INTRODUCTION
Clonazepam (CZP) is an anti-anxiety drug belonging to the benzodiazepine (BZP) family that affects gamma-aminobutyric acid (GABA) (Mikulecká et al., 2014). GABA is a neurotransmitter that obstructs brain function (a chemical used by nerve cells to interact with each other). Excessive brain activity is likely to contribute to anxiety or other psychological disorders (Goddard et al., 2004). CZP is used specifically to treat panic disorder and to inhibit some forms of convulsions (Morishita, 2009). On the other hand, it causes disturbance of many brain processes. Neurological impairments like in drug abusers are detected and may signify neural disorders and drug-induced neurotoxicity (Cunha-Oliveira et al., 2010). CZP has anxiolytic, sedative, and anticonvulsant effects and is a common drug of abuse due to its euphoric effect (Baselt, 2008). It is supposed that reversing amnesia and weakening memory would have some effect (Baselt, 2008; Storhaug et al., 2012).
CZP is utilized mainly to treat epilepticus though it is also commonly suggested for different neurological and psychological disorders containing drug abuse treatment, while CZP is known to be harmless. Addiction medicine specialists established that it is often commonly damaged as a drug lane (non-medicinal reasons) (Longo and Johnson, 2000). BZP is hardly the single or chosen medication for misuse (Steentoft and Linnet, 2009). Wilms et al. (2003) reported that BZP binding receptors are currently used to assess inflammation in diseases such as Alzheimer, while BZP peripheral receptors are plentiful in several kinds of cells and are stated physiologically merely at small levels in the central nervous system (CNS). Earlier studies indicate equivalent results after experimental injuries in animal models and inflammatory diseases and neurodegeneration in humans (Wilms et al., 2003).
Flavonoids are polyphenolic complexes that may be found in food products, brews, and herbs with numerous benefits to health in numerous studies (Li et al., 2008). Many flavonoids have antioxidant functions (Lambert and Elias, 2010). Chrysin (5,7-dihydroxyflavone) is a flavonoid that naturally exists and is extracted from various plants, including Passiflora caerulea (blue passionflower) and honey (Li et al., 2010). Chrysin has various pharmacological effects as other flavonoids, including anti-cancer (Li et al., 2010), antioxidant (Pushpavalli et al., 2010; Temel et al., 2020), antihypertensive (Villar et al., 2002), and anti-inflammations effects (Cho et al., 2004). Chrysin leaves an effect on the CNS, having an anxiolytic activity (Rodriguez-landa et al., 2019); it possesses antidepressant properties which can be beneficial in treating several complaints (Farkhondeh et al., 2020; Filho et al., 2016a); it improves memory disorders and cognitive deficits in various models of memory loss induction (Souza et al., 2015; Taslimi et al., 2019), where it affects the neurotransmitter systems like serotonergic, noradrenergic, dopaminergic, and GABAergic systems of brain constructions such as the prefrontal cortex, raphe nucleus amygdala, and hippocampus which are implicated in the pathophysiology of numerous neuropsychiatric disorders (Rodriguez-landa et al., 2022). Another work presented that Chrysin confidently reserved tunicamycin which results in the death of neural cells by suppression of mitochondrial apoptosis (Izuta et al., 2008). So, it acts as a neuroprotective mediator (Souza et al., 2015) and has a neuroprotective effect in chronic cerebral hypoperfusion (He et al., 2012). Moreover, Chrysin could be a neuroprotective agent in various models like Parkinson’s disease (Goes et al., 2018; Krishnamoorthy et al., 2019); it decreases neuroinflammation and increases the levels of neurotrophic factor (Filho et al., 2015). Additionally, Chrysin has been revealed to possess potential improvements in cerebral ischemia, preventing cognitive impairments and long-term mental potency owing to cerebral ischemia (Sarkaki et al., 2019) and managing the PI3K/Akt/mTOR pathway (Li et al., 2019). Recently, Chrysin has also been shown to ameliorate lead-acetate-induced toxicity in rats (Kucukler et al., 2021). It was also revealed that Chrysin suppresses HT-29 cell death induced by diclofenac through apoptosis and oxidative damage (Özbolat and Ayna, 2021). Additionally, Chrysin alleviates cyclophosphamide-induced toxicities in SH-SY5Y cells (Ayna et al., 2020).
This study explained the pathway of neurodegeneration induced by CZP through biochemical and behavioral studies and the impact of Chrysin treatment in the relief of neurodegeneration caused by CZP in male rats in the cortex, hippocampus, and striatum.
MATERIAL AND METHODS
Experimental animals
Forty adult male albino rats (Wister strain) (weighing 120 ± 5 g) were used in this study. The rats were obtained from the animal house of the National Organization for Drug Control and Research (NODCAR) and were housed in plastic cages, 10 rats per cage. Throughout the experiment, animals were preserved under the exact temperature of 25°C ± 2°C and 12 hours of light/12 hours of dark period. A pellet diet billboard was used during the experiment. 1 week before the experiment started, the animals were adapted to the laboratory conditions in compliance with the rules of the Scientific Research Ethics Committee of Ain Shams University and the NODCAR (with the approval number NODCAR/11/22/19) where the experimental procedures were carried out.
Drugs
Chrysin, 5,7-dihydroxyflavone, 98% (C15 H10 O4 molecular weight of 254.24) was manufactured by Alfa Aesar, Germany. CZP 2 mg oral tablets are available as generic drugs and the brand name drug is Apetryl. Apetryl tablets are manufactured by Multi-Apex for pharmaceutical industries S.A.E., Badr City, Egypt.
Experimental design
The animals were divided into four groups (10 rats each) as follows.
Group 1, the control group, was treated with a vehicle (1% w/v Tween 80) according to El Khashab et al. (2019). Group 2, CZP group, was treated with 2 mg/kg/day (Mohamed et al., 2015) and suspended in 1% w/v Tween 80 (Soca?a et al., 2018). Group 3, Chrysin group, was treated with 50 mg/kg/day (Mehri et al., 2014) suspended in 1% w/v Tween 80 according to El Khashab et al. (2019). Group 4, CZP + Chrysin group, was treated with both CZP (2 mg/kg/day) and Chrysin (50 mg/kg/day). All the animal groups were daily treated for 6 weeks starting on the first day of the experiment by oral gavage. The used doses are human equivalent pharmaceutical doses and were calculated as described previously by Reagan-Shaw et al. (2008). The open field and Y maze tests started on the 6th week of the experiment before decapitation.
Biochemical investigation
After 24 hours from the last dose, the rats were sacrificed by fast decapitation. Brains were dissected out and cut into two halves longitudinally. The cortex, hippocampus, and striatum were isolated from the first half and homogenized in 75% methanol high performance liquid chromatography grade for estimation of amino acids (glutamic acid and GABA) and monoamines [norepinephrine (NE), dopamine (DA), and serotonin (5HT)] and their metabolites [homovanillic acid (HVA), 3,4-dihydroxyphenylacetic acid (DOPAC), and hydroxy indoleacetic acid (5-HIAA), respectively] (Pagel et al., 2000) and 8-hydroxy-2–deoxyguanosine (8-HdG) (Lodovici et al., 1997). The second half was homogenized using a Polytron homogenizer at 40°C with a phosphate buffer of 0.05 M (PH 7). To remove cell waste, unbroken cells, nuclei, erythrocytes, and mitochondria, the homogenate was centrifuged at 10,000 rpm for 20 minutes. The supernatant was utilized by Enzyme-Linked Immuno-Sorbent Assay (ELISA) in conjunction with manual instructions for the determination of brain-derived neurotrophic factor (BDNF) and calcium ATPase (Ca-ATPase). All ELISA kits were measured by an ELISA reader. Color absorbance was read at an objective density range of 490 to 630 nm using an ELISA plate reader (Stat Fax 2200, Awareness Technologies, Palm City, FL).
Behavioral tests
Y maze test
The Y maze involved three identical arms labeled A, B, and C. The angle between the arms is 120 degrees; each arm of the maze has dimensions of 40 × 15 × 30 cm (Roghani et al., 2006). Both the floor and sides of all arms are made of wood. The maze is used to assess spontaneous changes. Each rat was placed in one of the arms and permitted to transfer freely among the three arms for 5 minutes. The number of arm entries and the number of trials are stated to evaluate the alteration percentage. The number of maximum random alterations is calculated as the total number of arms entered minus two, and the correct alteration is calculated as the consecutive overlapping entries into the three arms. The percentage of alternation is determined for three groups (i.e., ABC, CBA, and BAC). The percentage of alternation is calculated as [(actual alternations/maximum alternations) × 100], and the percentage of correct alternation is calculated as [(correct alternation/maximum alternations) × 100]. When the four limbs of a rat are in the arm, an entry is determined. The maze is cleaned with 70% ethyl alcohol and permitted to dry among sessions. The Y maze was used to assess the overall stereotypic behavior, locomotor activity, and short-term memory (Roghani et al., 2006).
Open field test
This test was utilized in this study to measure the locomotion activity. It was made of white plywood and measured 100 × 100 cm with 45 cm. A marker was used to draw black lines on the floor. The floor was divided into 25 parts with dimensions 20 × 20 cm by the black lines. The central square was drawn in the middle of the maze (20 × 20 cm). In their home cages, the rats were conceded to the practice chamber and were always handled by the base of their tails. Into one of the open field corners, rats were sited and permitted to discover the maze for 5 minutes. Then rats were returned to their cages. The maze was cleaned with 70% ethyl alcohol among trials and permitted to dry among tests. On two consecutive days, rats were exposed to the maze for 5 minutes to assess the process of acclimatization to the newness of the field according to Yang et al. (2013). The second-day trials were recorded and used in the statistics.
Statistical analysis
Values reported reflect means ± SEM. Statistical analysis was assessed by one-way ANOVA.
Least significant difference comparisons were conducted to determine the significant differences between different treated groups. SPSS for Windows software, release 23.0, was used with a significant value of p < 0.05.
RESULTS AND DISCUSSION
Biochemical investigation
Determination of glutamic acid, GABA, and 8-HdG contents
The data in Table 1 revealed a significant increase in glutamic acid contents in the cortex, hippocampus, and striatum of all treated groups except CZP + Chrysin rats in both brain cortex and hippocampus in comparison with the control rats at p < 0.05. Moreover, the GABA contents declined significantly in all treated groups in the cortex, hippocampus, and striatum when compared with the control values. Additionally, GABA content increased significantly in the brain hippocampus while it slightly increased in the brain cortex and striatum in CZP + Chrysin group rats when compared with the Chrysin-treated group at p < 0.05. Furthermore, all the CZP-treated groups exhibited a significant increase in the cortex, hippocampus, and striatum 8-HdG contents in comparison with the control group at p < 0.05. Otherwise, the group treated with Chrysin exhibited a remarkable decrease in cortex, hippocampus, and striatum 8-HdG contents as compared to CZP-treated rats. Also, CZP + Chrysin group rats demonstrated a significant decrease (p < 0.05) in the three brain areas of 8-HdG contents when compared with the CZP-treated group. These results were elucidated by Goddard et al. (2004) who revealed a significant decrease in occipital cortex GABA levels after chronic CZP as BZP family treatment and suggest that CZP may be capable of exerting a direct inhibitory effect on the activity of glutamate decarboxylase. CZP is highly potent and a long-acting BZP. It exerts pharmacological effects by acting as a positive allosteric modulator on GABA-A receptors. The GABA-A receptor is a ligand-gated chloride ion-selective channel whose endogenous ligand is GABA. BZPs facilitate GABA-A action by increasing the frequency of chloride channel opening, resulting in hyperpolarization of the neurons and decreased firing, thus producing calming effects on the brain by reducing the excitability of neurons (Griffin et al., 2013). Alternatively, BZP’s influence on other neuronal targets could alter both the excitatory drive for the neuronal activity of GABA and the availability of substrates for GABA synthesis, like glutamate and glutamine. In fact, after BZP administration, glutamic acid levels were slightly elevated, indicating an inhibitory effect of BZP administration on glutamic acid turnover. In addition, BZP enhances the GABA-A receptor-mediated impacts of exogenous and exogenous GABA (Mikulecká et al., 2014). This potential consists of an improvement in GABA’s apparent affinity for raising chloride conductance without increasing its effectiveness, which may be due to an increased probability of channel opening events at the single chloride channel level. So, recent studies suggest that BZP is a GABA receptor (GABA-R) chloride channel complex site that allosterically modulates the increase of the signal transducer feature of the latter. The exact contact with three groups of ligands is a special characteristic of this medication receptor, which is placed on a neurotransmitter receptor-gated ion channel (Kubová et al., 2020).
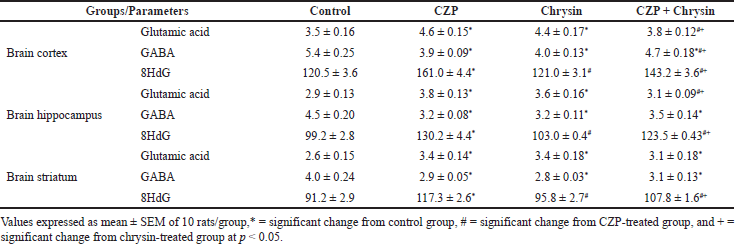 | Table 1. The effect of CZP and chrysin on glutamic acid, GABA (µg/g tissue), and 8-HdG (pg/g tissue) in brain cortex, hippocampus, and striatum. [Click here to view] |
Rats treated with Chrysin showed a significant elevation in glutamic acid in the three brain areas as compared with the control. These data were supported by El Khashab et al. (2019) who found that exposure to Chrysin was followed by Bcl2 elevation and a reduction in both Hsp90 and Bax levels and was also followed by aspartate and glutamate elevation. These records contribute to the understanding of the value of natural flavonoids as neuroprotective agents (Souza et al., 2015). Chrysin has been reported to exert neuroprotective effects through different mechanisms, including antioxidant, anti-inflammatory, and anti-apoptotic functions, monoamine oxidase inhibition, and GABA mimetic properties (Mishra et al., 2021). In addition, Bortolotto et al. (2020) confirmed that Chrysin normalizes the activity of Na+, K+- ATPase, and glutamate levels in the hippocampus, which may be related to memory impairment in reverse. The regulation of the glutamic acid level in rats treated with CZP + Chrysin compared to the CZP group can be addressed in this description. Also, the Chrysin-treated group revealed a marked decline in GABA levels in the brain cortex, hippocampus, and striatum, and the detected Chrysin anxiolytic effects in this work looked to be less partially mediated by GABAergic activity according to a previous study by Wang et al. (2002) which has revealed that Chrysin possesses a high GABAA receptor affinity. GABA is the most important inhibitory neurotransmitter in the brain. It exhibits a neuroprotective effect by inhibiting brain injury, neuronal damage, autophagy [via the upregulation of Bcl-2/Bax ratio and activating protein kinase B, glycogen synthase kinase-3β, and extracellular signal-regulated kinase 1 (ERK) signaling molecules], and neuronal cell death (Ngo and Vo, 2019). In this way, GABA demonstrates a therapeutic potential in various neurological disorders (Nuss, 2015); Chrysin has been shown to modulate the GABAA receptor and thus abrogate anxiety and depression-like behavior (Cueto-Escobedo et al., 2020; Rodriguez-Landa et al., 2021). Therefore, it can act as a neuroprotection via the modulation of GABAergic innervation. The chloride ions’ conductance increases when GABAergic agonists stimulate these receptors, thus hyperpolarizing the neuron and thereby inhibiting neuronal activity (Majewska et al., 1986). The anxiolytic effects of many materials, involving BZP, are associated with this neurophysiological effect, which arises through the chloride ion channels of the GABAA receptor. Moreover, Rashed et al. (2016) interpreted the decreased level of the inhibitory neurotransmitter GABA as Chrysin’s neuroprotective role.
Moreover, the CZP-treated group showed a significant increase in DNA fragmentation (8-HdG) in all of the brain cortex, hippocampus, and striatum. These results were supported by Girgis et al. (2010) who discovered that compared to the control group, CZP-treated rats displayed the highest optical density value of brain DNA degradation and discussed that through several mechanisms CZP can induce apoptosis. The peripheral non-GABAA receptors are one such mechanism. Another alleged mechanism is the oxidative stress caused by the overdose typically achieved in the state of abuse and tolerance, alteration of ion channel influx, and outflux activity modulations in the cell media through GABA-R that cause changes in normal chloride and other ions balance in the neural cells, and finally, activation of the caspase system and eventually apoptosis of cell death (Uren et al., 2000). Also, Bittigau et al. (2002) stated that at plasma concentrations important to seizure control in humans, CZP induces apoptotic neurodegeneration in the developing rat brain; neuronal death is related to the reduction of neurotrophin expression and decreased brain concentrations of survival-promoting proteins. Anti-epileptic drugs reduced the synthesis of BDNF and NT-3 neurotrophins and decreased levels of c-RAF, ERK12, and AKT active phosphorylated types. These changes indicate the inhibition of signals elevating survival and the difference between neuroprotective and neurodestructive mechanisms in the brain that facilitate apoptotic neurodegeneration during the developmental cycle of ongoing programmed neuronal death (Venters et al., 2000). Moreover, rats treated with Chrysin showed a significant reduction in brain cortex, hippocampus, and striatum 8HdG related to CZP-treated rats and showed amelioration impact in co-administration of Chrysin and CZP. These findings were verified by Rashno et al. (2019) who discovered that Chrysin prevented neuronal failure, decreased apoptotic index, and increased anti-apoptotic Bcl-2 protein; the expression of proapoptotic Bax protein in the tissues of the cerebral cortex and hippocampus decreased. In addition, Mehri et al. (2014) reported that Chrysin has been able to prevent the death of cells and inhibit apoptotic tissue damage by enhancing the activity of caspase 3. Chrysin also strengthened autophagic tissue damage by raising the light chain 3B expression (Kandemir et al., 2017). Also, Darendelioglu (2020) found that, through regulations on the mRNA expression of Bcl-2 and Bax protein, Chrysin plays a vital role in preventing apoptosis. Suppression of the activity of caspase 3 and the mitochondrial apoptotic pathway may at least partially involve the protective effects mechanism of Chrysin (Izuta et al., 2008).
Determination of monoamines and monoamines metabolites
The results in Table 2 indicated the treatment effect of CZP and Chrysin on monoamines NE, DA, and 5HT contents in brain cortex, hippocampus, and striatum in male rats. In comparison to the control rats, there was a significant elevation (p < 0.05) in NE, DA, and 5HT contents of the three brain areas in CZP- and Chrysin-treated groups. Also, CZP + Chrysin-treated rats showed a significant increment in monoamines contents of the brain areas except for DA contents of the brain cortex and striatum compared to control rats. Furthermore, the treatment with CZP + Chrysin induced a significant reduction in NE and DA contents of the brain areas except for hippocampus NE content when compared with the CZP-treated rats at p < 0.05.
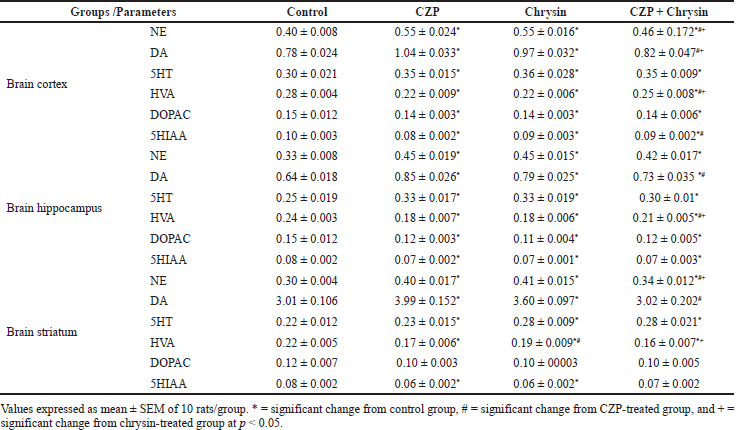 | Table 2. The effect of CZP and chrysin on monoamines NE, DA, and 5HT and their metabolites (HVA, DOPAC, and 5-HIAA, respectively) (µg/g tissue) in brain cortex, hippocampus, and striatum. [Click here to view] |
The monoamines metabolites, HVA, DOPAC, and 5-HIAA, of the three brain areas, were significantly decreased (p < 0.05) when comparing the treated groups with the control except for striatum 5-HIAA and DOPAC contents of CZP + Chrysin-treated groups and striatum DOPAC contents of CZP- and Chrysin-treated rats. Moreover, Chrysin treatment induced a significant elevation in the HVA contents in the brain striatum in comparison with the CZP-treated group. Moreover, there was an increase (p <0.05) in the HVA brain cortex and hippocampus content of the CZP + Chrysin-treated group compared with either CZP- or Chrysin-treated groups. However, rats treated with CZP + Chrysin showed a significant reduction in striatum HVA content as compared with the Chrysin-treated group.
CZP therapy demonstrated a significant rise in three forms of monoamines in the brain cortex, hippocampus, and striatum, as confirmed by Frey et al. (1991) who found that 5-HT levels were high in the midbrain and hemispheres and showed fluctuations in the cerebellum. Additionally, Inoue et al. (2007) proposed that CZP is a special high-power 1,4-BZP derivative and its activities are regulated not only by the GABA sub-type A receptor but also by the regulation of the metabolism of central 5-hydroxytryptamine (5-HT, serotonin). Also, Fennessy and Lee (1972) have reported the rise in DA and NE; many authors suggest that increases in 5-HT, DA, and NE production and improvement of 5-HT and monoamine activity mediated by the GABA system are linked to anti-depressive effects. While CZP increases the synthesis of serotonin, it was found that CZP decreased the release of 5-HIAA from the brain without changing 5-HT synthesis and clearly decreased the usage of 5-HT (Jenner et al., 1986). Based on their observations, Kishimoto et al. (1988) proposed that the anti-depressive effects might be due to the decrease in 5-HT use or the decline of the sensitivity of its receptors. In addition, it was suggested that GABA-B antagonists of the receptor possess antidepressant properties (Slattery et al., 2005). GABA-B receptor activation prevents serotonin production, so it has been proposed that serotonergic transmission plays a role in facilitating the antidepressant properties of the GABA-B receptor antagonist (Chaki et al., 2006). CZP reduced the GABA-B portion of inhibitory postsynaptic potential (Huguenard and Prince, 1994). Winkler et al. (2003) mentioned that CZP interacts with the GABA system and alters the humor phases. During the development of tolerance, monoamine transformation displayed an overall decline which, however, tended to be the most linked to tolerance in the case of NE in different brain areas and DA in the midbrain. There was also a transient rise in 5-HT turnover accompanied by a decline, except for the cerebellum (Frey et al., 1991). Vinkers et al. (2009) reported that BZP can produce its anxiolytic effects by stimulating alpha 3 subunits situated on serotonergic neurons. In support of that, serotonergic raphe nuclei obtain a protuberant GABAergic input from remote sources besides interneurons (Varga et al., 2001; Vinkers et al., 2009) and orders in these arches for novel anxiolytic drugs may be useful for the reaction of the GABA and 5-HT system in anxiety, together with the fact that BZP acutely decreases anxiety (Inoue et al., 2007).
Chrysin therapy also indicates a significant improvement in the brain areas of the cortex, hippocampus, and striatum, confirming the serotoninergic system’s role in the antidepressant-like impact of Chrysin (Bortolotto et al., 2018). Ye et al. (2015) mentioned that this finding is significant since both the frontal cortex and the hippocampus are involved in the organization of depression-related emotions, memory, and learning. Also, Filho et al. (2016a) related the serotoninergic system to the antidepressant-like consequence of Chrysin in mice exposed to changeable prolonged stress. Moreover, Chrysin has been efficient in normalizing the hippocampal DA level, and it works at the level of cerebral synaptogenesis, returning the levels of serotonin and dopamine in the synaptic cleft and synthesizing or taking up these neurotransmitters. So, the serotonergic and dopaminergic systems are involved in Chrysin antidepressant-like impact (Bortolotto et al., 2018). Otherwise, Angelopoulou et al. (2020) proved that by mono-amino-oxidase B inhibition, Chrysin increased DA levels in the striatum; this finding was verified by our results showing a significant decrease in the metabolites of monoamines in the three brain regions. Chrysin administration dramatically enhanced dopaminergic neuronal loss, leading to reduced shivering, stiffness, postural instability, rearing activity, and animal memory dysfunctions (Ahmed et al., 2018). Furthermore, in the study of Guo et al. (2016), in Parkinson’s disease mice models, Chrysin might save dopaminergic cell death in the substantia nigra pars compacta. In addition, oral therapy with Chrysin decreased the loss of tyrosine hydroxylase-immunoreactive striatal cells and preserved the morphology of 6-OHDA nigrostriatal neurons (Goes et al., 2018).
Determination of BDNF and Ca-ATPase contents
The data illustrated in Figure 1a and b displayed a significant reduction in whole BDNF and Ca-ATPase contents in the CZP group when compared to control rats at p < 0.05. Moreover, Chrysin and CZP + Chrysin treatment caused a significant increase in BDNF and Ca-ATPase compared to rats treated with CZP. Neurotrophic factors are the proteins family that is responsible for the development, survival, differentiation, and function of neuronal cells that produce and sustain mature neurons, including BDNF. BZPs are safe medications for the treatment of anxiety, sleeping disorders, and seizures; nevertheless, their usage has often led to unfavorable effects, containing memory dysfunctions and misuse (Licata et al., 2013). Huopaniemi et al. (2004) reported that BZP (Diazepam) decreases animal BDNF contents. Also, the work carried out by Huang and Hung (2009) revealed a reduction in serum BDNF contents in schizophrenia patients treated with lorazepam. This connection may be related to the unrecognized impact of the BDNF on the GABA neurotransmitter responsible for the relaxing and anxiolytic properties of BZP (Canas et al., 2004; Singh et al., 2006). The release pool that needs calcium entry via voltage-dependent calcium channels is affected by glutamate release from hippocampal synaptosomes and BDNFs. Moreover, the inhibitory effect of BDNFs on the liberation of potassium-evoked GABA from the synaptosomes of the hippocampus has been revealed to be independent of the admission of calcium into synaptosomes via voltage-sensitive calcium channels. Nevertheless, based on the operation of GABA transporters, it has been observed that BDNF acts as a result of transport reversal in the release pool (Canas et al., 2004). Accordingly, BDNF has been shown to inhibit the transport of GABA into synaptosomes (Vaz et al., 2008). Furthermore, Brünig et al. (2001) demonstrated a quick downregulation by BDNF of GABAA receptor surface expression levels. These results may interpret the significant decrease in whole brain BDNF according to its relationship with decreasing GABA levels in CZP-treated rats compared with the control group. On the other hand, while Chrysin decreased GABA levels in the three brain regions, it increased the whole brain BDNF level, which is confirmed by Sathiavelu et al. (2009) that Chrysin may confer a defensive effect by diminishing free radical production. These scientists also recorded that the fifth and seventh positions of the hydroxyl group can contribute to its strong antioxidant impact. Also, Souza et al. (2015) proved that the decline in cortical and hippocampal BDNF levels in aged mice was mitigated by Chrysin; these findings showed that the antioxidant compound flavonoid Chrysin was possibly able to inhibit age-related memory deficit through its free radical scavenger activity and BDNF output modulation. Thus, Chrysin may demonstrate a novel pharmacological strategy, performing as an anti-aging agent. Filho et al. (2015) reported that treatment with Chrysin caused upregulation of BDNF levels in the hippocampus of female mice. In the other work, they proposed that Chrysin’s antidepressant effect might be accompanied by the upregulation of this neurotrophin in the structures of the brain (Filho et al., 2016b). Also, Xu et al. (2023) suggested that Chrysin restrains the neuroinflammation throughout the signaling pathways of BDNF. Additionally, the flavonoid Chrysin close to the antidepressant fluoxetine increases 5-HT levels and decreases both indoleamine-2,3-dioxygenase and caspases 3 and 9 activities in the prefrontal cortex and hippocampus of mice accompanied by the antidepressant-like effect detected in the cortex and hippocampus of mice that were related to the antidepressant-like effect which was noticed in the suspension test (Filho et al., 2016a) with the participation of BDNF.
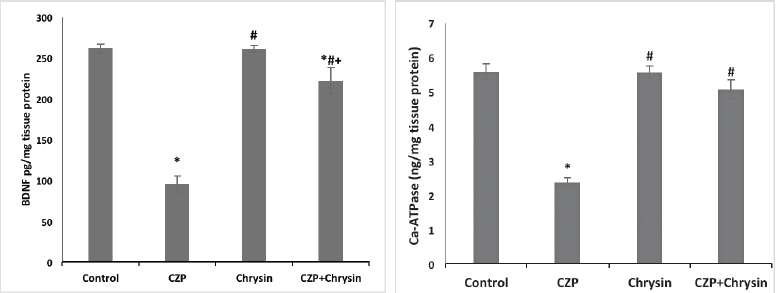 | Figure 1. (a): The effect of CZP and chrysin on BDNF (pg/mg tissue protein) in whole brain. Values expressed as mean ± SEM of 10 rats/group. * = significant change from control group, # = significant change from CZP-treated group, and + = significant change from chrysin-treated group at p < 0.05. (b): The effect of CZP and chrysin on Ca-ATPase (ng/mg tissue protein) in whole brain. Values expressed as mean ± SEM of 10 rats/group. * = significant change from control group and # = significant change from CZP-treated group at p < 0.05. [Click here to view] |
Mata et al. (2011) realized that Ca2+-ATPases existent in the plasma membrane are a strongly expressed brain multi-isoform family of proteins that are involved in maintaining low intraneural Ca2+ concentration. The increased concentration of monoamines in brain areas thus represents the increased concentration of intracellular Ca2+ and decreased intracellular Ca2+ ATPase, which was supported by our CZP-treated rats’ results related to the control rats. In addition, Ca2+ homeostasis changes are related to neurodegenerative diseases and brain aging (Jiang et al., 2012) associated with improved liberation of glutamate (Garside et al., 2009) and raised remaining calcium inside the presynaptic end associated with removal of protein misfolding cyclic amplification feature (Jensen et al., 2007) which may reflect increasing monoamines in our CZP results. BZPs have been identified as calmodulin inhibitors (Seckin et al., 2007) which are involved in Ca pump plasma membrane control (Vincenzi et al., 1980). The Ca2+-ATPase inhibition may be due to the collaboration between CZP and calmodulin. Evidence in support of the contribution of intracellular Ca2+ concentration changed homeostasis in several events leading to neural cell apoptosis was given in the same pathological settings (Annunziato et al., 2003).
Co-administration of Chrysin with CZP showed improvement in Ca-ATPase content, where Chrysin seems to preserve the components of the redox mechanism at close-to-normal standards and avoid the unusual expression of certain apoptotic cascade proteins, thereby avoiding calcium accumulation and cell death (Sundararajan et al., 2016). Furthermore, Chrysin can degrade intracellular Ca2+ levels by controlling the consumption of Ca2+ extracellular intake throughout calcium voltage-operated channels and obstructing the intracellular discharge of Ca2+ from the sarcoplasmic reticulum (Tew et al., 2023). Moreover, Ontiveros et al. (2019) proved that flavonoids modify intracellular Ca2+ homeostasis associated with mitochondrial Ca2+ channel and Ca2+ pump activity alterations. These results suggest the presence of the plasma membrane Ca2+-ATPase, as it actively transfers Ca2+ to the extracellular medium attached to ATP hydrolysis, thereby preserving ion cell homeostasis. The main calcium-regulating proteins involved in the calcium homeostasis equilibrium are Ca2+-ATPases, which are affected by oxidative/nitrosative stress and associated disorders or aging. Flavonoids not only work as antioxidants but can also bind directly to Ca2+-ATPases, thus altering their conformation and leading to enzyme activity modulation (Horáková, 2011).
Behavioral tests
Y maze test
The data depicted in Figure 2a revealed a notable upsurge in the number of arm entries/5 minutes and a decrease in correct alternation in the rats treated with CZP as compared to the control after 6 weeks, whereas Chrysin usage induced a reduction in the number of arm entries/5 minutes and a raise in the correct alternation in comparison to CZP-treated group at p < 0.05. The rats treated with CZP + Chrysin exhibited a marked increase in the number of arm entries/5 minutes related to the control group. Furthermore, the correct alternation in CZP + Chrysin-treated rats demonstrated a significant increase as compared to the CZP group after treatment at p < 0.05. The data in Figure 2b for the Y maze revealed a decrease in percent alternation and percent of correct alternation in the CZP group when compared to the control after treatment at p < 0.05. Although a notable increase was shown in the percent alternation in CZP + Chrysin-treated rats, there was a significant increase in the percent of correct alternation in rats that received Chrysin and CZP + Chrysin in comparison to those which received CZP only. Moreover, the CZP + Chrysin-treated group exhibited a marked decrease in the percent of correct alternation when compared to the Chrysin group (p < 0.05). The Y maze test is the general operation of locomotors, short-term memory, and stereotypic actions (Roghani et al., 2006).
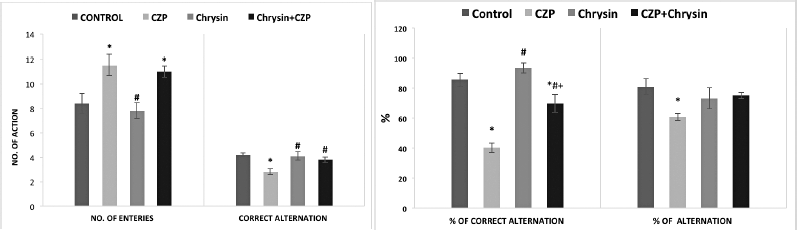 | Figure 2. (a): The effect of CZP and chrysin on Y maze (the number of entries and correct alternations/5 minutes) after 6 weeks of treatment. Values expressed as mean ± SEM of 10 rats/group. * = significant change from control group, # = significant change from CZP-treated group at p < 0.05. (b): The effect of CZP and chrysin on Y maze (percentage of alternations and correct alternations) after 6 weeks of treatment. Values expressed as mean ± SEM of 10 rats/group. * = significant change from control group, # = significant change from CZP-treated group, and + = significant change from chrysin-treated group at p < 0.05. [Click here to view] |
The data clarified by Mikulecká et al. (2014) suggested that chronic administration of CZP contributes to the development of motor action tolerance which is associated with disorganization of the GABA-A receptor, and the stopping of CZP leads to behavior disorder containing augmented activity related to the GABA-A receptor upregulation. CZP thus tends to be comparable to many other BZP in behavioral and neurochemical effects. Additionally, CZP is related to enhanced motor activity and upregulation of receptors after discontinuation (Lopez et al., 1990). The decrease in the correct alternation and percent of correct alternation in rats treated with CZP was confirmed by Crowe and Stranks (2018). They discovered that the highest deficiencies were detected in the operational memory regions, speed processing, attention division, vasoconstriction, new memory, and meaningful language not only for patients who used BZP for long periods but also for those who used CZP as a member of the drug class (Sussman, 2006). These results are largely supportive of the studies reported after the previous meta-analysis (Helmes and Østbye, 2015; Pomara et al., 2015). Moreover, the same authors found that BZP withdrawal from long-term use was still expressively reduced in all cognition regions except in the field of executive function. Thinking/memory impairment is considered the main side effect of CZP treatment. The way by which CZP caused impairment of memory remains unclear. The possible way may be through reducing brain levels of acetylcholine (Kalachnik et al., 2003). Also, Jisham et al. (2015) found that CZP administration exhibited significant memory deficiency in mice assessed by Morris Water Maze. On the other hand, there was an increase in correct alternation and percent of correct alternation in the Chrysin and CZP + Chrysin groups in comparison with the CZP group. These were discussed by Li et al. (2014) who proved that Chrysin improves learning and memory functions. Moreover, Bortolotto et al. (2020) have proposed that Chrysin attenuates memory deficiencies by modulating the activities of both glutamate and sodium-potassium ATPase.
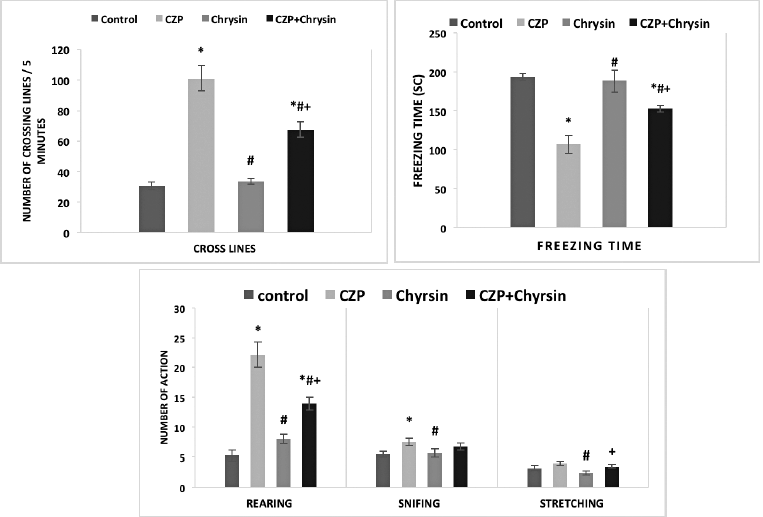 | Figure 3. (a): The effect of CZP and chrysin on open field (number of crossing lines movement/5 minutes) after 6 weeks of treatment. Values expressed as mean ± SEM of 10 rats/group. * = significant change from control group, # = significant change from CZP-treated group, and + = significant change from chrysin-treated group at p < 0.05. (b): The effect of CZP and chrysin on open field (freezing time (sc)/5 minutes) after 6 weeks of treatment. Values expressed as mean ± SEM of 10 rats/group. * = significant change from control group, # = significant change from CZP-treated group, and + = significant change from chrysin-treated group at p < 0.05. (c): The effect of CZP and chrysin on open field (rearing, sniffing, and stretching/5 minutes) after 6 weeks of treatment. Values expressed as mean ± SEM of 10 rats/group. * = significant change from control group, # = significant change from CZP-treated group, and + = significant change from chrysin-treated group at p < 0.05. [Click here to view] |
Open field test
The data demonstrated in Figure 3a and b showed at 6 weeks of treatment, a marked upsurge at p < 0.05 in the number of crossing lines as well as a reduction in freezing time in animals treated with both CZP and CZP + Chrysin when compared with the control. However, Chrysin- and CZP + Chrysin-treated groups exhibited a significant decline in the number of crossing lines and an increase in the freezing time in comparison with the CZP group at p < 0.05. Figure 3c showed that the CZP rats exhibited a significant upsurge in the number of rearing and sniffing times in comparison with control rats at p < 0.05, whereas the number of rearing and stretching times decreased significantly in the Chrysin-treated group in comparison to the CZP-treated rats at p < 0.05. In addition, the Chrysin rats decreased the number of sniffing times significantly compared to the CZP-treated group. Moreover, the rats treated with CZP + Chrysin proved a significant enhancement in the number of rearing and stretching times compared to the Chrysin rats at p < 0.05. The open field is a very common animal model of anxiety-like behavior and locomotion (Yang et al., 2013). A rise in the movement crossing lines, rearing, sniffing, stretching, and freezing time parameters is seen as a primary index of an anxiolytic-like impact (Germán-Ponciano et al., 2020). CZP treatment increased the number of rearing and sniffing times in open field tests which is confirmed by the study of Mikulecká et al. (2014) who discovered that higher locomotion was shown by the animals treated with CZP, which stayed more time in the central square of the open field, indicating decreased anxiety, and had impaired intersession habituations. But the administration of Chrysin with CZP showed an improvement in all these factors when compared with CZP treatment. Germán-Ponciano et al. (2020) indicated that Chrysin flavonoid therapy induced anxiolytic and protective effects against behavioral alterations triggered by a stressful condition and that Chrysin’s effects were partly correlated with cellular variations in the lateral septal nucleus. The behavioral stimulation observed with stimulant exposure can be mediated by different neural pathways. Dopamine is one potential pathway. Many brain regions that receive dopaminergic feedback, such as the dorsal striatum stimulants, increase extracellular dopamine, which in turn stimulates behavior (Anderson, 2014) and is conformed to our monoamines results.
CONCLUSION
This study concluded that Chrysin ameliorated neural behavior by adjusting the neurotransmitters and amino acids. Chrysin modulated the inhibition of BDNF and Ca-ATPase levels in the whole brain, modulated the neurotransmitters and their metabolites, and prevented DNA degradation in the cerebral cortex, hippocampus, and striatum induced with CZP. So, the co-administration of Chrysin with CZP creates pharmacological tolerance, which should be considered to validate or discard this possibility in potential experimental protocols.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the National Organization for NODCAR, Giza, Egypt, for aiding in carrying out the neurotransmitters of the present work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVALS
The animal study’s research protocol was approved by the Institutional Animal Care and Use Committee, Ain Shams University, and NODCAR (approval number NODCAR/11/22/19).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmed MR, Shaikh MA, Haq SHI, Nazir S. Neuroprotective role of chrysin in attenuating loss of dopaminergic neurons and improving motor, learning and memory functions in rats. Int J Health Sci, 2018; 12(3):35–43.
Anderson EM. 2014. Interaction of stress and stimulants in female rats: role of dopamine in the stress-induced reactivity to methamphetamine (ProQuest Dissertations Publishing: 1583548). Northern Illinois University, DeKalb, IL.
Angelopoulou E, Pyrgeli ES, Piperi C. Neuroprotective potential of chrysin in Parkinson’s disease, molecular mechanisms and clinical implications. Neurochem Int, 2020; 132:104612. CrossRef
Annunziatoa L, Amorosoa S, Pannaccionea A, Cataldia M, Pignataroa G, D’Alessioa A, Sirabellaa R, Secondoa A, Sibaudb L, Di Renzo GF. Apoptosis induced in neuronal cells by oxidative stress, role played by caspases and intracellular calcium ions. Toxicol Lett, 2003; 139(2–3):125–33. CrossRef
Ayna A, Özbolat SN, Darendelioglu E. Quercetin, chrysin, caffeic acid and ferulic acid ameliorate cyclophosphamide-induced toxicities in SH-SY5Y cells. Mol Bio Rep, 2020; 47(11):8535–43. CrossRef
Baselt RC. Disposition of toxic drugs and chemicals in man. 8th edition, Biomedical Publications, Foster City, CA, 2008.
Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietk M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegenerationin the developing brain. Proc Natl Acad Sci USA, 2002; 99(23):15089–94. CrossRef
Bortolotto VC, Araujo SM, Pinheiro FC, Poetini MR, de Paula MT, Meichtry LB, de Almeida FP, Musachio EAS, Guerra GP, Prigol M. Modulation of glutamate levels and Na+,K+-ATPase activity contributes to the chrysin memory recovery in hypothyroidism mice. Physiol Behav, 2020; 222:112892. CrossRef
Bortolotto VC, Pinheiro FC, Araujo SM, Poetini MR, Bertolazi BS, de Paula MT, Meichtry LB, de Almeida FP, Couto SF, Jesse CR, Prigo M. Chrysin reverses the depressive-like behavior induced by hypothyroidism in female mice by regulating hippocampal serotonin and dopamine. Eur J Pharmacol, 2018; 822:78–84. CrossRef
Brünig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci, 2001; 13(7):1320–8. CrossRef
Canas N, Pereira IT, Ribeiro JA, Sebastião AM. Brain-derived neurotrophic factor facilitates glutamate and inhibits GABA release from hippocampal synaptosomes through different mechanisms. Brain Res, 2004; 1016(1):72–8. CrossRef
Chaki S, Okubo T, Sekiguchi Y. Non-monoamine- based approach for the treatment of depression and anxiety disorders. Recent Pat CNS Drug Discov, 2006; 1(1):1–27. CrossRef
Cho H, Yun CW, Park WK, Kong JY, Kim KS, Park Y, Lee S, Kim BK. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol Res, 2004; 49(1):37–43. CrossRef
Crowe SF, Stranks EK. The residual medium and long-term cognitive effects of benzodiazepine use, an updated meta- analysis. Arch Clin Neuropsychol, 2018; 33(7):901–11. CrossRef
Cueto-Escobedo J, Andrade-Soto J, Lima-Maximino M, Maximino C, Hernandez-Lopez F, Rodriguez-Landa JF. Involvement of GABAergic system in the antidepressant-like effects of chrysin (5,7-dihydroxyflavone) in ovariectomized rats in the forced swim test: comparison with neurosteroids. Behav Brain Res, 2020; 386:112590. CrossRef
Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR. Neurotoxicity of heroin– cocaine combinations in rat cortical neurons. Toxicology, 2010; 276(1):11–7. CrossRef
Darendelioglu E. Neuroprotective effects of chrysin on diclofenac?induced apoptosis in SH?SY5Y cells. Neurochem Res, 2020; 45(5):1064–71. CrossRef
El Khashab IH, Abdelsalam RM, Elbrairy AI, Attia AS. Chrysin attenuates global cerebral ischemic reperfusion injury via suppression of oxidative stress, inflammation and apoptosis. Biomed Pharmacother, 2019; 112:108619.
Fennessy MR, Lee JR. The effect of benzodiazepines on brain amines of the mouse. Arch Int Pharmacodyn Ther, 1972; 197(1):37–44. CrossRef
Farkhondeh T, Jalali S, Samarghandian S, Samini F. Effects of chrysin on serum corticosterone levels and brain oxidative damages induced by immobilization in rat. Cardiovasc Hematol Disord Drug Targets, 2020; 20(1):47–53. CrossRef
Filho CB, Jesse CR, Donato F, Del Fabbro L, De Gomes MG, Goes ATR, Souza LC, Giacomeli R, Antunes M, Luchese C, Roman SS, Boeira SP. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur J Pharmacol, 2016a; 791:284–96. CrossRef
Filho CB, Jesse CR, Donato F, Giacomeli R, Del Fabbro L, Antunes M, De Gomes MG, Goes ATR, Boeira SP, Prigol M, Souza LC. Chronic unpredictable mild stress decreases BDNF and NGF levels and NA+K+-ATPase activity in the hippocampus and prefrontal cortex of mice, antidepressant effect of chrysin. Neuroscience, 2015; 289:367–80. CrossRef
Filho CB, Jesse CR, Lucian FD, Gome FM, Tiago GA, Leandro RG, Souza LC, Boeira SP. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulb ectomy in mice. Chem Biol Interact, 2016b; 260:154–62. CrossRef
Frey HH, Jung S, Scherk R. Monoamine turnover in the brain of mice during development of tolerance to the anticonvulsant effect of clonazepam. Epilepsy Res, 1991; 8(3):190–6. CrossRef
Garside ML, Turner PR, Austen B, Strehler EE, Beesley PW, Empson RM. Molecular interactions of the plasma membrane calcium ATPase PMCA2 at pre- and post-synaptic sites in rat cerebellum. Neuroscience, 2009; 162(2):383–95. CrossRef
Germán-Ponciano L, Puga-olguín A, Rovirosa-hernández M, Caba M, Meza E, Rodríguez-landa JF. Differential effects of acute and chronic treatment with the flavonoid chrysin on anxiety-like behavior and Fos immunoreactivity in the lateral septal nucleus in rats. Acta Pharm, 2020; 70(3):387–97. CrossRef
Girgis NF, Kamel S, Labib B, Hassab El, Naby SE. Cellular and DNA changes due to clonazepam abuse in brains of albino rats and role of clonidine during withdrawal period. MJFMCT, 2010; 18(1):25–49. CrossRef
Goddard AW, Mason GF, Appel M, Rothman DL, Gueorguieva R, Kevin L, Behar KL, Krystal JH. Impaired GABA neuronal response to acute administration in panic disorder. Am J Psychiatry, 2004; 161(12):2186–93. CrossRef
Goes ATR, Jesse CR, Antunes MS, Lobo Ladd FV, Lobo Ladd AAB, Luchese C, Boeira SP. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of parkinson’s disease, involvement of neuroinflammation and neurotrophins. Chem Biol Interact, 2018; 279:111–20.
Griffin CE, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J, 2013; 13(2):214–23. CrossRef
Guo B, Zheng C, Cai W, Cheng J, Wang H, Li H, Zhang Z. Multifunction of chrysin in parkinson’s model, anti-neuronal apoptosis, neuroprotection via activation of MEF2D, and inhibition of monoamine oxidase-B. J Agric Food Chem, 2016; 64(26):5324–33. CrossRef
He XL, Wang YH, Bi MG, Du GH. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur J Pharmacol, 2012; 680(1–3):41–8. CrossRef
Helmes E, Østbye T. Associations between benzodiazepine use and neuropsychological test scores in older adults. Can J Aging, 2015; 34(2):207–14. CrossRef
Horáková L. Flavonoids in prevention of diseases with respect to modulation of Ca pump function. Interdiscip Toxicol, 2011; 4(3):114–24. CrossRef
Huang TL, Hung YY. Lorazepam reduces the serum brain-derived neurotrophic factor level in schizophrenia patients with catatonia. Prog Neuro Psychopharmacol Biolo Psychiatry, 2009; 33(1):158–9. CrossRef
Huguenard JR, Prince DA. Clonazepam suppresses GABAB mediated inhibition in thalamic relay neurons through effects in nucleus reticularis. J Neurophysiol, 1994; 71(6):2576–81. CrossRef
Huopaniemi L, Keist R, Randolph A, Certa U, Rudolph U. Diazepam-induced adaptive plasticity revealed by α1 GABAA receptor-specific expression profiling. J Neurochem, 2004; 88(5):1059–67. CrossRef
Inoue K, Tanii H, Kaiya H, Okazaki Y. Possible therapeutic effect of clonazepam upon elderly depressive patients. Psychogeriatrics, 2007; 7(1):33–6. CrossRef
Izuta H, Shimazawa M, Tazawa S, Araki Y, Mishima S, Hara H. Protective effects of Chinese propolis and its component, chrysin, against neuronal cell death via inhibition of mitochondrial apoptosis pathway in SHSY5Ycells. J Agric Food Chem, 2008; 56(19):8944–53.
Jenner P, Pratt JA, Marsden CD. Mechanism of action of clonazepam in myoclonus in relation to effects on GABA and 5-HT. Adv Neurol, 1986; 143:629–43. CrossRef
Jensen TP, Filoteo AG, Knopfel T, Empsonm RM. Presynaptic plasma membrane Ca2+ ATPase isoform 2a regulates excitatory synaptic transmission in rat hippocampal CA3. J Physiol, 2007; 579:85–99. CrossRef
Jiang L, Bechtel MD, Nadezhda A, Galeva NA, Williams TD, Michaelis EK, Michaelis ML. Decreases in plasma membrane Ca2+-ATPase in brain synaptic membrane rafts from aged rats. J Neurochem, 2012; 123(5):689–99.
Jisham K, Ramprasad KL, Krishna KL, Ramesh B, Nidavani Mohan AJ, Kumar TM, Bhavana V. Assessment of memory impairment activity of clonazepam on mice. Int J Inst Pharm Life Sci, 2015; 5(2):197–210. CrossRef
Kalachnik JE, Hanzel TE, Sevenich R, Harder SR. Clonazepam behavioral side effects with an individual with mental retardation. J Autism Dev Disord, 2003; 33(3):349–54. CrossRef
Kandemir FM, Kucukler S, Eldutar E, Caglayan C, Gülçin I. Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy, a multi-biomarker approach. Sci Pharm, 2017; 85(1):4. CrossRef
Kishimoto A, Kamata K, Sugihara T, Ishiguro S, Hazama H, Mizukawa R, Kunimoto N. Treatment of depression with clonazepam. Acta Psychiatr Scand, 1988; 77(1):81–6. CrossRef
Krishnamoorthy A, Sevanan M, Mani S, Balu M, Balaji S, Ramajayan P. Chrysin restores MPTP induced neuroinflammation, oxidative stress and neurotrophic factors in an acute parkinson’s disease mouse model. Neurosci Lett, 2019; 709:134382. CrossRef
Kubová H, Bendová Z, Moravcová S, Pa?cesová D, Rocha L, Mareš P. Neonatal clonazepam administration induced long-lasting changes in GABAA and GABAB receptors. Int J Mol Sci, 2020; 21(9):3184. CrossRef
Kucukler S, Benzer F, Yildirim S, Gur C, Kandemir FM, Bengu AS, Dortbudak MB. Protective effects of chrysin against oxidative stress and inflammation induced by lead acetate in rat kidneys: a biochemical and histopathological approach. Biol Trace Elem Res, 2021; 199(4):1501–14. CrossRef
Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols, a role in cancer prevention. Arch Biochem Biophys, 2010; 501(1):65–72. CrossRef
Li X, Huang Q, Ong CN, Yang XF, Shen HM. Chrysin sensitizes tumor necrosis factor-alpha-induced apoptosisin human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett, 2010; 293(1):109–16. CrossRef
Li N, Liu JH, Zhang J, Yu BY. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J Agric Food Chem, 2008; 56(10):3876–83. CrossRef
Li T, Ma J, Han X, Jia Y, Yuan H, Shui S. Chrysin ameliorates cerebral ischemia/reperfusion (I/R) injury in rats by regulating the PI3K/Akt/mTOR pathway. Neurochem Int, 2019; 129:104496. CrossRef
Li R, Zang A, Zhang L, Zhang H, Zhao L, Qi Z, Wang H. Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol Sci, 2014; 35:1527–32. CrossRef
Licata SC, Shinday NM, Huizenga MN, Darnell SB, Sangrey GR, Rudolph U, James K, Rowlett JK, Sadri-Vakili G. Alterations in brain-derived neurotrophic factor in the mouse hippocampus following acute but not repeated benzodiazepine treatment. PLOS One, 2013; 8(12):e84806. CrossRef
Lodovici M, Casalini C, Briani C, Dolara P. Oxidative liver DNA damage in rats treated with pesticide mixtures. Toxicology, 1997; 117(1):55–60.
Longo LP, Johnson B. Addiction. Part I. benzodiazepines-side effects. Abuse risk and alternatives. Am Fam Physician, 2000; 61(7):2121–8. CrossRef
Lopez F, Miller LG, Greenblatt DJ, Chesley S, Schatzki A, Shader RI. Chronic administration of benzodiazepines. V. Rapid onset of behavioral and neurochemical alterations after alprazolam discontinuation. Neuropharmacology, 1990; 29(3):237–41. CrossRef
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science, 1986; 232(4753):1004–7. CrossRef
Mata AM, Berrocal M, Rosario Sep´ulveda M. Impairment of the activity of the plasma membrane Ca2+ -ATPase in Alzheimer’s disease. Biochem Soc Trans, 2011; 39(3):819–22.
Mehri S, Karami HV, Hassani FV, Hosseinzadeh H. Chrysin reduced acrylamide-induced neurotoxicity in both in vitro and in vivo assessments. Iran Biomed J, 2014; 18(2):101–6. CrossRef
Mikulecká A, Šubrt M, Pa?rízková M, Mareš P, Kubová H. Consequences of early postnatal benzodiazepines exposure in rats. II. Social behavior. Front Behav Neurosci, 2014; 8:169. CrossRef
Mishra A, Mishra PS, Bandopadhyay R, Khurana N, Angelopoulou E, Yam Nath Paudel YP, Piperi C. Neuroprotective potential of chrysin: mechanistic insights and therapeutic potential for neurological disorders. Molecules, 2021; 26(21):6456. CrossRef
Mohamed TM, Abdel Ghaffar HM, El Husseiny RMR. Effects of tramadol, clonazepam, and their combination on brain mitochondrial complexes. Toxicol Ind Health, 2015; 31(12):1325–33. CrossRef
Morishita S. Clonazepam as a therapeutic adjunct to improve the management of depression, a brief review. Human Psychopharmacol, 2009; 24(3):191–8. CrossRef
Ngo DH, Vo TS. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules, 2019; 24:2678. CrossRef
Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat, 2015; 11:165–75. CrossRef
Ontiveros M, Rinaldi D, Marder M, Espelt MV, Mangialavori I, Vigil M, Rossi JP, Ferreira-Gomes M. Natural flavonoids inhibit the plasma membrane Ca2+-ATPase. Biochem Pharmacol, 2019; 166:1–11. CrossRef
Özbolat SN, Ayna A. Chrysin suppresses HT-29 cell death induced by diclofenac through apoptosis and oxidative damage. Nutr Cancer, 2021; 73(8):1419–28. CrossRef
Pagel P, Blome J, Wolf HU. High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson’s disease. J Chromatogr B Biomed Sci Appl, 2000; 746(2):297–304. CrossRef
Pomara N, Lee SH, Bruno D, Silber T, Greenblatt DJ, Petkova E, Sidtis JJ. Adverse performance effects of acute lorazepam administration in elderly long-term users, pharmacokinetic and clinical predictors. Prog Neuro Psychopharmacol Biol Psychiatry, 2015; 56:129–35. CrossRef
Pushpavalli G, Kalaiarasi P, Veeramani C, Pugalendi KV. Effect of chrysin on hepatoprotective and antioxidant status in d-galactosamine-induced hepatitis in rats. Eur J Pharmacol, 2010; 631(1–3):636–41. CrossRef
Rashed LA, Al-Saeed HF, El-Shawwa MM. Neuroprotective effects of chrysin on adult male albino rats exposed to acrylamide and radiation. Al-Azhar Med J, 2016; 45(3):493–504. CrossRef
Rashno M, Sarkaki A, Farbood Y, Rashno M, Khorsandi L, Kazem M, Naseri G, Dianat M. Therapeutic effects of chrysin in a rat model of traumatic brain injury, a behavioral, biochemical, and histological study. Life Sci, 2019; 228:285–94. CrossRef
Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J, 2008; 22(3):659–61. CrossRef
Rodriguez-Landa JF, German-Ponciano LJ, Puga-Olguín A, Olmos-Vázquez OJ. Pharmacological, neurochemical, and behavioral mechanisms underlying the anxiolytic- and antidepressant-like effects of flavonoid chrysin. Molecules, 2022; 27(11):3551. CrossRef
Rodriguez-Landa JF, Guillen-Ruiz G, Hernandez-Lopez F, Cueto-Escobedo J, Rivadeneyra-Dominguez E, Bernal-Morales B, Herrera-Huerta EV. Chrysin reduces anxiety-like behavior through actions on GABAA receptors during metestrus-diestrus in the rat. Behav Brain Res, 2021; 397:112952. CrossRef
Rodriguez-landa JF, Hernandez-lopez F, Cueto-escobedo J, Herrera-huerta EV, Rivadeneyra-dominguez E, Bernal-morales B, Romero-Avendano E. Chrysin (5,7-dihydroxyflavone) exerts anxiolytic-like effects through GABAA receptors in a surgical menopause model in rats. Biomed Pharmacother, 2019; 109:2387–95.
Roghani M, Joghataie MT, Jalali MR, Baluchnejadmojarad T. Time course of changes in passive avoidance and Y – maze performance in male diabetic rats. Iran Biomed J, 2006; 10(2):99–104. CrossRef
Sarkaki A, Farbood Y, Mohammad S, Mansouri T, Badavi M, Khorsandi L, Dehcheshmeh MG, Shooshtari MK. Chrysin prevents cognitive and hippocampallong-term potentiation deficits and inflammation in rat with cerebral hypoperfusionand reperfusion injury. Life Sci, 2019; 226:202–9. CrossRef
Sathiavelu J, Senapathy GJ, Devaraj R, Namasivayam N. Hepatoprotective effect of chrysin on prooxidant-antioxidant status during ethanol-induced toxicity in female albino rats. J Pharm Pharmacol, 2009; 61(6):809–17. CrossRef
Seckin S, Alsancak E, Basaran-kucukgergin C, Uysal M. The effect of chronic diazepam administration on lipid peroxidation and Ca2+-atpase activity in rat liver. Acta Biol Hung, 2007; 58(4):441–3. CrossRef
Singh B, Henneberger C, Betances D, Arevalo MA, Rodríguez-Tébar A, Meier JC, Grantyn R. Altered balance of glutamatergic/GABAergic synaptic input and associated changes in dendrite morphology after BDNF expression in BDNF-deficient hippocampal neurons. J Neurosci, 2006; 26(27):7189–200. CrossRef
Slattery DA, Desrayaud S, Cryan JF. GABAB receptor antagonist mediated antidepressant-like behavior is serotonin-dependent. J Pharmacol Exp Therap, 2005; 312(1):290–6.
Soca?a K, Nieoczym D, Pieróg M, Wyska E, Szafarz M, Doboszewska U, Wla? P. Effect of tadalafil on seizure threshold and activity of antiepileptic drugs in three acute seizure tests in mice. Neurotox Res, 2018; 34(3):333–46. CrossRef
Souza LC, Antunes MS, Filho CB, Del Fabbro L, De Gomes MG, Goes ATR, Donato F, Prigol M, Boeira SP, Jesse CR. Flavonoid chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levelsin aged mouse brain. Pharmacol Biochem Behav, 2015; 134:22–30. CrossRef
Steentoft A, Linnet K. Blood concentrations of clonazepam and 7-amino clonazepam in forensic cases in Denmark for the period 2002–2007. Forensic Sci Int, 2009; 184(1–3):74–9. CrossRef
Storhaug LW, Enger A, Hjelmel K, Øiestad EL, Vindenes V. Prolonged excretion of 7-amino clonazepam in urine after repeated ingestion of clonazepam, a case report. Forensic Sci Int, 2012; 222(1–3):e33–5. CrossRef
Sundararajan M, Thomas PA, Teresa A, Anbukkarasi M, Geraldine P. Regulatory effect of chrysin on expression of lenticular calcium transporters, calpains, and apoptotic-cascade components in selenite-induced cataract. Mol Vis, 2016; 22:401–23.
Sussman N. Memory impairment as an under recognized medication side effect. Prim Psychiatry, 2006; 13(8):13–4.
Taslimi P, Kandemir FM, Demir Y, ?leritürk M, Temel Y, Caglayan C, Gulçin I. The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: pharmacological evaluation of some metabolic enzyme activities. J Biochem Mol Toxicol, 2019; 33:e22313. CrossRef
Temel Y, Kucukler S, Y?ld?r?m S, Caglayan C, Kandemir FM. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn Schmiedebergs Arch Pharmacol, 2020; 393:325–37. CrossRef
Tew WY, Tan CS, Yan CS, Loh HW, Wen X, Wei X, Yam MF. Evaluation of vasodilatory effect and antihypertensive effect of chrysin through in vitro and sub-chronic in vivo study. Biomed Pharmacother, 2023; 157:114020. CrossRef
Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KH. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol, 2000; 10(21):1319–28. CrossRef
Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA inter neurons in the cortical modulation of mid brain5-hydroxytryptamine neurones. Neuroscience, 2001; 106(4):783–92. CrossRef
Vaz SH, Cristóvão-Ferreira S, Ribeiro JA, Sebastião AM. Brain-derived neurotrophic factor inhibits GABA uptake by the rat hippocampal nerve terminals. Brain Res, 2008; 1219:19–25. CrossRef
Venters HD, Dantzer R, Kelley KW. A new concept in neurodegeneration, TNFalpha is a silencer of survival signals. Trends Neurosci, 2000; 23(4):175–80. CrossRef
Villar IC, Jimenoz R, Galisteo M, Garcio–Saura MF, Zarzuelo A, Luarte J. Effect of chronic chysin treatment in spontaneously hypertensive rats. Planta Med, 2002; 68(9):847–50. CrossRef
Vincenzi FF, Hinds TR, Raess BU. Calmodulin and the plasma calcium pump. Ann N Y Acad Sci, 1980; 356:232–44. CrossRef
Vinkers CH, Klanker M, Groenink L, Korte SM, Cook JM, Van Linn ML, Hopkins SC, Olivier B. Dissociating anxiolytic and sedative effects of GABAAergic drugs using temperature and locomotor responses to acute stress. Psychopharmacology (Berl.), 2009; 204(2):299–311. CrossRef
Wang H, Hui KM, Chen Y, Xu S, Wong JTF, Xue H. Structure-activity relationships of flavonoids, isolated from Scutellariabaicalensis, binding to benzodiazepine site of GABAA receptor complex. Planta Med, 2002; 68(12):1059–62. CrossRef
Wilms H, Claasen J, Röhl C, Sievers J, Deuschl G, Lucius R. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases, evidence from activated microglial cells in vitro. Neurobiol Dis, 2003; 14(3):417–24. CrossRef
Winkler D, Willet M, Wolf R. Clonazepam in the long-term treatment of patients with unipolar depression, bipolar and schizoaffective disorder. Eur Neuropsychopharmacol, 2003; 13(2):129–34. CrossRef
Xu M, Yang Y, Peng J, Zhang Y, Wu B, He B, Jia Y, Yan T. Effects of Alpinae Oxyphyllae fructus on microglial polarization in a LPS-induced BV2 cells model of neuroinflammation via TREM2. J Ethnopharmacol, 2023; 302:115914. CrossRef
Yang C, Hu YM, Zhou ZQ, Zhang GF, Yang JJ. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci, 2013; 118(1):3–8. CrossRef
Ye M, Yang TL, Qing P, Lei X, Qiu J, Liu GY. Changes of functional brain networks in major depressive disorder, a graph theoretical analysis of resting state fMRI. PLOS One, 2015; 10(9):e0133775. CrossRef