United States Pharmacopoeia. General Chapter, Residual Solvents, 2016; USP39–NF34:339.
Zhao YY, Jing ZZ, Wang H, Zhang HS, Yu JX. N-Hydroxysuccinimidylphenylacetate as a novel derivatizing reagent for aliphatic amines in gas chromatography. Anal Chim Acta. 2003;468:255–61 CrossRef