INTRODUCTION
Sepsis is a life-threatening emergency due to multiple organ dysfunction resulting from systemic host dysregulation in response to infection (Gyawali et al., 2019). The central mechanism of sepsis is acute inflammation involving multiple organs (Hotchkiss et al., 2017). Data from the World Health Organization (WHO) showed that the global incidence of sepsis was 189 per 100,000 people/year with a mortality rate of 26.7%, and the mortality of sepsis patients in intensive care units was 42% (WHO, 2020). Fifteen percent of sepsis patients experienced septic shock, with a mortality rate of more than 50% (Dugar et al., 2020).
Sepsis pathophysiology and pathogenesis come from the reaction of infection, and bacterial infection is still the main cause (Prescott and Angus, 2018). The occurrence of infection can also be influenced by environmental factors such as air, water, food, or changes in climate and seasons. In sepsis, excessive inflammation and oxidative stress cause degranulation and release of proteases, resulting in local and systemic endothelial damage. Complement released as an immune response is known to induce endothelial dysfunction (Chang, 2019; Jacobi, 2021). Endothelial dysfunction can be used as a predictor of patients’ mortality in sepsis, where endothelial dysfunction plays a role in the pathophysiology of septic shock and organ dysfunction (Boisramé-Helms et al., 2013). Current management of sepsis does not specifically target inflammation and oxidative stress. Therefore, effective adjuvant therapy is needed to reduce inflammation and oxidative stress (Lin et al., 2018).
The kecombrang plant (Etlingera elatior) is one of the plants widely found in Asia, especially in Indonesia, and has commonly been used as traditional medicine (Anzian et al., 2017). Kecombrang fruit contains bioactive alkaloids, flavonoids, tannins, and terpenoids (Juwita et al., 2018; Leorita et al., 2018). Many experiments have shown that E. elatior (EE) has extensive benefits, such as anti-inflammatory (Srey et al., 2014), antioxidant (Isyanti et al., 2019), antibacterial for gram-negative or gram-positive microbes (Sahidin et al., 2019), and also antihyperglycemic and antihyperuricemic effects (Dewi et al., 2016; Tundis et al., 2010). A study by Srey et al. (2014) showed that EE extract could act as an anti-inflammatory by suppressing nitric oxide production by up to 91% at concentrations up to 100 g/ml (Srey et al., 2014). At doses of 300–400 mg/kgBW, ethanol extract of kecombrang fruit has the potential to act as an immunomodulator (Wahyuni et al., 2018).
Sepsis is still a health problem with high mortality rates. Prompt and appropriate treatment can improve the prognosis in sepsis patients (Cecconi et al., 2018). An in vivo study is needed to assess the potential anti-inflammatory and antioxidant effects of the ethanol extract of EE fruit in the treatment of sepsis. The aim of this study was to identify and evaluate the potential of local plants, namely ethanol extract of kecombrang fruit in reducing IL-1ß and caspase-3 levels in experimental animal models.
MATERIAL AND METHODS
The use of experimental animals in this study has complied with the 3R principles according to the provisions of the National Center for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs) and has been ethically approved by the Health Research Ethics Committee of Dr. Moewardi General Hospital (serial number: 477/IV/HREC/2021). This study was conducted at the Laboratory of Food and Nutrition Study of PAU, Universitas Gadjah Mada, Yogyakarta, and the Biomedical Laboratory of Universitas Sebelas Maret, Surakarta.
Experimental animals
Adult male Mus musculus Balb/C strain 3–4 months old and weighed 20–30 g were used in this research. Male mice were obtained and kept in experimental animal care at PAU Universitas Gadjah Mada, Yogyakarta. Throughout the observation, all animals were kept at room temperature of 32°C with light controlled in 12-hour light:12-hour dark cycle, fed standard with BR 1 diet according to body weight, and given water ad libitum for 7 days.
Preparation of extract
EE fruits were obtained from Langkaplancar, Pangandaran, and Jawa Barat. Extraction is conducted by maceration methods. The chemicals used are 70% ethanol. The fruits were peeled, washed, and dried, then crushed with a blender. The first maceration process was conducted for 24 hours, followed by another 2 hours at room temperature. The sample was centrifuged at 4°C at a 4,200 rpm speed for 20 minutes. The filtrate obtained was put into an extraction flask and evaporated with a rotary evaporator at 50°C. The extract was then tested using high- performance liquid chromatography examination (Farida and Maruzy, 2016). According to an analysis of the levels of vanillic acid and ethanol extract, 1 gram of ethanol extract of E. elatior fruit contained 255 mg of vanillic acid).
Experimental design
The research laboratory with post-test only group design was used in this study. The number of Mus musculus mice in this study was calculated using the Federrer formula to get at least 5 mice per group for 4 groups. The mice were divided into four groups at random (n = 8 per group). Each group of mice received an intraperitoneal injection of 0.3 mg/kgBW lipopolysaccharide (LPS) to induce sepsis.
Control group: sepsis control mice received LPS alone.
EE-1 group: given ethanol extract of EE starting from 5 days before LPS induction.
EE-2 group: given ethanol extract of EE 5 days after LPS induction.
EE-3 group: given ethanol extract of EE simultaneously LPS induction.
Each experimental animal received a daily dose of 4.2 mg/20 g ethanol extract of EE. All treatments were administered orally every day until 7 days before the sacrifice. The IL-1β level was measured before the mice were killed and caspase-3 expression was examined after mice were sacrificed.
Measurement of IL-1β levels and caspase-3 expression
The venous blood sample of each mice was collected into a separated serum tube before the sacrificed day. The research protocol of IL-1β and Caspase-3 levels (Sigma Aldrich, USA) used the ELISA method and were conducted as a manufacturer’s instruction. Each plate contained 100μl of mice serum that has been diluted with serum diluent (1:50) and incubated at 37°C for 45 minutes. A conjugate solution and 100μl of ABTS peroxidase were also added to the plate. Each plate then received a 25 μl stop solution treatment. If the difference in optical density (OD) between the (+) and (−) is ≥0.300 at a wavelength of 405 nm, the result is positive.
Statistical analysis
Statistical analysis in this study was done using SPSS version 22.0 for Windows. A descriptive analysis was conducted to determine the characteristics of the research sample. The numerical data were tested for normality with Shapiro–Wilk and homogeneity of variance with Levene’s test. The ANOVA F-test was used to determine differences in each group of data with a normal and homogeneous distribution, followed by the post-hoc least significant difference test to determine the difference in mean between groups. Meanwhile, for data that is not normally distributed, the Kruskal–Wallis test is carried out, followed by the Mann–Whitney test.
 | Table 1. Effects of the ethanol extract of EE fruit on caspase-3 and IL-1ß levels in various treatment preparations. [Click here to view] |
RESULT
Effects of the ethanol extracts of Kecombrang fruit on caspase-3 levels in sepsis model
Table 1 shows the ANOVA test results for differences in caspase-3 levels in the EE-1, EE-2, EE-3, and control groups. According to Table 1, an ethanol extract of kecombrang fruit at 4.2 mg/20 g significantly (p = 0.001) reduced caspase-3 levels (Fig. 1), with the best results in the EE-1 group. Table 2 shows significant difference in caspase-3 levels in the EE-1, EE-2, and EE-3 groups, and partially in the control group (p < 0.05). Based on the description above, it is clear that the ethanol extract of kecombrang fruit is effective in reducing caspase-3, with the best results in the group (EE-1) treated with the extract 5 days before LPS.
Effects of the ethanol extract of Kecombrang fruit on IL-1ß levels in sepsis model
Table 1 shows the effect of the ethanol extract on the IL-1ß levels in the EE-1, EE-2, EE-3, and control groups. The ethanol extract significantly (p = 0001) reduced IL-1ß levels in all the groups (Fig. 2), with the best results in the EE-1 group. Table 2 shows significant differences in IL-1ß levels in the EE-1, EE-2, and EE-3 groups and partially in the control group (p < 0.05). Based on the result, it is clear that the ethanol extract of kecombrang fruit can reduce the IL-1ß levels and that it can do so more effectively when administered prior to LPS (EE-1).
DISCUSSION
Sepsis is a medical emergency induced by a systemic immune response to infection that can lead to septic shock, where bacterial infections are still the main cause of sepsis (Dugar et al., 2020; Gyawali et al., 2019; Hotchkiss et al., 2017; Prescott and Angus, 2018). A mortality rate above 50% is associated with sepsis and septic shock (Dugar et al., 2020). In sepsis, severe endothelial cell dysfunction may lead to multiorgan disorders’ progression (Ince et al., 2016). The release of proinflammatory cytokines such as TNF-a, IL-1, and Il-6 from macrophages may exacerbate systemic infection and endothelial dysfunction in sepsis (Aziz et al., 2012).
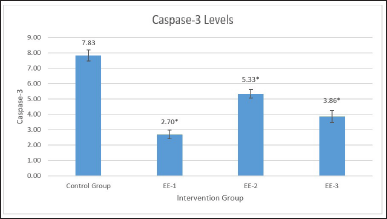 | Figure 1. Description of caspase-3 levels in each treatment preparation. EE extract decreased caspase-3 in experimental groups. Data represent the mean of three independent experiments and the best results in the group (EE-1) treated with the extract 5 days before LPS (EE-1 mean: 2.70, *p < 0.001). [Click here to view] |
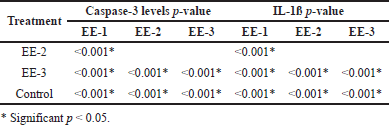 | Table 2. Post-hoc test of caspase-3 and IL-1ß levels in the EP1 group, the EP2 group, the EP3 group, and the control group. [Click here to view] |
In septic conditions, endothelial apoptosis is involved in endothelial injury and dysfunction. The Shioiri et al. (2009) research found that LPS in sepsis induces a significant increase in caspase-3 activity (Shioiri et al., 2009). The main executor of the apoptotic process is caspase-3. Nonsurvivor septic patients were recorded to have higher serum caspase-3 concentrations than survivors. Compared to healthy ones, septic patients have higher caspase-3 in their lymphocytes, spleen samples, and plasma (Lorente et al., 2018).
IL-1ß is known to cause vasodilation and hypotension and increase integrin synthesis, leading to the adherence of leukocytes and also stimulates the expression of intercellular adhesion molecules to promote neutrophil adhesion to the endothelial cells (Namas et al., 2012; Nasronudin, 2011). This phenomenon may trigger endothelial leakage and lead to septic shock. Thus, IL-1ß and caspase-3 can be the apparent markers of endothelial dysfunction in sepsis (Aziz et al., 2012; Luan et al., 2015; Namas et al., 2012).
In this study, the level of caspase-3 expression was reduced in subjects who received the ethanol extracts treatment at various times, with treatment group 1 (EE-1) having the lowest caspase-3 value. IL-1ß also significantly decreased compared to the baseline value (control group). The EE-1 had the lowest IL-1ß values. It shows that administering EE 5 days prior to mice-induced sepsis protects against endothelial dysfunction by inhibiting caspase-3 and IL-1ß expression. In sepsis, decreased caspase-3 expression and IL-1ß levels reduce the likelihood of endothelial dysfunction. This is consistent with the findings of Juwita et al. (2020), who found that a 1,000 mg/kgBW dose of EE extract reduced the expression of nuclear factor-B (NF-kB) in gastric ulcer model rats (Juwita et al., 2020).
Kecombrang fruit extract contains bioactive compounds such as vanillic acid that plays a role in fighting inflammation. The inflammatory process is believed to be inhibited through the suppression of cyclooxygenase-2 expression and the inhibition of transcriptional activation (NF-kB) and caspase-1 (Ernilasari et al., 2021; Hu et al., 2022). In addition, the alkaloids, phenolics, and flavonoids of kecombrang fruit extract can inhibit the growth of Staphylococcus aureus and Escherichia coli bacteria at a concentration of 2% through cell wall destruction (Ernilasari et al., 2021; Ghasemzadeh et al., 2015). Based on the results of the study, the potential of EE to inhibit bacterial growth is shown. Administration of EE before the induction of sepsis may have a protective effect on the endothelium by suppressing bacterial growth. Sepsis will affect various organs, and endothelial cells are the first to be exposed due to the inflammatory cascade by various cytokines (Dolmatova et al., 2021). It was reported that 48.4% of septic patients based on the Sepsis-3 criteria had bacteremia (Yu et al., 2022)
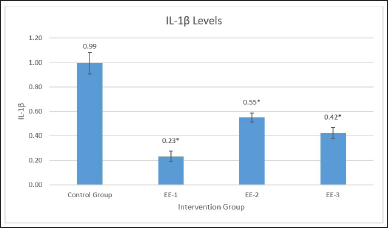 | Figure 2. Description of IL-1ß levels in each treatment preparation, EE extract decreased caspase-3 in experimental groups with the best results in the group (EE-1) treated with the extract 5 days before LPS (EE-1 mean: 0.23, *p < 0.001). [Click here to view] |
In sepsis, there is also an increase in the activity of the enzyme NADPH (nicotinamide adenine dinucleotide phosphate) oxidase, which synthesizes reactive oxygen species (ROS) in blood vessels (Heerebeek et al., 2002; Wu et al., 2007). Increased ROS triggers an increase in caspase-3 and causes apoptosis. Flavonoids can inhibit the action of the NADPH oxidase enzyme and capture ROS (Yousefian et al., 2019). Inhibition of the expression of NADPH oxidase, NF-kB, and IL-1β can reduce the apoptotic process which is characterized by a decrease in caspase-3 levels and prevent endothelial damage. Bacterial infection can trigger apoptosis in endothelial cells (Dolmatova et al., 2021; Ince et al., 2016). EE plant-containing flavonoids may have an anti-inflammatory effect and inhibit endothelial cells apoptosis which can be the target of therapeutic development of sepsis.
Further study awaits to determine the exact mechanism of EE suppressing the level of caspase-3 and IL-1β and also the effect of the daily routine of EE administration. To determine the effective dose, studying different doses of EE is necessary to prevent endothelial damage. This study will benefit the development and utilization of EE ethanolic extract and provide reliable evidence for its future clinical applications for sepsis treatment.
CONCLUSION
The intervention of kecombrang fruit ethanol extract on caspase-3 and IL-1β levels in various preparation treatments showed that kecombrang fruit ethanol extract (4.2 mg/20gr) can reduce caspase-3 and IL-1β, with the best results in the group given ethanol extract before LPS induction. This demonstrates that kecombrang extract given before induction can significantly reduce caspase-3 and IL-1β expression levels, demonstrating the potential benefits of kecombrang extract in averting endothelial dysfunction in sepsis. As a result, kecombrang extract may be utilized as an adjuvant therapy in treating sepsis.
ACKNOWLEDGMENT
The authors are grateful to the internal medicine specialist of Dr. Moewardi General Hospital for providing feedback and technical support.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study have been ethically approved by the Health Research Ethics Committee of Dr. Moewardi General Hospital (serial number: 477/IV/HREC/2021).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Anzian AB, Sukor R, Saari N, Sapawi CW, Hussin AS. Chemical composition and antioxidant activity of Torch Ginger (Etlingera elatior) flower extract. Food Appl Biosci J, 2017; 5:32–49.
Aziz M, Varghese AJ, Health N, Yang W, Matsuda A. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol, 2012; https://doi.org/10.1189/jlb.0912437.
Boisramé-Helms J, Kremer H, Schini-Kerth V, Meziani F. Endothelial dysfunction in sepsis. Curr Vasc Pharmacol, 2013; 11:150–60.
Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet, 2018; 392:75–87; https://doi.org/10.1016/S0140-6736(18)30696-2.
Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J, 2019; 17:1–19; https://doi.org/10.1186/s12959-019-0198-4.
Dewi AR, Nur’Aini I, Bahri IS, Afifah HN, Fattah A, Tunjung WAS. Antihyperuricemic activity of ginger flower (Etlingera elatior Jack.) extract in beef broth-induced hyperuricemic rats (Rattus norvegicus). AIP Conf Proc, 2016; 1755; https://doi.org/10.1063/1.4958573.
Dolmatova EV, Wang K, Mandavilli R, Griendling KK. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res, 2021; 117:60–73; https://doi.org/10.1093/cvr/cvaa070.
Dugar S, Choudhary C, Duggal A. Sepsis and septic shock: guideline-based management. Cleve Clin J Med, 2020; 87:53–64; https://doi.org/10.3949/ccjm.87a.18143.
Ernilasari, Walil K, Fitmawati, Roslim DI, Zumaidar, Saudah, Rayhannisa. Antibacterial activity of leaves, flowers, and fruits extract of Etlingera elatior from Nagan Raya district, Indonesia against Escherichia coli and Staphylococcus aureus. Biodiversitas, 2021; 22:4457–64; https://doi.org/10.13057/biodiv/d221039.
Farida S, Maruzy A. Kecombrang (Etlingera elatior): sebuah tinjauan penggunaan secara tradisional, fitokimia dan aktivitas farmakologinya. J Tumbuh Obat Indones, 2016; 9:19–28; https://doi.org/10.22435/toi.v9i1.6389.19-28.
Ghasemzadeh A, Jaafar HZE, Rahmat A, Ashkani S. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) R.M.Sm grown in different locations of Malaysia. BMC Complement Altern Med, 2015; 15:1–10; https://doi.org/10.1186/s12906-015-0838-6.
Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: the evolution in definition, pathophysiology, and management. SAGE Open Med, 2019; 7:205031211983504; https://doi.org/10.1177/2050312119835043.
Heerebeek L, Meischl C, Stooker W, Meijer CJLM, Niessen HWM, Roos D. NADPH oxidase(s): new source(s) of reactive oxygen species in the vascular system?? J Clin Pathol, 2002; 55(8):561–8.
Hotchkiss R, Moldawer LL, Opal SM, Reinhart K, Tumbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Prim, 2017; 2:1–46; https://doi.org/10.1038/nrdp.2016.45.Sepsis.
Hu R, Wu S, Li B, Tan J, Yan J, Wang Y, Tang Z, Liu M, Fu C, Zhang H, He J. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim Nutr, 2022; 8:144–52; https://doi.org/https://doi.org/10.1016/j.aninu.2021.06.009.
Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, Hernandez G, Murray P, De Backer D. The endothelium in sepsis. Shock, 2016; 45:259–70; https://doi.org/10.1097/SHK.0000000000000473
Isyanti M, Andarwulan N, Faridah DN. Karakteristik Fisik dan Fitokimia Buah Kecombrang (Etlingera elatior). J Agro-Based Ind, 2019; 36:96–105.
Jacobi J. The pathophysiology of sepsis-2021 update: Part 1, immunology and coagulopathy leading to endothelial injury. Am J Health Syst Pharm. 2022 Feb 18;79(5):329-337. doi: 10.1093/ajhp/zxab380. PMID: 34605875; PMCID: PMC8500113.
Juwita T, Pakpahan WHP, Puspitasari IM, Saptarini NM, Levita J. Anti-inflammatory activity of Etlingera elatior (Jack) r.m. smith flower on gastric ulceration-induced wistar rats. Pakistan J Biol Sci, 2020; 23:1193–200; https://doi.org/10.3923/pjbs.2020.1193.1200.
Juwita T, Puspitasari IM, Levita J. Torch ginger (Etlingera elatior): a review on its botanical aspects, phytoconstituents and pharmacological activities. Pak J Biol Sci, 2018; 21:151–65; https://doi.org/10.3923/PJBS.2018.151.165.
Leorita M, Mardikasari SA, Wahyuni, Malaka MH, Sartinah A, Sahidin. Aktivitas Antioksidan dan Toksisitas Akut Ekstrak Etanol Buah, Daun, Batang dan Rimpang Tanaman Wualae (Etlingera elatior (Jack) R.M. Smith). J Farm Sains Dan Kesehat, 2018; 4:36–9.
Lin J, Li H, Wen Y, Zhang M. Adjuvant administration of vitamin C improves mortality of patients with sepsis and septic shock: a systems review and meta-analysis. Open J Intern Med, 2018; 08:146–59; https://doi.org/10.4236/ojim.2018.82015.
Lorente L, Martín MM, Pérez-Cejas A, González-Rivero AF, López RO, Ferreres J, Solé-Violán J, Labarta L, Díaz C, Palmero S, Jiménez A. Sustained high serum caspase-3 concentrations and mortality in septic patients. Eur J Clin Microbiol Infect Dis, 2018; 37:281–8; https://doi.org/10.1007/s10096-017-3129-y.
Luan YY, Yao YM, Xiao XZ, Sheng ZY. Insights into the apoptotic death of immune cells in sepsis. J Interf Cytokine Res, 2015; 35:17–22; https://doi.org/10.1089/jir.2014.0069.
Namas R, Zamora R, Namas R, An G, Doyle J, Dick TE, Jacono FJ, Androulakis IP, Nieman GF, Chang S, Billiar TR, Kellum JA, Angus DC, Vodovotz Y. Sepsis: something old, something new, and a systems view. J Crit Care, 2012; 27:314. e1–314e.11; https://doi.org/10.1016/j.jcrc.2011.05.025.
Nasronudin. Penyakit infeksi di Indonesia Solusi Kini dan Mendatang. 2nd edition, Percetakan UNAIR, Surabaya, Indonesia, 2011.
Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA J Am Med Assoc, 2018; 319:62–75; https://doi.org/10.1001/jama.2017.17687.
Sahidin S, Salsabila S, Wahyuni W, Adryan F, Imran I. Potensi Antibakteri Ekstrak Metanol dan Senyawa Aromatik dari Buah Wualae (Etlingera elatior). J Kim Val, 2019; 5:1–7; https://doi.org/10.15408/jkv.v5i1.8658.
Shioiri T, Muroi M, Hatao F, Nishida M, Ogawa T, Mimura Y, Seto Y, Kaminishi M, Tanamoto KI. Caspase-3 is activated and rapidly released from human umbilical vein endothelial cells in response to lipopolysaccharide. Biochim Biophys Acta Mol Basis Dis, 2009; 1792:1011–8; https://doi.org/10.1016/j.bbadis.2009.06.006.
Srey C, Sontimuang C, Thengyai S, Ovatlarnporn C, Puttarak P. Anti α-glucosidase, anti α-amylase, anti-oxidation, and anti-inflammation activities of Etlingera elatior rhizome. J Chem Pharm Res, 2014; 6:885–91. Available via WwwJocprCom
Tundis R, Loizzo MR, Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini-Rev Med Chem, 2010; 10:315–31; https://doi.org/10.2174/138955710791331007.
Wahyuni W, Malik F, Ningsih A, Zubaydah WOS, Sahidin S. Antimicrobial activities of ethanol extract of Wualae (Etlingera elatior (JACK) R.M. Smith). J Pharm Med Sci, 2018; 3:14–8.
Wu F, Schuster DP, Tyml K, Wilson JX. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic Biol Med, 2007; 42:124–31; https://doi.org/10.1016/j.freeradbiomed.2006.10.033.
Yani Y, Tri Arianto A, Sudjito M. Pengaruh Pemberian Propofol Intravena terhadap Ekspresi Kaspase 3 Hipokampus pada Mencit Balb/C dengan Cedera Kepala. J Neuroanestesi Indones, 2013; 2:81–8; https://doi.org/10.24244/jni.vol2i2.160.
Yousefian M, Shakour N, Hosseinzadeh H, Hayes AW, Hadizadeh F, Karimi G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine, 2019; 55:200–13; https://doi.org/10.1016/j.phymed.2018.08.002.
Yu D, Unger D, Unge C, Parke Å, Sundén-Cullberg J, Strålin K, Özenci V. Correlation of clinical sepsis definitions with microbiological characteristics in patients admitted through a sepsis alert system; a prospective cohort study. Ann Clin Microbiol Antimicrob, 2022; 21:1–9; https://doi.org/10.1186/s12941-022-00498-3.