INTRODUCTION
Dyslipidemia or hyperlipidemia is a condition characterized by rising total cholesterol (TC), the “bad” low-density lipoprotein (LDL) cholesterol, and the triglyceride (TG) concentrations and decreasing of the “good” high-density lipoprotein (HDL) cholesterol concentration in the blood, such that they lose their normal levels and result in an imbalance of their levels (Qinna et al., 2012). Therefore, dyslipidemia is considered hypercholesterolemia when the rate of TC exceeds more than 240 mg/dl and it is combined dyslipidemia when the TC is around 240–260 mg/dl, TG around 200–400 mg/dl, and LDL cholesterol above 190 mg/dl (Agarwal and Parsad, 2016; Ashavaid et al., 2000; Tai et al., 2017). Furthermore, a condition where HDL cholesterol is lower than 35 mg/dl is called hypoalphalipoproteinemia, while it will be known as hypertriglyceridemia if the fasting serum TG ratio is above 200 mg/dl (Corris, 2001; Berglund et al., 2012).
High cholesterol levels are considered a common modifiable hazard factor of hyperlipidemia, and treatment can be done through changes in lifestyle along with lipid-regulated pharmacotherapy, which mainly involves statins which are considered the top-tolerated medications in lowering the TC and LDL cholesterol concentrations, through blocking the rate-limiting enzyme of cholesterol synthesis (HMG-CoA) reductase (Gotto, 2002; Liu et al., 2010).
Rosuvastatin (known as ZD4522) is a fully synthetic compound that consists of a single enantiomer that has the empirical formula (C22H27FN3O6S)2Ca; it can be obtained on the market under “Rosuvastatin Calcium” with 1,001.14 g/mol of molecular weight (McTaggart et al., 2001; Olsson et al., 2002). Comparable to other statins, rosuvastatin was alleged to be a “super-statin” in lowering LDL cholesterol along with raising HDL cholesterol levels, taken orally once daily (Shahin et al., 2020). Rosuvastatin treatment might be progressed to muscle spasms, pain, and feebleness in around 5%–10% of the sick people, and in acute conditions, symptoms such as myalgia, myopathy, and rhabdomyolysis probably occur (McTaggart et al., 2001).
Artichoke is an edible plant that is commonly used in treating high levels of cholesterol or any other fats (lipids) in the blood, where its leaves include diverse phenolic compounds including cynarin (1.62%), chlorogenic acid (CGA) (4.71%), cymaroside (0.25%), luteolin (0.42%), and 1-caffeoylquinic acid (0.13%) (Negro et al., 2012; Sánchez-Rabaneda et al., 2003). According to a previous clinical study, using artichoke leaves extract (ALE) in patients diagnosed with elementary mild hypercholesterolemia for 2 months resulted in effectively lowering T-C and LDL-C and raising HDL-C levels (Rondanelli et al., 2013). Therapeutically, artichoke is used in food as it is a fundamental agent for reinforcement diuresis, as well as an antiseptic for the care of gout and rheumatism. Leaf extracts of the artichoke are used in herbal medicines like hepatoprotective and choleretic and have also recently been reported to act as antibacterial, antioxidative, anticarcinogenic, and antihuman immunodeficiency virus (Macua, 2007; Negro et al., 2012).
However, there are few studies that evaluated the combined effect of artichoke with conventional drugs. For example, the study of Crevar-Sakac et al. (2016) examined the combined effect of artichoke on lipid levels with atorvastatin in experimental hypercholesterolemic conditions and concluded that the combination of them has a small effect on the baseline lipid parameters for Wistar albino rats and the artichoke is less efficient when used in combination with atorvastatin.
Our research team previously studied the combined effect of rosuvastatin drug with a number of natural herbs such as milk thistle and flaxseed (Abdullah, 2019; Shahin et al., 2020), where the results showed a significant reduction in lipid profile parameters in rats suffering from dyslipidemia. Therefore, our aim in this research is to examine the effect of ALE administration on serum lipid levels when used alone or in synergism with both doses of rosuvastatin (10 and 20 mg/kg). We also added CGA to the experiment, one of the main constituents in the artichoke, so that we could know if it was the ingredient responsible for lowering cholesterol lipids in ALE.
MATERIALS AND METHODS
Chemicals
Rosuvastatin drug powder was acquired from Dar Al Dawa Company (Jordan). ALE was purchased from Gehrlicher Pharmaceutical Extract (Gmbh) Company (Eurasburg, Germany). Cholesterol was purchased from Biosynth Carbosynth® (USA). Biochemical reagents and the necessary buffers were obtained from AWDT Company (Netherlands). Trifluoroacetic acid was obtained from Tedia company (CA, USA). Corn oil was sourced from the domestic market.
Instruments
This study was divided into two major parts: pharmacodynamics interaction of ALE with rosuvastatin and pharmacokinetics interactions. Biochemical quantitative analysis was performed to study the pharmacodynamics of each rat using a fully automated chemical analyzer (Snibe® Biossays 240 Plus) manufactured by Shenzhen New Industries Biomedical Engineering Company, China.
A pharmacokinetic study analysis was performed using high-pressure liquid chromatography (HPLC) (FINNIGAN SURVEYOR) equipped with UV-VIS plus detector (ChromQuest software 4.2.3.4), solvent delivery system pump (LC Pump Plus), and an automatic sampling system (Autosampler Plus).
Preparation of stock solutions
Rosuvastatin solution
The stock solution of rosuvastatin was primed weekly. About 200 mg of drug powder was dissolved in 100 ml of distilled water to obtain a 0.2% (w/v) concentration of drug solution, which is equivalent to 2 mg/ml per rat (Dizaye and Chalaby, 2015; Susic et al., 2003).
ALE and CGA extract solutions
Artichoke leaves standardized extract (1.4% m/m CGA, Gehrlicher Pharmaceutical Extracts GmbH, Germany) stock solution was prepared by dissolving 700 mg of artichoke extract in 100 ml distilled water to obtain a concentration of 0.7% (w/v) of extract solution, which is equivalent to 7 mg/ml per rat. The daily dose of ALE was calculated in accordance with the EMA (2018) Cynara scolymus L. monograph.
A CGA solution was prepared by dissolving 10 mg of CGA (Sigma-Aldrich, USA) in 100 ml distilled water to obtain a concentration of 0.01% (w/v), which is equivalent to 0.1 mg/ml per rat. The dose of CGA was equivalent to that calculated amount in the standardized extract (i.e., 7 mg/ml ALE contains 0.1 mg/ml CGA).
Cholesterol stock solution
Cholesterol oily solution was primed every week. About 40 g of cholesterol amount was dissolved in 100 ml of corn oil to be strenuously mingled in a blender, in order to obtain a homogeneous oily solution of 40% (w/v) of cholesterol concentration, tantamount to 400 mg/ml per rat (Hirunpanich et al., 2006).
Preclinical study
Animal handling and study protocol of pharmacodynamic study
Both genders of Wistar albino laboratory rats (5 weeks old, weighing 110–140 g at the beginning of the experiment) were used for the experiment. Animals were obtained from the ASPU University (Amman, Jordan), in which they were housed and divided into groups of seven in a monitored environment with 12-hour light and dark cycles and free access to food and water.
Rats were separated into seven groups: group #1 included the rats of the control group, which were fed with a normal diet and 1 ml of cholesterol solution at a dose of 2g/kg for 8 weeks (Hirunpanich et al., 2006).
In order to induce dyslipidemia, rats in the other six groups (2, 3, 4, 5, 6, and 7) were fed a normal diet with 1ml of cholesterol oily solution per rat daily for 4 weeks. After 4 weeks, dyslipidemia was induced in all rats that were given the cholesterol solution. Subsequently, for another 4 weeks, the rats were therapeutic with either rosuvastatin, artichoke extract, or a combination of the two, and CGA. All these groups of rats with their treatment regimens are briefly described in Table 1.
Samples collection
After 14 hours of fasting, blood samples were drawn through the rat’s optic vein and left in labeled Eppendorf tubes for around 1 hour in order to separate the serum from other blood contents very well. Then, immediate centrifugation was performed at 5,000 rpm for 10 minutes to obtain a clear, impurity-free serum blood sample which was transferred to further labeled Eppendorf tubes where it is stored at −20°C until biochemical analysis. These samples were collected at time zero to determine zero-line measurements, then on every week for monitoring and assessment of the induction and treatment of dyslipidemia.
Quantitative biochemical analysis
Lipid profile parameters were quantified for each individual rat including TC, TG, and HDL, while LDL and very low-density lipoprotein (VLDL) concentrations were calculated by applying the modified Friedewald formula, as follows (Chen et al., 2010):
LDL-Cholesterol = Non-HDL-Cholesterol × 90% – TG × 10%(1)
Non-HDL-Cholesterol = TC – HDL-Cholesterol(2)
However, the VLDL concentration was estimated by being the fifth of TG concentration:
VLDL = TG/5(3)
Animal handling and study protocol of pharmacokinetics study
The pharmacokinetic effect was studied in two separate groups without cholesterol by a daily intragastric administration of ALE for 3 days in the second group (ALE + RL) only. On the fourth day, a low dose of 10 mg/kg rosuvastatin solution was administered to both groups. Both rat groups (7 weeks old, weighing 140–160 g) along with their therapeutic regimens are described briefly in Table 2.
Samples collection
Blood samples were drawn through the rat’s optic vein after 12 hours of fasting at different times (0 hour, 30 minutes, 1 hour, 2 hours, 2.5 hours, 3.5 hours, 5 hours, 8 hours). Then, the same procedures in the pharmacodynamic study were applied to these samples until quantitative analysis by HPLC. The full HPLC conditions used in the experiment are described in Table 3.
Sacrificing the rats
For the purpose of sacrifice, rats were anesthetized with 10% of chloral hydrate by injecting it into the abdomen of the rat.
Statistical analysis
Statistical analysis was conducted by using Statistical Package for the Social Sciences statistics software (version 23), including an independent t-test, in which each data point represents the mean ± the standard error of the mean (M ± SEM). Each probability value equal to 0.05 or lower was deemed significant (p-value ≤ 0.05).
RESULTS
Dyslipidemia inducement
The following results were based on the parameters of the zero line before the start of the study and were then compared with the parameters after 4 weeks of cholesterol induction, in which TC, TG, HDL, LDL, and VLDL levels were measured for all rats to evaluate their lipid profile. After 4 weeks of intragastric intake of cholesterol oily solution (2 g/kg) per day, dyslipidemia was induced in 56 rats.
The data showed that this paradigm was efficient in causing dyslipidemia in all experimental rats by significantly (p ≤ 0.01) increasing the TG from 66.1 to 107.3 and VLDL from 13.2 to 21.4, along with significantly (p ≤ 0.05) increasing the TC from 52.1 to 69.8 and LDL from 2.3 to 15.5. However, no significant effect on HDL was noticed (Table 4).
Treatment of dyslipidemia
The treatment was given as either rosuvastatin, ALE, or a combination of the two, and CGA. The results of all rats were statistically analyzed and compared before (BT) and after (AT) treatment.
 | Table 1. Preclinical study design and rats grouping. [Click here to view] |
 | Table 2. Pharmacokinetics study design and rats grouping. [Click here to view] |
 | Table 3. The HPLC conditions used in the experiment. [Click here to view] |
 | Table 4. Effect of cholesterol administration on lipid profile parameters of all rats, at the zero line and after 4 weeks of dyslipidemia induction (mean ± SEM). [Click here to view] |
Rosuvastatin therapy
Both doses of rosuvastatin were significantly efficacious in curing dyslipidemia (p ≤ 0.05), where the low dose of rosuvastatin 10 mg/kg decreased the TC from 66.8 to 52.8 with no significant effect on TG, HDL, LDL, and VLDL, while the high dose of rosuvastatin 20 mg/kg reduced the TG from 142.1 to 102.5 with no significant effect on other lipid profile parameters (Table 5).
Combined therapy: ALE and rosuvastatin (ALE + RL, ALE + RH)
The effect of combination therapy (ALE + RL) on serum lipid profile significantly (p ≤ 0.01) reduced the TC from 78.6 to 60.1, TG from 111.3 to 81.2, LDL from 22.2 to 16.2, and VLDL from 21.3 to 7.5 with no significant effect on HDL, whereas the effect of combined therapy (ALE + RH) significantly (p ≤ 0.05) reduced the levels of the TG from 138.8 to 112.8, LDL from 17.7 to 7.4, and VLDL from 27.7 to 22.5 and reduced (p ≤ 0.01) the TC from 77.1 to 54.3 with no significant effect on HDL (Table 6).
CGA therapy
The administration of CGA significantly (p ≤ 0.01) reduced the levels of the TC from 69.6 to 54.6 and LDL (p ≤ 0.05) from 12.6 to 3.4 with no significant effect on TG, VLDL, and HDL (Table 7).
ALE therapy
The effect of artichoke extract on serum lipid profile significantly (p ≤ 0.05) reduced the TG from 157.1 to 87.1 and VLDL from 31.4 to 17.4 with no significant effect on TC, LDL, and HDL (Table 8).
The effect of the gender factor on the study
The results were further analyzed and divided depending on the gender of rats.
Effect of both doses of rosuvastatin (RL; 10 mg/kg) and (RH; 20 mg/kg) on gender factor
The administration of rosuvastatin 10 mg/kg in male rats showed a significant (p ≤ 0.05) reduction of the TC from 66.8 to 52.8 only, while the administration of rosuvastatin 20 mg/kg in male rats significantly (p ≤ 0.05) reduced the TC from 78.8 to 63.2 and LDL from 18.4 to 10.9 with no significant effect on TG, HDL, and VLDL. Moreover, there was no significant effect for both doses of rosuvastatin on female rats (Table 9).
The effect of both combination therapy (ALE + RL, ALE + RH) on gender factor
The administration of combined therapy (ALE + RL) in male rats showed a significant (p ≤ 0.05) reduction in the levels of the TC from 79.2 to 60.1, TG from 116.1 to 81.8, and VLDL from 23.2 to 16.3, with no significant effect on HDL and LDL. However, only LDL levels were significantly reduced (p ≤ 0.05) in female rats. While the administration of combined therapy (ALE + RH) in male rats showed a significant (p ≤ 0.01) reduction of the TC from 81.2 to 54.1 and a reduction (p ≤ 0.05) of the TG from 135.4 to 116.1, LDL from 19.9 to 7.6, and VLDL from 27.8 to 23.2, with no significant effect on HDL. On the other hand, no significant effect was noticed in female rats (Table 10).
 | Table 5. Effect of both doses of rosuvastatin (RL; 10 mg/kg) and (RH; 20 mg/kg) on lipid profile parameters before (BT) and after (AT) treatment (mean ± SEM). [Click here to view] |
 | Table 6. The effect of both combination therapy (ALE + RL, ALE + RH) on lipid profile parameters before and after treatment (mean ± SEM). [Click here to view] |
 | Table 7. Effect of CGA on lipid profile parameters before and after treatment (mean ± SEM). [Click here to view] |
 | Table 8. Effect of ALE on lipid profile parameters before and after treatment (mean ± SEM). [Click here to view] |
 | Table 9. Effect of both doses of rosuvastatin (RL, RH) on male rats before and after treatment (mean ± SEM). [Click here to view] |
The effect of CGAon gender factor
The administration of CGA in male rats showed a significant (p ≤ 0.05) reduction in the levels of the TC from 71.1 to 55.1 and LDL from 11.1 to 4.2, with no significant effect on the TG, HDL, and VLDL. However, no significant effect was noticed on the lipid profile in female rats (Table 11).
The effect of ALE on gender factor
The administration of ALE in male rats only increased the levels of HDL (p ≤ 0.05), with no significant effect on TC, TG, LDL, and VLDL. On the other hand, artichoke significantly (p ≤ 0.05) reduced levels of TG from 103.1 to 45.1 and VLDL from 20.6 to 9.1 along with increased (p ≤ 0.01) levels of HDL from 36.2 to 57.5 in female rats (Table 12).
Pharmacokinetics study
Quantitative analysis of rosuvastatin using HPLC
First, a calibration curve was plotted using seven concentrations. The calibration process was carried out regularly for 3 days prior to the analysis of the pharmacokinetic samples. R2 was within the accepted range (0.9984). Atorvastatin was used as the internal standard for rosuvastatin (Table 13). Furthermore, quality control testing procedures (high, mid, and low) were conducted to ensure the accuracy of the analysis process and to obtain more reliable results. The Appendix clearly illustrates each calibration curve alone and quality control (high, mid, and low) tests (Abdullah, 2019).
Two groups of rats were studied. Table 14 and Figure 1 show the sampling times and the effect of the pharmacokinetic study.
It was evident that Cmax and AUC were significantly (p ≤ 0.01) altered in group #2, where artichoke extract was combined with a low dose of rosuvastatin (Table 15). The pharmacokinetic parameters for each rat are clearly described in the Appendix.
The effect of gender factor on pharmacokinetics study
The results for male and female rats were separated in the pharmacokinetics study. Male rats were more susceptible to being affected by the combination treatment protocol (ALE + RL) than female rats as shown in Tables 16 and 17.
DISCUSSION
The experimental model was efficient in casing dyslipidemia in all studied rats after intragastric administration of cholesterol for 4 weeks, by significantly increasing TG and VLDL (p ≤ 0.01) and TC and LDL (p ≤ 0.05). However, there was a slight decrease in HDL cholesterol. Several studies have investigated and implemented the use of a cholesterol-rich diet for the induction of dyslipidemia (Fazio and Linton, 2001; Matos et al., 2005; Xie et al., 2005).
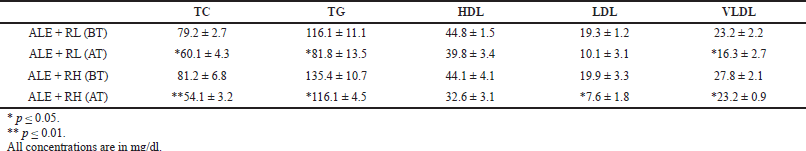 | Table 10. The effect of both combination therapy (ALE + RL, ALE + RH) on male rats before and after treatment (mean ± SEM). [Click here to view] |
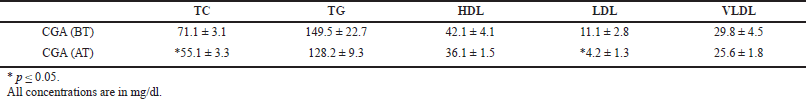 | Table 11. Effect of CGA on male rats before and after treatment (mean ± SEM). [Click here to view] |
 | Table 12. Effect of artichoke extract (ALE) on female rats before and after treatment (mean ± SEM). [Click here to view] |
 | Table 13. Average of the three calibrations curve data for rosuvastatin (all concentrations are in ng/ml). [Click here to view] |
 | Table 14. Sampling times for pharmacokinetics study. [Click here to view] |
 | Figure 1. Effect of groups 1 and 2 in pharmacokinetics study. [Click here to view] |
All studied therapeutic protocols had a hypolipidemic effect including rosuvastatin (10 and 20 mg/kg), ALE (7 mg/kg), or a combination of the two, and CGA (0.1 mg/kg), which reduced lipid profile parameters (TC, TG, LDL, and VLDL) differently in each protocol. Each dose of rosuvastatin (low and high) showed a hypolipidemic impact by reducing TC and TG cholesterol levels.
CGA was effective in significantly reducing lipids profile parameters, especially TC and LDL cholesterol, which indicates that CGA (one of the main components in artichoke extract) may be responsible for reducing lipids. Chemically, CGA is an ester formed between caffeic acid and quinic acid and is the largest ingredient in artichoke leaves at 4.71% (Negro et al., 2012; Sato et al., 2011). CGA has been reported to be more effective in reducing body weight and regulating fat metabolism than caffeic acid, according to Cho et al. (2010), in their study on a high-fat-diet induced in obese mice, in which administration of CGA significantly reduced TG (in blood serum, heart, and liver) and TC (in blood serum, heart, and fatty tissue) concentrations, body weight, visceral fat mass, plasma leptin, and insulin levels compared to the high-fat control group while they increased fatty acid b-oxidation activity and peroxisome proliferator-activated receptors, causing an expression in the liver compared to the high-fat group.
ALE was effective in the treatment of dyslipidemia, as it exhibits hypolipidemic impact by significantly decreasing TG and VLDL. Artichoke leaves are rich in diverse phytochemically active substances that could have contributed to controlling dyslipidemia, e.g., phenolic compounds includingCGA, cynarin, cynaroside, luteolin, and flavonoids. It may be effective as hepatoprotective, antioxidative, antiatherosclerotic, choleretic, antitoxicant, anti-inflammatory, and anticancer, which may indicate an effective alternative treatment for dyslipidemia rather than rosuvastatin to some extent with fewer side effects (Ali et al., 2016; Gezer et al., 2015).
Combining ALE with rosuvastatin showed more effective treatment than each alone in reducing all parameters of the lipid profile except HDL cholesterol, in treating dyslipidemia and lipid disorders. Moreover, the combined therapy (ALE + RL) has the most hypolipidemic effect (p ≤ 0.01) comparable to all therapeutic protocols in the experiment. Previous studies have reported that the use of natural drugs for the protection and prevention of conventional drugs’ associated side effects and toxicities was extremely essential, e.g., Baravalia et al. (2011), who studied the protective effect of Woodfordia fruticosa (L.) Kurz flowers against diclofenac sodium caused by liver toxicity in rats, which was shown to be effective for the treatment of liver disorders. In addition, other researchers investigated the nephroprotective effect of 16-herbal remedies such as pomegranate, garlic, grapes, and green tea, on the nephrotoxic effects of chemotherapeutic agents (Heidari-Soreshjani et al., 2017).
The pharmacokinetics study was conducted to evaluate the interaction effect between ALE and a low dose of rosuvastatin in rats’ plasma. It is known that the simultaneous administration of two medicinal substances increases or decreases the pharmacological potential of each other. Combinational therapy may sometimes produce beneficial effects via synergism or addition while antagonism has also been reported in a previous study (Asdaq et al., 2009). Like any other medicinal substance, herbal remedies might be harmful if used in combination with a possible conventional regimen without their validated concomitant use. Serious adverse interactions have been reported in several studies when herbal remedies are combined with conventional drugs (Asher et al., 2017; Parvez and Rishi, 2019). Therefore, it is necessary to determine the role of commonly used herbal remedies or their bioactive constituents in the presence of potential conventional medicines to confirm the safety index of their combined use. Earlier studies with drug–drug and drug–herb interactions with rosuvastatin had revealed mixed effects. Some medications such as bezafibrate, gemfibrozil, and raltegravir and herbs such as red yeast rice have been reported to increase the myopathic effect of rosuvastatin (Bajaj and Giwa, 2021), while flaxseed extract has been reported to increase the bioavailability of rosuvastatin without increasing its adverse effects (Abdullah, 2019). Moreover, the coadministration of rosuvastatin with drugs that increase its bioavailability is known to lead to adverse effects on the kidneys (Kostapanos et al., 2010). Hence, the combination of ALE and rosuvastatin should be used with caution until the adverse effects of this combination have been determined.
 | Table 15. Pharmacokinetics parameters for group #1 and group #2. [Click here to view] |
 | Table 16. The effect of male rats in both groups in the pharmacokinetic study. [Click here to view] |
 | Table 17. The effect of female rats in both groups in the pharmacokinetic study. [Click here to view] |
In our results, coadministration of artichoke extract with a low dose of rosuvastatin reduced its concentration in blood serum from 755 to 388 ng/ml and changed Cmax and AUCinf significantly (p ≤ 0.01). By correlating these findings with the results of the lipid profile study, we found that the combination of ALE and a low dose of rosuvastatin significantly affected all parameters of the lipid profile along with decreased rosuvastatin bioavailability. Thereby, their combination together may protect the body from the adverse effects of the drug in the body with a more potent impact in reducing lipid profile parameters than rosuvastatin alone, thereby enhancing the treatment of the disease besides protecting the body.
The lipid profile study highlighted the fact that all treatment protocols including rosuvastatin, ALE, or a combination of the two, and CGA did not significantly affect HDL cholesterol levels. These results are consistent with several studies that demonstrated that artichoke extract lowers lipid profile parameters except for HDL cholesterol (Englisch et al., 2000; Lupattelli et al., 2004; Pittler et al., 2002; Soliman and Saad, 2009).
The effect of rats’ gender was evident in the results; male rats were more susceptible to all therapeutic protocols, in contrast to the results of female rats. None of the groups of female rats showed significant changes in the lipid profile parameters, except for the group that was administered artichoke alone, in which TG, VLDL, and HDL decreased significantly. These findings are in line with a study (Küskü-Kiraz et al., 2010) which showed that the use of artichoke in female Wistar rats led to a decrease in TC and TG. However, we found that HDL and VLDL levels significantly increased and TC level was not affected, which controverted the findings of the previous study. Also, the effect of the gender factor of rats on the pharmacokinetic study showed that the administration of rosuvastatin with artichoke decreases the concentration of rosuvastatin in male rats more than in females. Moreover, the HDL cholesterol was significantly increased by using ALE alone in both males and females or in combination with high-dose rosuvastatin in males only, which indicated that gender played an important role in better determining the results and their accuracy.
CONCLUSION
This study has highlighted and supported the effect of rosuvastatin and artichoke alone and its combination in controlling hyperlipidemia significantly (p ≤ 0.01 and p ≤ 0.05). It would serve as preliminary evidence for both researchers and healthcare providers in combinational therapy between conventional and natural drugs. Furthermore, CGA has proven its efficiency (p ≤ 0.05) in decreasing lipids, and this indicates that CGA, one of the main components in ALE, may be responsible for reducing lipids. In addition, the pharmacokinetics study showed that coadministration between artichoke extract and a low dose of rosuvastatin decreased rosuvastatin’s bioavailability, and significantly (p ≤ 0.01) affected Cmax and AUC along with decreasing its concentration in the serum blood from 755 to 388 ng/ml. After the separation of the results into males and females, the gender factor showed an important role in better determining the results and their accuracy.
ACKNOWLEDGMENTS
We thank the Deanship of Faculty of Pharmacy at both University of Petra and Applied Science Private University for material and logistical support.
LIST OF ABBREVIATIONS
TC: total cholesterol; TG: triglyceride; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very low-density lipoprotein; ALE: artichoke leaf extract; CGA: chlorogenic acid; RL: rosuvastatin low-dose; RH: rosuvastatin high-dose; BT: before therapy; AT: after therapy; G1: group 1; G2: group 2.
AUTHORS’ CONTRIBUTIONS
Ahmad M Al Masalmeh, Eyad Mallah, and Kenza Mansoor played major role in designing the research proposal, writing, and editing the research paper. Luay Abu-Qatouseh and Feras Darwish El-Hajji participated in the technical and material support. Nasir Idkaidek performed the statistical calculations. Israa Haj Issa and Saba Aws participated in data analysis and interpretation. Ahmad Z Al Meslamani contributed to data acquisition. Iman Al-Bashiti generally supervised.
FINANCIAL SUPPORT
The study did not receive any funding.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
The study received approval from the Scientific Committee of the Higher Research Council at the College of Pharmacy, University of Petra (12-2019-PHA-45).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdullah SM. Evaluation of the lipid lowering effect of flaxseeds (Linum usitatissimum L.) extract, combined with rosuvastatin, by using a fully automated chemistry analyzer (ACE~ Alera®) and HPLC. Dissertation, University of Petra, Amman, Jordan, 2019, p 166.
Agarwal A, Prasad GV. Post-transplant dyslipidemia: mechanisms, diagnosis and management. World J Transplant, 2016; 6(1):125–34.
Ali F, Rahul, Naz F, Jyoti S, Siddique Y. Health functionality of apigenin: a review. Int J Food Prop, 2016; 20(6):1197–23.
Asdaq SM, Inamdar MN, Asad M. Effect of conventional antihypertensive drugs on hypolipidemic action of garlic in rats. Indian J Exp Biol, 2009; 47(3):176–81.
Ashavaid TF, Altaf AK, Nair KG. Molecular basis of familial hypercholesterolemia: an Indian experience. Indian J Clin Biochem, 2000; 15(1):11–9.
Asher GN, Corbett AH, Hawke RL. Common herbal dietary supplement-drug interactions. Am Fam Phys, 2017; 96(2):101–7.
Bajaj T, Giwa AO. Rosuvastatin. StatPearls Publishing, Treasure Island, FL, 2021.
Baravalia Y, Vaghasiya Y, Chanda S. Hepatoprotective effect of (Woodfordia fruticose L.) Kurz flowers on diclofenac sodium induced liver toxicity in rats. Asian Pacific J Trop Med, 2011; 4(5):342–6.
Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, Stalenhoef AF. Evaluation and treatment of hypertriglyceridemia: an endocrine society clinical practice guideline J Clin Endocrinol Metab, 2012; 97(9):2969–89.
Chen Y, Zhang X, Pan B, Jin X, Yao H, Chen B, Zou Y, Ge J, Chen H. A modified formula for calculating low-density lipoprotein cholesterol values. Lipids Health Dis, 2010; 9:52.
Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol, 2010; 48(3):937–43.
Corris PA. A practical approach to the diagnosis of venothromboembolism. Clin Med J (Lond), 2001; 1(4):274–81.
Crevar-Sakac M, Vuji? Z, Kotur-Stevuljevi? J, Ivanisevi? J, Jeli?-Ivanovi? Z, Milenkovi? M, Markeli? M, Vujci? Z. Effects of atorvastatin and artichoke leaf tincture on oxidative stress in hypercholesterolemic rats. Vojnosanit Pregl, 2016; 73(2):178–87.
Dizaye K, Chalaby L. Hypolipidemic efficacy of trigonella foenum seeds in comparison with rosuvastatin and fenofibrate in hyperlipidemic rats. World Fam Med J/Middle East J Fam Med, 2015; 13(6):30–8.
EMA. European Medicines Agency. 2018. Available via https://www.ema.europa.eu/en/documents/herbal monograph/final-european-union-herbal-monograph-cynara cardunculus-l-syn-cynara-scolymus-l-folium_en.pdf (Accessed 17 March 2018).
Englisch W, Beckers C, Unkauf M, Ruepp M, Zinserling V. Efficacy of artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung, 2000; 50(3):260–5.
Fazio S, Linton MF. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci, 2001; 6:D515–25.
Gezer C, Yücecan S, Rattan SI. Artichoke compound cynarin differentially affects the survival, growth, and stress response of normal, immortalized, and cancerous human cells. Turk J Biol, 2015; 39:299–305.
Gotto AM. Management of dyslipidemia. Am J Med, 2002; 112(8):10–8.
Heidari-Soreshjani S, Asadi-Samani M, Yang Q, Saeedi-Boroujeni A. Phytotherapy of nephrotoxicity-induced by cancer drugs: an updated review. J Nephropathol, 2017; 6(3):254–63.
Hirunpanich V, Utaipat A, Morales NP, Bunyapraphatsara N, Sato H, Herunsale A, Suthisisang C. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J Ethnopharmacol, 2006; 103(2):252–60.
Kostapanos MS, Milionis HJ, Elisaf MS. Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia. Am J Cardiovasc Drug, 2010; 10(1):11–28.
Küskü-Kiraz Z, Mehmetçik G, Dogru-Abbasoglu S, Uysal M. Artichoke leaf extract reduces oxidative stress and lipoprotein dyshomeostasis in rats fed on high cholesterol diet. Phytother Res, 2010; 24(4):565–70.
Liu Y, Manchekar M, Sun Z, Richardson PE, Dashti N. Apolipoprotein B-containing lipoprotein assembly in microsomal triglyceride transfer protein-deficient McA-RH7777 cells. J Lipid Res, 2010; 51(8):2253–64.
Lupattelli G, Marchesi S, Lombardini R, Roscini AR, Trinca F, Gemelli F, Vaudo G, Mannarino E. Artichoke juice improves endothelial function in hyperlipemia. Life Sci J, 2004; 76(7):775–82.
Macua J. New horizons for artichoke cultivation. Acta Hortic, 2007; 730:39–48.
Matos SL, Paula HD, Pedrosa ML, Santos RC, Oliveira EL, Chianca Júnior DA, Silva ME. Dietary models for inducing hypercholesterolemia in rats. Braz Arch Biol Technol, 2005; 48(2):203–9.
McTaggart F, Buckett L, Davidson R, Holdgate G, McCormick A, Schneck D, Smith G, Warwick M. Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor. Am J Cardiol, 2001; 87(5A):28B–32B.
Negro D, Montesano V, Grieco S, Crupi P, Sarli G, De Lisi A. Polyphenol compounds in artichoke plant tissues and varieties. J Food Sci, 2012; 77(2):C244–52.
Olsson AG, McTaggart F, Raza A. Rosuvastatin: a highly effective new HMG-CoA reductase inhibitor. Cardiovasc Drug Rev, 2002; 20(4):303–28.
Parvez MK, Rishi V. Herb-drug interactions and hepatotoxicity. Curr Drug Metab, 2019; 20(4):275–82.
Pittler MH, Thompson CO, Ernst E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst Rev, 2002; (3):CD003335.
Qinna N, Kamona B, Alhussainy T, Taha H, Badwan A, Matalka K. Effects of prickly pear dried leaves, artichoke leaves, turmeric and garlic extracts, and their combinations on preventing dyslipidemia in rats. Int Sch Res Notices, 2012; 2012:1–7.
Rondanelli M, Giacosa A, Opizzi A, Faliva MA, Sala P, Perna S, Riva A, Morazzoni P, Bombardelli E. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia: a double-blind, randomized, placebo-controlled trial. Int J Food Sci Nutr, 2013; 64(1):7–15.
Sánchez-Rabaneda F, Jáuregui O, Lamuela-Raventós RM, Bastida J, Viladomat F, Codina C. Identification of phenolic compounds in artichoke waste by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A, 2003; 1008(1):57–72.
Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, Sugawara M, Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm, 2011; 403(1–2):136–8.
Shahin F, Mallah E, Emad R, Abu-Qatouseh L, Abu Dayyih W, Ei- Hajji FD Mansoor K Bustam M Sweidan K Arafat T. Evaluation of lipid lowering effect of milk thistle (silybum marianum L.) in comparison with rosuvastatin in rats by using ACE-Alera® analyzer. Int J Biol Biomed Eng, 2020; 14:89–95.
Soliman G, Saad MMT. Effect of Cynara scolymus L.(artichoke) extract on lipid profile of hyperlipidemic male rats. Egypt J Hosp Med, 2009; 371:733–41.
Susic D, Varagic J, Ahn J, Slama M, Frohlich ED. Beneficial pleiotropic vascular effects of rosuvastatin in two hypertensive models. J Am Coll Cardiol, 2003; 42(6):1091–7.
Tai ES, Chia BL, Bastian AC, Chua T, Ho SC, Koh TS, Low LP, Tey JS, Poh KK, Tan CE, Ting P, Tham TY, Toh SA, van Dam RM. Ministry of health clinical practice guidelines: lipids. Singap Med J, 2017; 58(3):155–66.
Xie W, Xing D, Sun H, Wang W, Ding Y, Du L. The effects of Ananas comosus L. leaves on diabetic-dyslipidemic rats induced by alloxan and a high-fat/high-cholesterol diet. Am J Chin Med, 2005; 33(1):95–105.