INTRODUCTION
Strychnos ligustrina Blume commonly known as bidara laut in Indonesia is a deciduous tree and belongs to Loganiaceae family (Rahayu et al., 2022; Syafii et al., 2016). The plants taste bitter in all parts of the plant, especially the roots. In Indonesian people, this plant is commonly used as a traditional medicine: its bark for toothache and burns, root for stomach aches, and seeds and wood for antimalarial effect (Setiawan et al., 2014). Virtual screening found that bioactive compounds, like chlorogenic acid, phyllamycin A, strychnine, and brucine N-oxide, were predicted to have antidiabetic agents (Setiawansyah et al., 2022).
Phytochemical analysis showed that S. ligustrina wood extract was detected to contain alkaloids, phenol, hydroquinone, tannins, flavonoids, saponins, steroids, and terpenes (Manurung et al., 2019; Setiawan et al., 2014). The wood of S. ligustrina Blume syn. S. lucida R. Br. in East Nusa Tenggara (NTT), Indonesia, was traditionally used to treat people with malaria (Setiawan et al., 2014; Syafii et al., 2016). Syafii et al. (2016) successfully identified that two fractions contained from ethanol extract of S. ligustrina wood were noted as an antimalarial activity with IC50 values of 1.99 (F3) and 0.39 (F4) μg ml−1. The bioactive profiling compound using gas chromatography-mass spectrometry revealed the presence of alkaloids, 4-methyl-5-[3-trifluoromethylphenoxy]-6-methoxy-8-nitroquinoline, and phenolic compounds in F3 fraction and furan and phenolic compounds, fatty acid, and alkaloid in F4 fraction.
Malaria is a disease caused by the protozoan, Plasmodium sp., with the female Anopheles mosquito as a vector. The World Health Organization (WHO) (2015) stated that future antimalarial medicine should be treated with at least two effective medicines with different mechanisms of action to prevent and reduce drug resistance. Furthermore, WHO recommended artemisinin-based combination therapy (ACT) to treat malaria. One of the ACTs commonly used is dihydroartemisinin-piperaquine phosphate (DHP). Artocarpus champeden Spreng. extract was used in combination with DHP to increase the effectiveness as an antimalarial treatment and possibly slow down the parasite resistance to the drug (Hafid et al., 2011). Until now, there have been no reports of subchronic toxicity of the combination of S. ligustrina extract and DHP. Therefore, this study aimed to investigate the subchronic toxicity of S. ligustrina extract and DHP in mice.
MATERIAL AND METHODS
Extraction and preparation
Strychnos ligustrina wood was harvested from West Nusa Tenggara (NTB), Indonesia, at April, 2021. A total of 30 logs (1 m) were cut from 15 trees. The wood of logs was cut and dried under sunlight. Then, the wood was shaved and ground to get powder. The powder was macerated in distilled water (1:8, b/v) for 24 hours. The maceration was conducted three times in the same powder. The filtrate was performed in a vacuum evaporator to get the dry extract and then was put in a capsule using a semiauto capsule filler. The average weight of the extract was 459.6 ± 24.56 mg.
The sample test was the combination of S. ligustrina extract and DHP-Frimal®. The basis for combinations was determined based on the effectivity test in the antimalarial inhibition test, which showed that the best ratio was 1 (S. ligustrina extract 200 mg/Kg BW): 0.5 (DHP 111 mg/kg BW) (Cahyaningsih et al., 2022). The tests were divided into three doses (S. ligustrina extract+DHP): the low dose was the effective dose (200 + 111 mg/kg BW); the medium dose was a 2-fold effective dose (400 + 222 mg/kg BW); the high dose was a 4-fold effective dose (800 + 333 mg/kg BW).
Animals
Adult albino mice (DDY strain; 6–8 weeks) were used for the subchronic studies and were obtained from the National Agency of Drug and Food Control (NA-DFC), the Republic of Indonesia. A total of 150 mice were 75 males and 75 females. Males were separated from females, and every cage was housed with five mice. All mice were acclimatized for 14 days in controlled conditions: 12-hours light/12-hours dark cycle, temperature 24°C ± 1°C, and humidity 55%–63%. The fed mice were given 5 g/mice, and they were free to access water (ad libitum). All procedures in the experiment were approved by the Animal Ethics Committee, IPB University (approval number: 154-2019).
Subchronic toxicity study
The assay of the subchronic study was performed according to the NA-DFC, Republic of Indonesia (BPOM, 2014). Each male and female mice were divided into the following:
Treatment groups (n = 10):
a. Group B: baseline (n = 5)
b. Group I: control (AQUADEST)
c. Group II: low dose (S. ligustrina 200 + DHP 111 mg/kg BW)
d. Group III: medium dose (S. ligustrina 400 + DHP 222 mg/kg BW)
e. Group IV: high dose (S. ligustrina 800 + DHP 333 mg/kg BW)
Satellite groups (n = 10):
a. Group V: low dose (S. ligustrina 200 + DHP 111 mg/kg BW)
b. Group VI: medium dose (S. ligustrina 400 + DHP 222 mg/kg BW)
c. Group VII: high dose (S. ligustrina 800 + DHP 333 mg/kg BW)
The satellite groups were used to evaluate the recovery process from toxic effects. The subchronic toxicity test was performed for 28 days. The S. ligustrina extract + DHP was administrated orally. The dead mice during the treatment were recorded. The bodyweight was weighed on days 0, 1, 7, 14, 21, and 28.
Blood and organ collection
Blood collection was conducted from group B at 0 days as a normal parameter. At 29 days, the treatment groups (I, II, and III) were anesthetized with ketamine-xylazine (70 mg/kg to 20 mg/kg BW), and blood (1 ml) was collected via intracardiac, followed by a secondary means of euthanasia, exsanguination. The blood was stored in a vacutainer with a heparin anticoagulant. After death, mice were sacrificed, and their organs were isolated to evaluate relative organ weights, namely liver, lung, kidney, brain, heart, lymph, ulcer, testis, ovarium, and uterus. The satellite groups (I, V, VI, and VII) were sacrificed 14 days after treatment.
Hematological and biochemistry study
Hematological analysis was performed using a hematology analyzer (Mindray BC-2800vet) to evaluate erythrocyte (RBC), hemoglobin (Hb), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular Hb (MCH), MCH concentration (MCHC), red-cell distribution width (RDW), platelet (PLT), mean platelet volume (MPV), platelet distribution width (PDW), procalcitonin (PCT), leukocyte (WBC), lymphocyte, monocyte, and granulocyte (Zhang et al., 2021). Biochemistry analysis was performed using a clinical chemistry analyzer (SPOTCHEM™ EZ SP-4430) with the following parameters: alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), total cholesterol total, glucose, urea (BUN), and creatinine (Lee et al., 2021). Blood electrolytes were evaluated using SPOTCHEM™ EL SE-1520 to calculate sodium (Na), potassium (K), and chlorine (Cl) (Okamoto et al., 2021).
Histopathology study
The liver, lung, kidney, brain, heart, lymph, ulcer, testis, ovarium, and uterus were fixed in buffer neutral formalin 10% for 7 days and were stored at the stopping point (ethanol 70%). Then, this tissue was trimmed and dehydrated in a graded ethanol series (80%, 90%, 95%, and 100%). The clearing of tissues was carried out using xylene, paraffin infiltration, and paraffin embedding. The embedded tissues were cut using a rotary microtome at a thickness of 5 μm. The sections were stained with hematoxylin-eosin and observed under a light microscope (400×). The analysis was examined in the following parameters: degeneration, necrosis, necrosis/degeneration, inflammation, regeneration, pneumonia, bronchitis, peribronchitis, and edema. The score was 0: no damage, 1: <25% damage, 2: ≥25%–50% damage, 3: ≥50%–75% damage, and 4: ≥75% damage.
Statistical analysis
The data were analyzed using one-way ANOVA followed by Duncan’s multiple comparison test (Duncan’s test). If the data were not in normal distribution and homogeneity after transformation, Kruskal–Wallis test followed by the Mann–Whitney test was used for analysis.
RESULT
Mortality
The ratio of animals with mortality after S. ligustrina extract+DHP treatments was shown in Table 1. In the male groups, the total ratio of dead/treated animals for 5 weeks from the highest to the lowest was high dose (11/20), low dose (5/20), medium dose (3/20), and control groups (1/10). The mortality in male groups was unrelated to the dose. In female groups, high-dose groups (12/10) exhibited the highest ratio of animal mortality, followed by medium-dose groups (5/20). In contrast, control and low-dose groups (2/20) had the same mortality ratio in animals.
Bodyweight and relative organ
Data related to relative organ weight in male and female mice treated with S. ligustrina extract+DHP were shown in Tables 2 and 3. The body weight in high-dose treatment groups significantly differed from control on the 7th–28th day for male mice (Table 2). In female mice of treatment groups, high doses showed a significant increase in body weight from the 21st to 28th compared to control, low, and medium doses. The body weight in the high-dose satellite groups was not significantly different from the 35th to the 42nd. The research showed that S. ligustrina extract+DHP in high dose significantly decreased bodyweight, but these effects were not permanent (reversible), as indicated by increasing bodyweight in satellite groups.
The relative organ weight of control and treatment groups, including liver, lymph, ulcer, lung, and heart, did not reveal significant change after S. ligustrina extract+DHP administration (Table 3). A significant change in the relative weight of the kidney was found in male and female treatment groups, but it was a non-dose-dependent increase. The relative organ weight of the kidney (1.54% ± 0.11%) in high dose increased significantly compared to in control (1.22% ± 0.11%) and low dose (1.17 ± 0.08). Brain and gonad in female mice of treatment groups showed a significant change in relative organ weight, but not in male mice of treatment groups. The relative organ weight of the brain in control was not different from in other doses. However, a high dose (0.42 ± 0.30) showed a decrease in the relative weight of gonad compared to the control (1.33 ± 0.47). There was no significant change in relative organ weight in all organs observed in satellite groups. The study showed that the administration of S. ligustrina extract+DHP significantly affected the relative weight of kidneys and gonads of mice, but their effects were reversible.
Hematological analyses
The results of hematological parameter analysis in male and female mice were presented in Tables 4 and 5, respectively. No significant change among male mice in treatment and satellite groups was noted in the level of Hb, MCV, MCH, MCHC, RDW, PLT, MPV, PDW, PCT, WBC, lymphocyte, monocyte, and granulocyte. The level of erythrocyte (RBC) in male mice (treatment and satellite groups) showed a significant change. The level of RBC in the high dose (11.64 ± 0.056 × 10^6/μl) of the treatment group increased significantly compared to the control (8.67 ± 0.15 × 10^6/μl). HCT level in satellite groups showed a significant change but not in treatment groups. However, RBC and HCT in medium (8.02 ± 0.83 × 10^6/μl; 36.73% ± 2.86%) and low dose (9.87 ± 0.43 × 10^6/μl; 44.87% ± 3.63%, respectively) were not significantly different in comparison with control (8.71 ± 0.72 × 10^6/μl; 41.37% ± 2.78%) in satellite groups. These changes were not dose related. In the treatment groups, the level of lymphocyte and granulocyte in medium and high doses (52.93% ± 11.7% and 41.63% ± 10.72%; 38.90% ± 0.95% and 57.47% ± 1.36%, respectively) was significantly different from the control (62.73% ± 2.55% and 33.60% ± 2.78%, respectively), but not for granulocyte in medium dose (41.63% ± 10.72%).
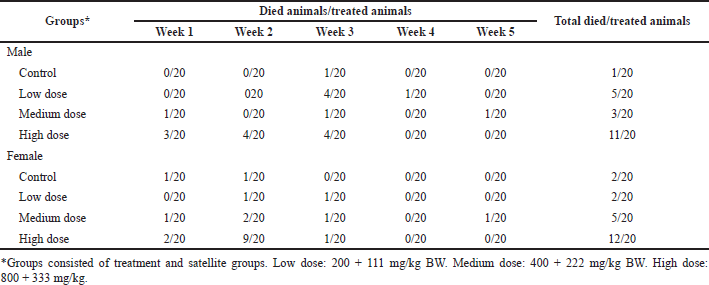 | Table 1. The mortality of mice after the combination of S. ligustrina extract and DHP treatments. [Click here to view] |
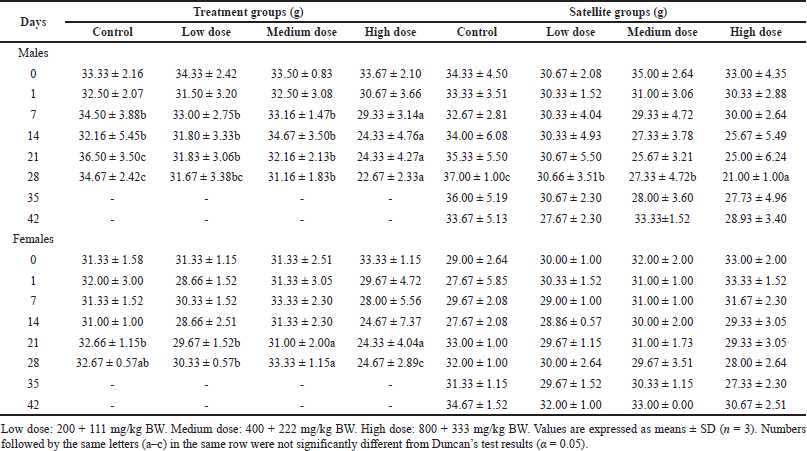 | Table 2. Effect of combination of S. ligustrina extract and DHP on body weights in mice. [Click here to view] |
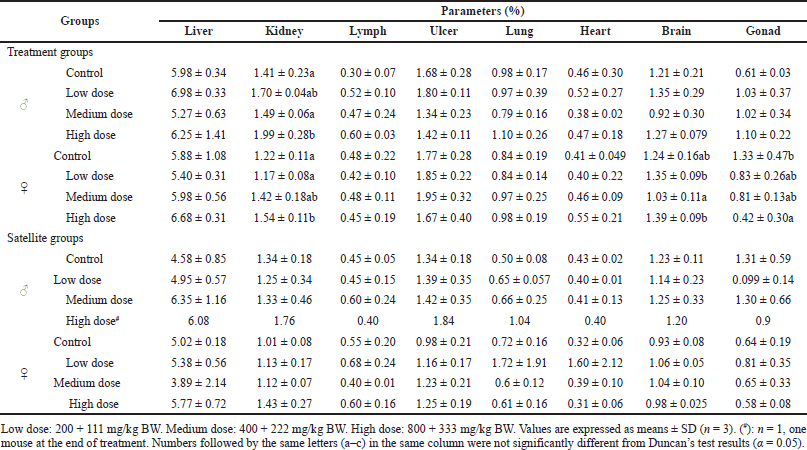 | Table 3. Effect of combination of S. ligustrina extract and DHP on the relative organ weights in mice. [Click here to view] |
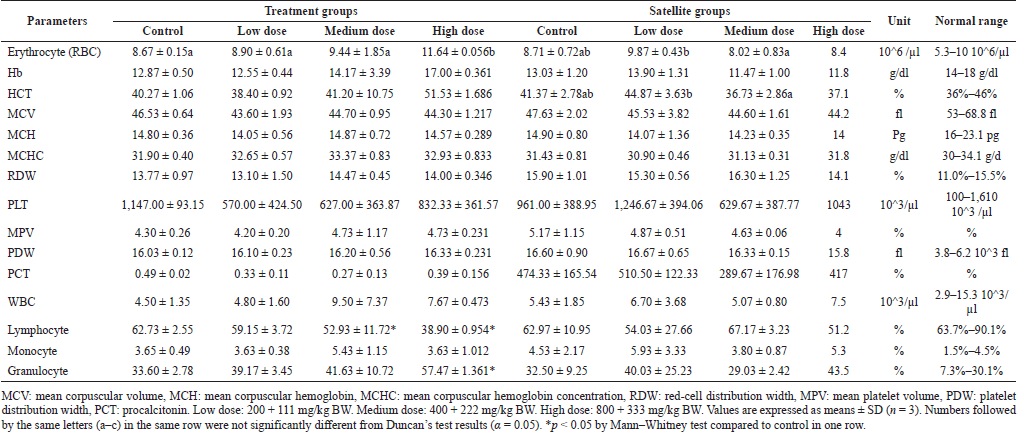 | Table 4. Effect of combination of S. ligustrina extract and DHP on hematological parameters in male mice. [Click here to view] |
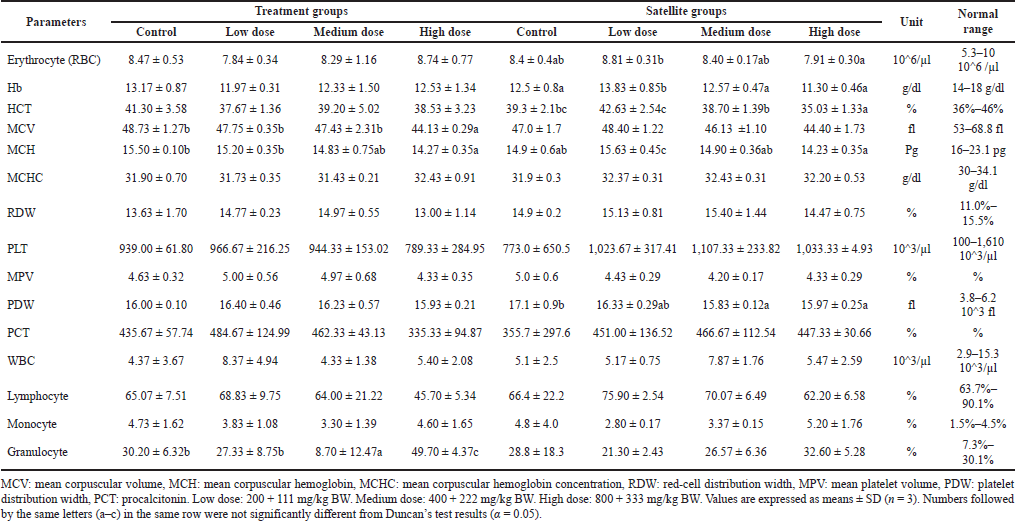 | Table 5. Effect of combination of S. ligustrina extract and DHP on hematological parameters in female mice. [Click here to view] |
The hematological analysis in female treatment groups revealed a statistical change in some parameters, i.e., MCV, MCH, and granulocyte 2, while other parameters showed no significant change (Table 5). The value of MCV and MCH in high dose of treatment groups (44.13 ± 0.29 fl and 14.27 ± 0.35 Pg) decreased significantly compared to control (48.73 ± 1.27 fl and 15.50 ± 0.10 Pg). In addition, the high dose (49.70% ± 4.37%) in treatment groups showed a significant increase in granulocyte compared to the control (30.20% ± 6.32%). A significant change in the level of RBC, Hb, HCT, MCH, and PDW was noted in female satellite groups treated with three-level doses compared to the control groups. However, these changes were not dose related. The level of HCT and PDW in the high dose of satellite groups (35.03% ± 1.33% and 15.97 ± 0.25fl) decreased significantly compared to the control (39.3% ± 2.1% and 17.1 ± 0.9fl). The result showed that S. ligustrina extract+DHP in the high dose significantly changed the hematological parameters of treatment groups, but these changes recovered in male and female mice of satellite groups. The best recovery was in hematological parameters found in the male mice.
Biochemical analyses
The effect of subchronic toxicity of S. ligustrina extract+DHP on biochemical parameters was shown in Tables 6 and 7. The treatment groups in male mice showed a significant increase in ALT, AST, and creatinine after increasing the dose level (Table 6). The level of ALT and AST in the high dose of treatment groups (275.00 ± 90.510 IU/l and 411.00 ± 104.652 IU/l) increased significantly compared to the control (26.50 ± 2.12 and 76.50 ± 20.51, respectively). Likewise, high and medium doses of treatment groups (0.43±0.404 mg/dl and 0.20 ± 0.10 mg/dl) showed a significant increase in creatinine levels compared to the control (0.07 ± 0.06 mg/dl). On the other hand, the satellite group in male mice only exhibited a significant change in Na+ ion.
In female mice of treatment groups, a significant increase in ALT and AST levels was noted in the high dose (147 ± 21.21 IU/l and 240.5 ± 41.72 IU/l) compared to the control (78.50 ± 55.86 IU/l and 77.00 ± 28.28 IU/l, respectively). Also, the satellite groups in female mice showed a significant change in ALT and glucose levels. The level of ALT in the high dose (84.33 ± 12.74 IU/l) of the satellite group was significantly different from the control (24.7 ± 23.5 IU/l). The result showed that S. ligustrina extract+DHP in the high-dose treatment groups significantly changed biochemical parameters (ALT and AST), but these changes recovered in the satellite groups. The best recovery in biochemical parameters was found in the male mice.
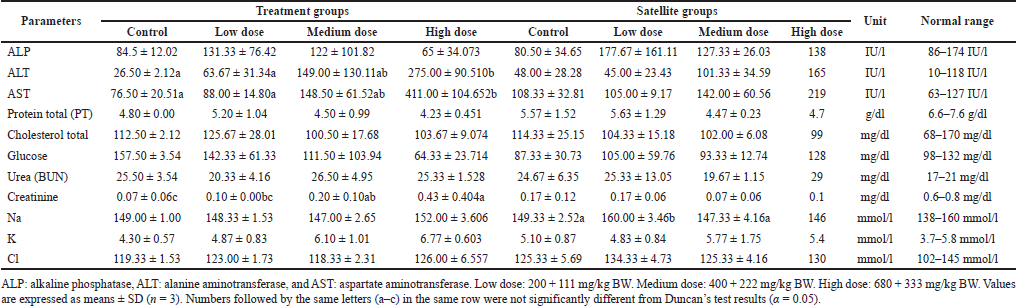 | Table 6. The effect of the combination of S. ligustrina extract and DHP on biochemical parameters in male mice. [Click here to view] |
Histopathological analyses
The vital organs at the end of treatments, including liver, lung, kidney, brain, heart, lymph, ulcer, testis, ovarium, and uterus, were evaluated with the histopathological examination (Table 8). In general, the liver (0–1.3), kidney (0–2.3), and ulcer (0–2) exhibited higher abnormalities such as necrosis, degeneration, and inflammation than other organs in all doses. The medium abnormality was found in the brain (0–1.67) and lung (0–2.33). Ovarium (0–1), heart (0–1.5), testis (0–0.5), and lymph (0–1.5) showed a low abnormality. No abnormality was noted in the uterus (0). Interestingly, no degeneration in the liver was observed in the satellite groups at all doses, but necrosis (0.67–1.33) and inflammation (0.67–1.00) in the liver increased at low and medium doses. Interestingly, regeneration cell in the liver was observed in medium (1.00) and high dose (1.33). In the kidney, the treatment groups exhibited necrosis/degeneration (0–1.3) and inflammation (0–1.3), while the satellite groups showed an increase in necrosis/degeneration (0–2.33) and inflammation (0–2.33). The abnormality of ulcers, brain, and lung in satellite groups increased compared to the treatment groups. In the ovarium (0–1.67) and lymph (0–1.67), the abnormality only was found in satellite groups. Based on the data, it can be concluded that S. ligustrina extract+DHP had a strong effect on the kidney and ulcer compared to other organs.
DISCUSSION
The combination of S. ligustrina and DHP may increase antimalarial potential. However, no toxicity study of this combination has been reported. This study was the first report about the subchronic study of S. ligustrina+DHP. All doses (low, medium, and high doses) induced mortality in mice but had a non-dose-dependent increase. Previous research reported that methanol extract of S. ligustrina had a toxic effect on Nile tilapia (Oreochromis niloticus), such as decreasing HCT and leucocyte levels (Zubaidah et al., 2018). The high mortality was shown at the high doses in male and female groups, while the lowest mortality was in control groups (Table 1). The mortality in control groups was affected by cannibalism in mice.
Bodyweight changes, as sensitivity indication, are commonly used as an indicator and marker of side effects in toxicological evaluation (Traesel et al., 2014). In this study, the high dose in satellite groups consistently decreased body weight from the 7th day to the 28th day in treatment groups and on the 28th day in male satellite groups (Table 2). Interestingly, the body weight increased after S. ligustrina+DHP administration was discontinued for 2 weeks in satellite groups. Relative organ weight is to examine pathological changes in disturbed organs (Nugroho et al., 2020). The significant relative organ weights were noted in the kidney (female and male mice), brain (female mice), and gonad (female mice) (Table 3). Interestingly, there was no significant change in the relative organ weights in satellite groups. The result showed that S. ligustrina+DHP was toxic in body weight and relative organ weight, but its effect was not permanent in mice.
Hematological and biochemical parameters were measured to evaluate the organ disorder. Nonetheless, the repeated administration of S. ligustrina+DHP for 28 days may induce temporary damage to the hematopoietic system. The hematological parameter can be used as an indicator to determine the toxic effect of compounds and provide pathophysiological information about mammals (Diaz et al., 2016). Erythrocyte levels showed a significant increase at the high dose compared to other groups in male treatment groups (Table 4). In the male satellite groups, erythrocyte levels in low and medium doses were not significantly different compared to those in the control group. A previous study also found an inconsistent pattern of increasing doses with hematological change (Chung et al., 2017). This result demonstrated that the recovery after S. ligustrina+DHP administration in hematological parameters of male mice was better than in female mice. Previous research found that male rats showed a fast recovery from acute nickel toxicity (Alcon et al., 1991). El Kabbaoui et al. (2017) found that female mice were more sensitive to hematological parameters than male mice after C. ladaniferus extract administration so the recovery may be slow.
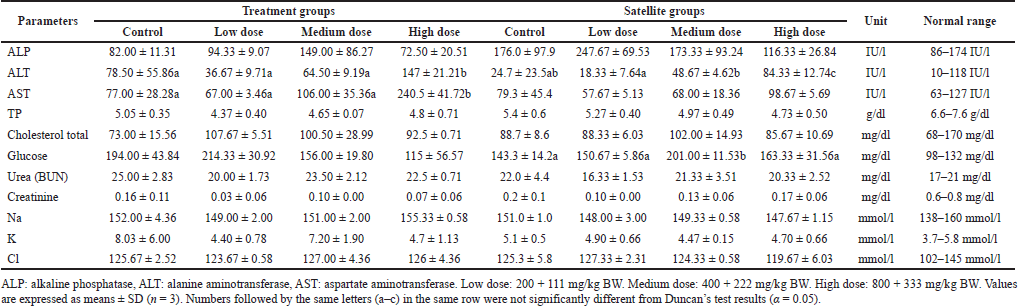 | Table 7. The effect of the combination of S. ligustrina extract and DHP on biochemical parameters in female mice. [Click here to view] |
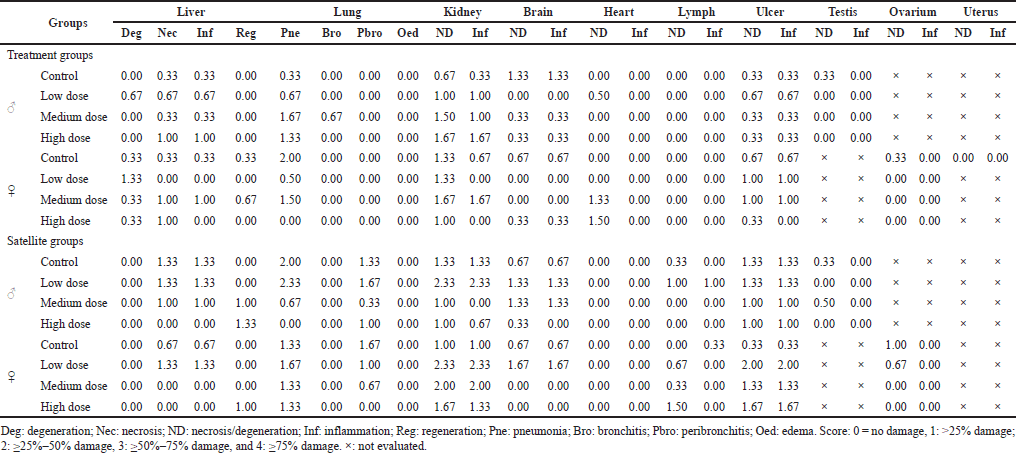 | Table 8. Histopathological examination of mice organ on subchronic toxicity test after the combination of S. ligustrina extract and DHP administration. [Click here to view] |
Blood biochemical parameters were measured to evaluate the toxic effect on liver and kidney function because both organs are vital organs for survival (Nugroho et al., 2020). The treatment groups showed a significant increase in ALT and AST levels in male and female mice (Tables 6 and 7). Then, the level of ALT and AST decreased after the treatments were stopped for 2 weeks. It could be concluded that the liver damage after S. ligustrina+DHP treatments was self-repaired. ALT and AST are enzymes used as an indicator to evaluate liver damage (Tran and Tran, 2021). Both enzymes will be massively released into the bloodstream when a large number of liver cells are damaged (Al-Habori et al., 2002). The kidney function was evaluated via urea and creatinine measurement. The kidney is responsible for excreting metabolic waste and regulating osmotic and electrolyte concentrations (Tran and Tran, 2021). Creatinine is produced by the daily movement of muscle tissue, while urea is the end product of metabolism. A significant increase in creatinine, urea, Na2+, and glucose levels in blood indicated the nephrotoxic effect of S. ligustrina+DHP administration, as supported by an increase in necrosis-degeneration and inflammation. The liver and kidney damage has been related to phytotherapeutics present in plants, such as alkaloids, phenol, hydroquinone, tannins, flavonoids, saponins, steroids, and terpenes, if administrated at a high dose (Evans, 1996; Manurung et al., 2019).
A histopathological examination was performed to evaluate microscopic damage to these organs. All groups showed more abnormalities (necrosis, degeneration, and inflammation) in the liver, kidneys, and ulcers than in other organs (Table 8). These results were confirmed by elevated ALT, AST, creatinine, and glucose levels in Tables 6 and 7. Although some parameters recovered after stopping treatment, organ damage was not completely repaired at the microstructure level. These findings may indicate that S. ligustrina+DHP had microstructure damage in the vital organs of mice.
CONCLUSION
In conclusion, subchronic oral administration of S. ligustrina and DHP for 28 days was safe in low doses (200 + 111 mg/kg BW). High doses (800 + 333 mg/kg BW) and medium doses (400 + 222 mg/kg BW) showed toxic effects including body weight, relative organ weight, hematology, biochemistry, and histopathology. However, the toxic effect of S. ligestrina+DHP was recovered after stopping treatments for 2 weeks.
ACKNOWLEDGMENTS
The authors thank Animal Facilities Tropical Biopharmaca Research Center—IPB University and Pathology Laboratory of Indonesia Research Centre for Veterinary Science.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was funded by the Directorate of Higher Education of the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia with the IPB Higher Education Research Grant scheme 2021 (contract No. 1/EI/KP.PT.PTNBH/2021) for funding support for this research.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
All procedures in the experiment were approved by the Animal Ethics Committee, IPB University (approval number: 154-2019).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alcon MP, Arola L, Mas A. Response to acute nickel toxicity in rats as a function of sex. Biol Met, 1991; 4:136–40. CrossRef
Al-Habori M, Al-Aghbari A, Al-Mamary M, Baker M. Toxicological evaluation of Catha edulis leaves: a long term feeding experiment in animals. J Ethnopharmacol, 2002; 83:209–17. CrossRef
BPOM. Peraturan Kepala Badan Pengawas Obat dan Makanan Republik Indonesia nomor 7 tahun 2014 tentang pedoman uji toksisitas nonklinik secara in vivo. BPOM RI, Indonesia: 2014.
Cahyaningsih U, Sa’diah S, Syafii W, Sari RK, Maring AJ, Nugraha AB. Antimalarial efficacy of aqueous extract of Strychnos ligustrina and its combination with dihydroartemisinin and piperaquine phosphate (DHP) against Plasmodium berghei infection. Korean J Parasitol, 2022; 60:339–44. CrossRef
Chung JY, Lee J, Lee D, Kim E, Shin JH, Seok PR, Yoo SH, Kim Y. Acute and 13-week subchronic toxicological evaluations of turanose in mice. Nutr Res Pract, 2017; 11:452–60. CrossRef
Diaz D, Hartley DP, Kemper R. Issue investigation and practices in discovery toxicology. In: Will Y, McDuffie E, Olaharski AJ, Jeffy BD (eds.). Drug discovery toxicology: from target assessment to translational biomarkers, John Wiley & Sons, Inc, Hoboken, NJ, pp 530–9, 2016. CrossRef
El Kabbaoui M, Chda A, El-Akhal J, Azdad O, Mejrhit N, Aarab L, Bencheikh R, Tazi A. Acute and sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats. J Ethnopharmacol, 2017; 209:147–56. CrossRef
Evans G. Animal clinical chemistry a practical handbook for toxicologists and biomedical researchers. 2nd edition, CRC Press, Boca Raton, FL, 1996.
Hafid AF, Tyas MW, Widyawaruyanti A. Model terapi kombinasi ekstrak etanol 80 % kulit batang cempedak ( Artocarpus Champeden Spreng.) dan artesunat pada mencit terinfeksi parasit malaria. J Indones Med Assoc, 2011; 61:161–7.
Lee JY, Mori C, Tokumoto M, Satoh M. Changes in DNA-binding activity of transcription factors in the kidney of mice exposed to cadmium. J Toxicol Sci, 2021; 46:125–9. CrossRef
Manurung H, Sari RK, Syafii W, Cahyaningsih U, Ekasari W. Antimalarial activity and phytochemical profile of ethanolic and aqueous extracts of bidara laut (Strychnos ligustrina Blum) wood. J Korean Wood Sci Technol, 2019; 47:587–96. CrossRef
Nugroho RA, Aryani R, Manurung H, Rudianto R, Prahastika W, Juwita A, Alfarisi AK, Pusparini NAO, Lalong A. Acute and subchronic toxicity study of the ethanol extracts from ficus deltoidea leaves in male mice. Open Access Maced J Med Sci, 2020; 8:76–83. CrossRef
Okamoto M, Ichii O, Mie K, Nishida H, Akiyoshi H. Altered clinicopathology and renal pathology in dogs treated with a clinical dose of cisplatin. Jpn J Vet Res, 2021; 69:109–24.
Rahayu AAD, Prihantini AI, Krisnawati, Nugraheni YMMA. Chemical components of different parts of Strychnos ligustrina, a medicinal plant from Indonesia. IOP Conf Ser Earth Environ Sci, 2022; 959:0–11. CrossRef
Setiawan O, Wahyuni N, Susila WW, Rahayu AAD, Rostiwati T. Bidara Laut (Strychnos ligustrina Blume) syn. S. lucida R. Br: Sumber Bahan Obat Potensial di Nusa Tenggara Barat dan Bgali. FORDA PRESS, Bogor, Indonesia, 2014.
Setiawansyah A, Reynaldi MA, Tjahjono DH, Sukrasno S. Molecular docking-based virtual screening of antidiabetic agents from Songga (Strychnos lucida R.Br.): an Indonesian native plant. Curr Res Biosci Biotechnol, 2022; 3:208–14. CrossRef
Syafii W, Sari RK, Cahyaningsih U, Anisah LN. Antimalarial activity of the fractions from ethanol extract of Strychnos ligustrina blume. Wood. Res J Med Plant, 2016; 10:403–8. CrossRef
Traesel GK, de Souza JC, de Barros AL, Souza MA, Schmitz WO, Muzzi RM, Oesterreich SA, Arena AC. Acute and subacute (28 days) oral toxicity assessment of the oil extracted from Acrocomia aculeata pulp in rats. Food Chem Toxicol, 2014; 74:320–5. CrossRef
Tran PNT, Tran TTN. Evaluation of acute and subchronic toxicity induced by the crude ethanol extract of Plukenetia volubilis Linneo leaves in Swiss albino mice. Biomed Res Int, 2021; 2021 :1–13. CrossRef
WHO. Guidelines for the treatment of malaria. 3rd edition, WHO, Geneva, Switzerland, 2015.
Zhang Z, He F, Yang W, Yang L, Huang S, Mao H, Hou Y, Xiao R. Pu-erh tea extraction alleviates intestinal inflammation in mice with flora disorder by regulating gut microbiota. Food Sci Nutr, 2021; 9:4883–92. CrossRef
Zubaidah A, Faidah KR, Samsundari S. Effectiveness of Strychnos ligustrina Bl. Extract as feed supplementation to increase immune system of Nile tilapia (Oreochromis niloticus) wich againts Streptococcus agalactiae. IJOTA Indones J Trop Aquat, 2018; 1:1–8. CrossRef