INTRODUCTION
Triterpenoids are oxygen derivatives of triterpenes, often having pentacyclic or tetracyclic structures. Characterized by their five isoprene units, pentacyclic triterpenoids are of the friedelane, lupane, oleanane, and ursane types (Furtado et al., 2017; Garg et al., 2020; Ghante and Jamkhande, 2019; Wo?niak et al., 2015). These compounds possess a broad spectrum of pharmacological properties including anti-cancer, antioxidant, antimicrobial, antiviral, antidiabetic, anti-inflammatory, anti-ulcerogenic, anti-obesity, anti-aging, analgesic, immunomodulatory, anti-hyperglycemia, anti-hypertensive, hypolipidemic, neuroprotective, hepatoprotective, and cardioprotective activities. The molecular mechanisms underlying the anti-cancer activities of triterpenoids range between cytotoxicity, inhibition of tumor cell proliferation, induction of apoptosis, change in signal transduction, and suppression of angiogenesis and metastasis (Ghante and Jamkhande, 2019; Shanmugam et al., 2012). These activities are well-documented through in vitro and in vivo models and chemically induced tumor xenograft models, and they include both naturally occurring triterpenoids and their synthetic derivatives (Patlolla and Rao, 2012). Of the different types of triterpenoids, the ursane-type pentacyclic triterpenoids are a useful source of anti-cancer drugs (Salvador et al., 2012). They include asiatic acid, boswellic acid, corosolic acid, pomolic acid (PA), and ursolic acid.
PA was chosen as the compound for review in this short article as there is none in the literature. Only some reviews on pentacyclic triterpenoids such as Salvador et al. (2012), Ghante and Jamkhande (2019), and Mioc et al. (2022) have included PA. Topics covered in this short review included chemistry, plant sources, synthesis, pharmacological properties, and patents. PA is reported in many plant species and is rich in bioactivities.
CHEMISTRY
PA or 3-β,19α-dihydroxy-urs-12-en-28-oic acid is a pentacyclic triterpenoid of the ursane type. PA is also known as benthamic acid (BA). It has a molecular formula of C30H48O4, and molecular weight of 473 g/mol. Its chemical structure has a 30-carbon skeleton comprising five six-membered rings A–E (Fig. 1).
PA has a carboxyl (–COOH) group at C17, and methyl (–CH3) groups are attached to C4, C8, C10, C14, C19, and C20. C4 has two –CH3 groups. There are four oxygen atoms, one at C3, two at C17, and one at C19. At C3, C17, and C19 are the hydroxyl (–OH) groups. Other triterpenoids of the ursane type include ursolic acid, asiatic acid, corosolic acid, and β-boswellic acid. Unlike PA which has –H at C2 and –OH group at C19, ursolic acid has –H at C2 and –H at C19 (Chan et al., 2019), and corosolic acid has –OH group at C2 and –H at C19 (Chan et al., 2022). The existence of –OH and –COOH groups in the PA molecule enables its intermolecular hydrogen bonding (Hou et al., 2022). The ability of PA to self-assemble via non-covalent interactions has been attributed to the presence of seven –CH3 groups.
PLANT SOURCES
PA was first isolated by Brieskorn and Wunderer (1967) from the peels of apples. Subsequently, it has been reported in 39 plant species from 19 families (Table 1). Families with more than one species are Rosaceae (10), Lamiaceae (7), Chrysobalanaceae (4), Aquifoliaceae (3), and Styracaceae (2). Species reported more than once are Diospyros kaki (Ebenaceae), Ocimum gratissimum (Lamiaceae), Osmanthus fragrans (Oleaceae), Weigela subsessilis (Caprifoliaceae), and Ziziphus jujuba (Rhamnaceae).
Although PA has been reported in many plant species, its content is very low. The content of PA in the roots and rhizomes of Potentilla species from Poland ranged from 0.09 mg/g in Potentilla reptans to 1.63 mg/g in Potentilla neumanniana (Jó?wiak et al., 2014). In China, the contents of PA in the fruits of different Chaenomeles species have been reported to range from 0.10% (Chaenomeles lagenaria) to 0.24% (Chaenomeles sinensis), 0.17% to 0.36% in C. sinensis from different prefectures, and 0.04% in the roots to 0.16% in the fruits of C. sinensis (Yang et al., 2009).
A practical approach to enhance the availability of PA is to synthesize PA from structurally similar compounds such as tormentic acid and euscaphic acid via regioselective acylation followed by Saito photochemical reduction (Kraft et al., 2019; Wiemann et al., 2016).
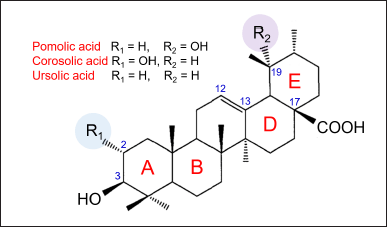 | Figure 1. Chemical structure of PA. [Click here to view] |
PHARMACOLOGICAL PROPERTIES
Anti-cancer properties
Against tumor cells, PA inhibited SK-MEL-28 melanoma, A549 lung, and U-373 MG glioblastoma cancer cells via the suppression of NF-κB and HIF-1α pathways (Apaza et al., 2021). PA inhibited HIF-1α in the SK-MEL-28 (IC50 = 3.0 μM), A549 (IC50 = 10 μM), and U-373MG (IC50 = 6.3 μM) cancer cells. In addition, PA inhibited NF-κB in SK-MEL-28 (IC50 = 1.0 μM), A549 (IC50 = 3.6 μM), and U-373 MG (IC50 = 2.5 μM) cancer cells.
PA was identified as a potent inhibitor of SUMO-specific protease 1 (SENP1) with an IC50 value of 5.1 μM (Wei et al., 2022). SENP1 is a member of the SENP family of proteins that can be used to detect bladder, colon, or prostate cancer in a biological sample (Uzoigwe et al., 2012). When combined with cisplatin, PA (IC50 = 3.7 μM) exhibited potent inhibitory activity compared to cisplatin alone (IC50 = 28 μM) against SK-OV-3 ovarian cancer cells.
Against MK-1 squamous, HeLa cervical, and B16F10 melanoma cancer cells, cytotoxicity of PA based on GI50 was 55, 59, and 29 μM (Yoshida et al., 2005). Against other cancer cells, PA exerted cytotoxic effects on A549 lung cancer cells with IC50 values of 5.6 μM (Lei et al., 2014) and 10 μM (Yang et al., 2022), and on leukemia cells (THP-1) with IC50 value of 3.2 μM (Nganteng et al., 2022).
The anti-cancer effects and molecular mechanisms of PA are listed in Table 2. They involved breast, lung, ovarian, and prostate cancer cells, including leukemia and glioma. Most susceptible are breast cancer and leukemia cells.
Against breast cancer cells, anti-cancer effects include induction of apoptosis and inhibition of cell proliferation, metastasis, angiogenesis, and invasion. Molecular mechanisms against breast cancer cells involve activation of AMP-activated protein kinase (AMPK), poly (ADP-ribose) polymerase (PARP), and caspases-3 and -9; suppression of CXC chemokine receptor type-4 (CXCR4), matrix metalloproteinase-9 (MMP-9), focal adhesion kinase (FAK), and extracellular signal-regulated kinase (ERK); blocking nuclear factor-κB (NF-κB)/ERK/ mammalian target of rapamycin (mTOR) signaling pathways; targeting p38-mitogen-activated protein kinase (MAPK) and mTOR signaling.
Against leukemia cells, anti-cancer effects include inhibition of cell growth, promotion of cell death, and induction of apoptosis. Molecular mechanisms against leukemia cells involve activation of the caspase pathway (caspases-3 and -9) and checking multidrug resistance (MDR) by over-expression of anti-apoptotic Bcl-2 proteins.
Other bioactivities
The other bioactivities of PA are anti-human immunodeficiency virus (HIV), anti-diabetic, anti-fibrosis, anti-human platelet aggregation (HPA), hypotensive, new multi-target cardiovascular agent (MCA), anti-osteoclastogenesis (OCG), anti-aging, anti-inflammatory, new natural product gel (NPG), apoptosis, anti-obesity, and anti-rheumatoid arthritis (RA) properties (Table 3).
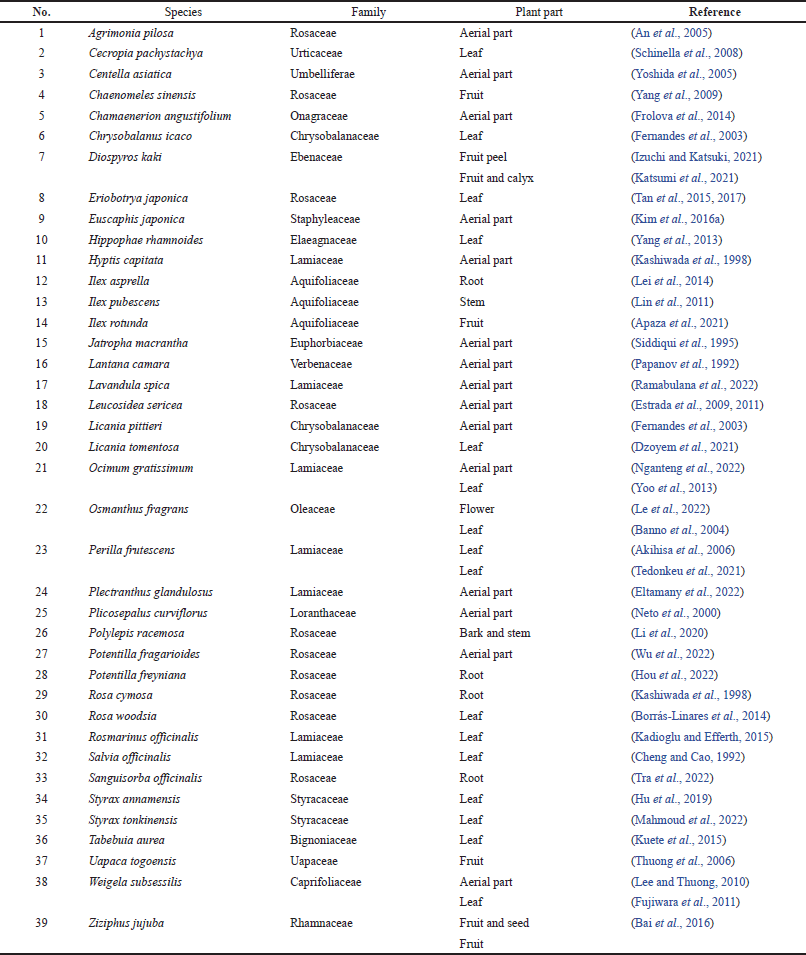 | Table 1. Plant sources of PA or BA. [Click here to view] |
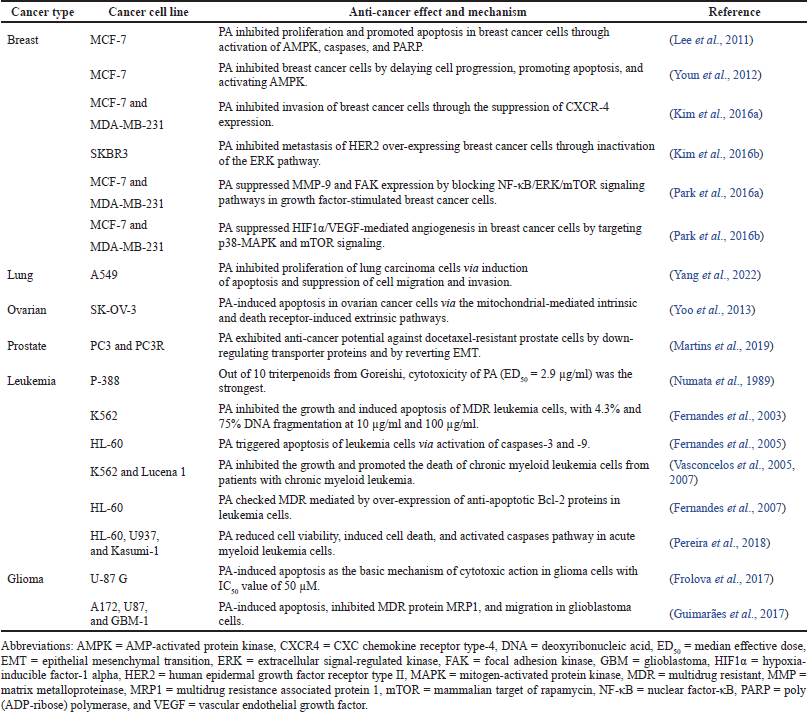 | Table 2. Anti-cancer effects and molecular mechanisms of PA. [Click here to view] |
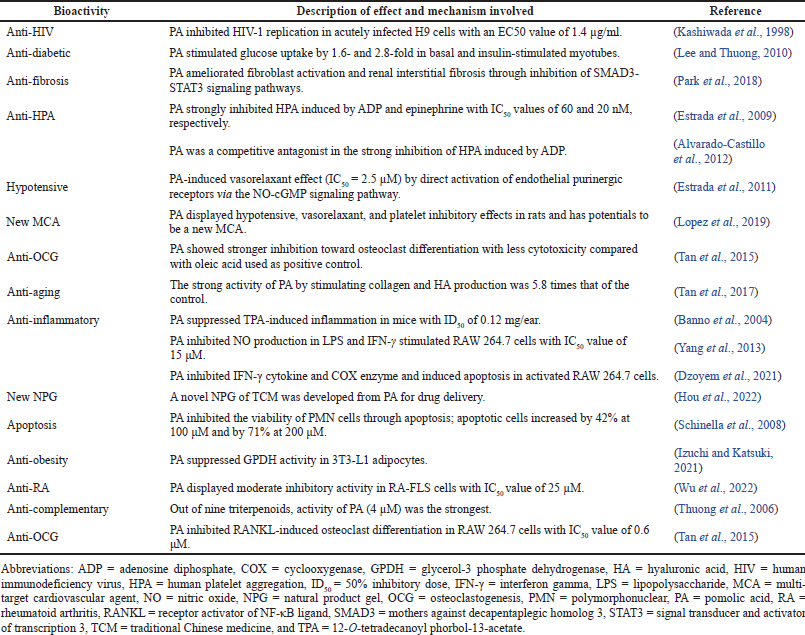 | Table 3. Other bioactivities of PA. [Click here to view] |
PATENTS
PA has two patents filed by the same group of scientists from the Federal University of Rio de Janeiro in Brazil (Gattass et al., 2004, 2008). The two patents are entitled, ‘PA, its isomers, derivatives and their uses, pharmaceutical composition, method to prepare the pharmaceutical composition, and method for treating MDR tumors’ and ‘PA for treating MDR tumors’. The former (WO 2004/030682 A1) was a World Intellectual Property Organization (WIPO) Patent published in April 2004, while the latter (EP 1 549 330 B1) was a European Patent (EP) published in January 2008. The WIPO invention was specifically related to the identification of PA, its isomers, and derivatives as anti-neoplastic drugs, to be used in the treatment of patients suffering from tumors intrinsically MDR or tumors that acquired this resistance as a result of chemotherapy treatment (Gattass et al., 2004). The EP invention was related to the use of PA for the preparation of medicaments for the treatment of cancer with MDR (Gattass et al., 2008).
CONCLUSION
PA is an ursane-type pentacyclic triterpenoid. It is commonly reported in species of the families Rosaceae and Lamiaceae. Anti-cancer activities are the major pharmacological properties of PA with breast cancer and leukemia cells being most susceptible. A wide array of other pharmacological properties of PA have been reported. In view of the very low content of PA in plant species, more research on the synthesis of PA from structurally similar compounds is recommended to enhance its availability. The correlation of the bioactivities of PA with its structural properties, i.e., structure-activity relationship studies, is worthy of more in-depth investigation. Analysis of the bioactivities and drug delivery of PA-gel holds great promise especially when its bioactivity is equal to or stronger than that of non-gel PA. Greater effort deserves to be accorded to strengthening the MDR potential of PA.
AUTHORS’ CONTRIBUTIONS
All the authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revised it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; agreed to be accountable for all aspects of the work.
FUNDING
The authors are grateful to the Malaysian Ministry of Higher Education for the financial support of this publication (Grant No. FRGS/1/2022/STG04/UCSI/02/2).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akihisa T, Kamo S, Uchiyama T, Akazawa H, Banno N, Taguchi Y, Yasukawa K. Cytotoxic activity of Perilla frutescens var. japonica leaf extract is due to high concentrations of oleanolic and ursolic acids. J Nat Med, 2006; 60(4):331−3.
Alvarado-Castillo C, Estrada O, Carvajal E. Pomolic acid, triterpenoid isolated from Licania pittieri, as competitive antagonist of ADP-induced aggregation of human platelets. Phytomedicine, 2012; 19(6):484−7.
An RB, Kim HC, Jeong GS, Oh SH, Oh HC, Kim YC. Constituents of the aerial parts of Agrimonia pilosa. Nat Prod Sci, 2005; 11(4):196−8.
Apaza T, Antognoni F, Potente G, Sánchez ÁR. Triterpenoids isolated from Jatropha macrantha (Müll. Arg.) inhibit the NF-κB and HIF-1α pathways in tumor cells. Nat Prod Res, 2021; 35(24):5843−7.
Bai L, Zhang H, Liu Q, Zhao Y, Cui X, Guo S, Zhang L, Ho CT, Bai N. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food Funct, 2016; 7(6):2870−7.
Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa JI, Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem, 2004; 68(1):85−90.
Borrás-Linares I, Stojanovi? Z, Quirantes-Piné R, Arráez-Román D, Švarc-Gaji? J, Fernández-Gutiérrez A, Segura-Carretero A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int J Mol Sci, 2014; 15(11):20585−606.
Brieskorn CH, Wunderer H. Chemical composition of apple peels. IV. Pomolic and pomonic acids. Chem Ber, 1967; 100:1252–65.
Chan EWC, Soon CY, Tan JBL, Wong SK, Hui YW. Ursolic acid: an overview on its cytotoxic activities against breast and colorectal cancer cells. J Integr Med, 2019; 17(3):155−60.
Chan EWC, Wong SK, Chan HT. An overview of the phenolic constituents and pharmacological properties of extracts and compounds from Lagerstroemia speciosa leaves. Trop J Nat Prod Res, 2022; 6(4):470−9.
Cheng DL, Cao XP. Pomolic acid derivatives from the root of Sanguisorba officinalis. Phytochemistry, 1992; 31(4):1317−20.
Dzoyem JP, Nganteng DN, Melong R, Wafo P, Ngadjui B, Allémann E, Delie F. Bioguided identification of pentacyclic triterpenoids as anti-inflammatory bioactive constituents of Ocimum gratissimum extract. J Ethnopharmacol, 2021; 268:113637.
Eltamany EE, Goda MS, Nafie MS, Abu-Elsaoud AM, Hareeri RH, Aldurdunji MM, Elhady SS, Badr JM, Eltahawy NA. Comparative assessment of the antioxidant and anticancer activities of Plicosepalus acacia and Plicosepalus curviflorus: metabolomic profiling and in silico studies. Antioxidants, 2022; 11(7):1249−71.
Estrada O, Alvarado-Castillo C, Fernandez AZ, López M, Romero-Vecchione E, Vásquez J, Mendez J, Conde D, Cardozo A. Pomolic acid isolated from the leaves of Licania pittieri inhibits ADP- and epinephrine-induced platelet aggregation and has hypotensive effect on rats. Curr Bioact Compd, 2009; 5(3):219−25.
Estrada O, González-Guzmán JM, Salazar-Bookaman M, Fernández AZ, Cardozo A, Alvarado-Castillo C. Pomolic acid of Licania pittieri elicits endothelium-dependent relaxation in rat aortic rings. Phytomedicine, 2011; 18(6):464−9.
Fernandes J, Castilho RO, Costa MR, Wagner-Souza K, Kaplan MA, Gattass CR. Pentacyclic triterpenes from Chrysobalanaceae species: cytotoxicity on multidrug resistant and sensitive leukemia cell lines. Cancer Lett, 2003; 190(2):165−9.
Fernandes J, Weinlich R, Castilho RO, Amarante-Mendes GP, Gattass CR. Pomolic acid may overcome multidrug resistance mediated by overexpression of anti-apoptotic Bcl-2 proteins. Cancer Lett, 2007; 245(1–2):315−20.
Fernandes J, Weinlich R, Castilho RO, Kaplan MA, Amarante-Mendes GP, Gattass CR. Pomolic acid triggers mitochondria-dependent apoptotic cell death in leukemia cell line. Cancer Lett, 2005; 219(1):49−55.
Frolova TS, Lipeeva AV, Baev DS, Tsepilov YA, Sinitsyna OI. Apoptosis as the basic mechanism of cytotoxic action of ursolic and pomolic acids in glioma cells. Mol Biol, 2017; 51(5):705−11.
Frolova TS, Sal’nikova OI, Dudareva TA, Kukina TP, Sinitsyna OI. Isolation of pomolic acid from Chamaenerion angustifolium and the evaluation of its potential genotoxicity in bacterial test systems. Russ J Bioorg Chem, 2014;40(1):82−8.
Fujiwara Y, Hayashida A, Tsurushima K, Nagai R, Yoshitomi M, Daiguji N, Sakashita N, Takeya M, Tsukamoto S, Ikeda T. Triterpenoids isolated from Zizyphus jujuba inhibit foam cell formation in macrophages. J Agric Food Chem, 2011; 59(9):4544−52.
Furtado NAJC, Pirson L, Edelberg H, Miranda LM, Loira-Pastoriza C, Preat V, Larondelle Y, André CM. Pentacyclic triterpene bioavailability: an overview of in vitro and in vivo studies. Molecules, 2017; 22(3):400−24.
Garg A, Sharma R, Dey P, Kundu A, Kim HS, Bhakta T, Kumar A. Analysis of triterpenes and triterpenoids. In: Silva AS, Nabavi SF, Saeedi M, Nabavi SM, (eds.). Recent advances in natural products analysis, Elsevier, Amsterdam, The Netherlands, pp 393−426, 2020.
Gattass CR, Rumjanek VMD, Fernandes J, Castilho RO, Kaplan MAC. Pomolic acid, its isomers, derivatives and their uses, pharmaceutical composition, method to prepare the pharmaceutical composition, and method for treating multidrug resistant tumors. World Intellectual Property Organization (WIPO) Patent WO 2004/030682 A1. 2004-04.
Gattass CR, Rumjanek VMD, Fernandes J, Castilho RO, Kaplan MAC. Pomolic acid for treating multidrug resistant tumors. European Patent EP 1 549 330 B1. 2008-01.
Ghante MH, Jamkhande PG. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: an overview on targets and underling mechanisms. J Pharmacopunct, 2019; 22(2):55−67.
Guimarães LP, Rocha GD, Queiroz RM, Martins CA, Takiya CM, Gattass CR. Pomolic acid induces apoptosis and inhibits multidrug resistance protein MRP1 and migration in glioblastoma cells. Oncol Rep, 2017; 38(4):2525−34.
Hou Y, Chen M, Ruan H, Sun Z, Wu H, Xu X, Yang J, Ma G, Zhou X. A new supramolecular natural product gel based on self-assembled pomolic acid from traditional Chinese medicine. Colloid Interface Sci Commun, 2022; 46:100583.
Hu WL, Li ZL, Chen QJ, Sun YW, Zhai S, Lu F, Li F, Zhang CF. Triterpenes and lignans from the leaves of Styrax tonkinensis. Biochem Syst Ecol, 2019; 86:103891.
Izuchi R, Katsuki T. Pomolic acid in persimmon peel suppresses the increase in glycerol-3 phosphate dehydrogenase activity in 3T3-L1 adipocytes. Biosci Biotechnol Biochem, 2021; 85(3):691−6.
Jó?wiak A, Jó?wiak G, Waksmundzka-Hajnos M. Simultaneous HPLC determination of pomolic, ursolic and euscaphic/tormentic acids in roots and rhizomes of various Potentilla species. Acta Chromatogr, 2014; 26(1):97−110.
Kadioglu O, Efferth T. Pharmacogenomic characterization of cytotoxic compounds from Salvia officinalis in cancer cells. J Nat Prod, 2015; 78(4):762−75.
Kashiwada Y, Wang HK, Nagao T, Kitanaka S, Yasuda I, Fujioka T, Yamagishi T, Cosentino LM, Kozuka M, Okabe H, Ikeshiro Y. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J Nat Prod, 1998; 61(9):1090−5.
Katsumi E, Oshima N, Kagawa N, Ohara H, Hada N. Changes in the extracted amounts and seasonally variable constituents of Diospyros kaki at different growth stages. J Nat Med, 2021; 75(1):105−15.
Kim B, Kim JH, Park B. Pomolic acid inhibits invasion of breast cancer cells through the suppression of CXC chemokine receptor type 4 expression. J Cell Biochem, 2016a; 117(6):1296−307.
Kim B, Kim YC, Park B. Pomolic acid inhibits metastasis of HER2 overexpressing breast cancer cells through inactivation of the ERK pathway. Int J Oncol, 2016b; 49(2):744−52.
Kraft O, Wiemann J, Al-Harrasi A, Csuk R. En route to anti-glioblastoma active pomolic acid. Phytochem Lett, 2019; 32:29−32.
Kuete V, Sandjo LP, Seukep JA, Zeino M, Mbaveng AT, Ngadjui B, Efferth T. Cytotoxic compounds from the fruits of Uapaca togoensis towards multifactorial drug-resistant cancer cells. Planta Med, 2015; 81(1):32−8.
Le DD, Lee YE, Lee M. Triterpenoids from the leaves of Osmanthus fragrans var. aurantiacus with their anti-melanogenesis and anti-tyrosinase activities. Nat Prod Res, 2022; 1−7; DOI: 10.1080/14786419.2022.2035384
Lee JS, Lee MS, Kim JR, Chang ES. Pomolic acid enhances the growth inhibition and apoptosis in human breast cancer cell. Breast, 2011; 20(1):S26.
Lee MS, Thuong PT. Stimulation of glucose uptake by triterpenoids from Weigela subsessilis. Phytother Res, 2010; 24(1):49−53.
Lei Y, Shi SP, Song YL, Bi D, Tu PF. Triterpene saponins from the roots of Ilex asprella. Chem Biodiver, 2014; 11(5):767−75.
Li Y, Li K, Yao H. Chemical constituents from Potentilla fragarioides L. Biochem Syst Ecol, 2020; 93:104172.
Lin LP, Wei QU, Liang JY. Chemical constituents from the stems of Ilex pubescens var. glabra. Chin J Nat Med, 2011; 9(3):176−9.
Lopez R, Bolanos P, Guillen A, Fernandez MC, Ramos M, Granados S, Milan AF, Caputo C, Alvarado-Castillo C, Estrada O, Calderon JC. Pomolic acid reduces contractility and modulates excitation-contraction coupling in rat cardiomyocytes. Eur J Pharmacol, 2019; 851:88−98.
Mahmoud AH, Mahmoud BK, Samy MN, Fouad MA, Kamel MS, Matsunami K. Cytotoxic and anti-leishmanial triterpenes of Tabebuia aurea (Silva Manso) leaves. Nat Prod Res, 2022; 1−5; doi:10.1080/14786419.2022.2062350
Martins CD, Rocha GD, Gattass CR, Takiya CM. Pomolic acid exhibits anticancer potential against a docetaxel?resistant PC3 prostate cell line. Oncol Rep, 2019; 42(1):328−38.
Mioc M, Prodea A, Racoviceanu R, Mioc A, Ghiulai R, Milan A, Voicu M, Mardale G, ?oica C. Recent advances regarding the molecular mechanisms of triterpenic acids: a review (Part II). Int J Mol Sci, 2022; 23(16):8896−944.
Nakatani M, Miyazaki Y, Iwashita T, Naoki H, Hase T. Triterpenes from Ilex rotunda fruits. Phytochemistry, 1989; 28(5):1479−82.
Neto CC, Vaisberg AJ, Zhou BN, Kingston DG, Hammond GB. Cytotoxic triterpene acids from the Peruvian medicinal plant Polylepis racemosa. Planta Med, 2000; 66(5):483−4.
Nganteng DN, Melong R, Mbiekop EP, Maffo T, Allémann É, Delie F, Wafo P, Tchaleu BN, Dzoyem JP. Chemical constituents and cytotoxic activity of Ocimum gratissimum L. S Afr J Bot, 2022; 150:330−3.
Numata A, Yang P, Takahashi C, Fujiki R, Nabae M, Fujita E. Cytotoxic triterpenes from a Chinese medicine, Goreishi. Chem Pharm Bull, 1989; 37(3):648−51.
Papanov G, Bozov P, Malakov P. Triterpenoids from Lavandula spica. Phytochemistry, 1992; 31(4):1424−6.
Park JH, Cho YY, Yoon SW, Park B. Suppression of MMP-9 and FAK expression by pomolic acid via blocking of NF-κB/ERK/mTOR signaling pathways in growth factor-stimulated human breast cancer cells. Int J Oncol, 2016a; 49(3):1230−40.
Park JH, Jang KM, An HJ, Kim JY, Gwon MG, Gu H, Park B, Park KK. Pomolic acid ameliorates fibroblast activation and renal interstitial fibrosis through inhibition of SMAD-STAT signaling pathways. Molecules, 2018; 23(9):2236−47.
Park JH, Yoon J, Park B. Pomolic acid suppresses HIF1α/VEGF-mediated angiogenesis by targeting p38-MAPK and mTOR signaling cascades. Phytomedicine, 2016b; 23(14):1716−26.
Patlolla JMR, Rao CV. Triterpenoids for cancer prevention and treatment: current status and future prospects. Curr Pharm Biotechnol, 2012; 13(1):147−55.
Pereira MX, Hammes AS, Vasconcelos FC, Pozzo AR, Pereira TH, Caffarena ER, Gattass CR, Maia RC. Antitumor effect of pomolic acid in acute myeloid leukemia cells involves cell death, decreased cell growth and topoisomerases inhibition. Anti Cancer Agents Med Chem, 2018; 18(10):1457−68.
Ramabulana T, Ndlovu M, Mosa RA, Sonopo MS, Selepe MA. Phytochemical profiling and isolation of bioactive compounds from Leucosidea sericea (Rosaceae). ACS Omega, 2022; 7(14):11964−72.
Salvador JA, Moreira VM, Gonçalves BM, Leal AS, Jing Y. Ursane-type pentacyclic triterpenoids as useful platforms to discover anticancer drugs. Nat Prod Rep, 2012; 29(12):1463−79.
Schinella G, Aquila S, Dade M, Giner R, Carmen Recio M, Spegazzini E, Buschiazzo P, Tournier H, Ríos JL. Anti-inflammatory and apoptotic activities of pomolic acid isolated from Cecropia pachystachya. Planta Med, 2008; 74(3):215−20.
Shanmugam MK, Nguyen AH, Kumar AP, Tan BK, Sethi G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: potential role in prevention and therapy of cancer. Cancer Lett, 2012; 320(2):158−70.
Siddiqui BS, Raza SM, Begum S, Siddiqui S, Firdous S. Pentacyclic triterpenoids from Lantana camara. Phytochemistry, 1995; 38(3):681−5.
Tan H, Ashour A, Katakura Y, Shimizu K. A structure–activity relationship study on anti-osteoclastogenesis effect of triterpenoids from the leaves of loquat (Eriobotrya japonica). Phytomedicine, 2015; 22(4):498−503.
Tan H, Sonam T, Shimizu K. The potential of triterpenoids from loquat leaves (Eriobotrya japonica) for prevention and treatment of skin disorder. Int J Mol Sci, 2017; 18(5):1030−42.
Tedonkeu AT, Tamokou JD, Mpetga JD, Nzogong RT, Kengne IC, Hao XJ, Tene M. A new antimicrobial nor-friedelane-type triterpenoid and other constituents from Plectranthus glandulosus Hook. (Lamiaceae). Nat Prod Res, 2021; DOI: 10.1080/14786 419.2021.1999946.
Thuong PT, Min BS, Jin W, Na M, Lee J, Seong R, Lee YM, Song K, Seong Y, Lee HK, Bae K. Anti-complementary activity of ursane-type triterpenoids from Weigela subsessilis. Biol Pharm Bull, 2006; 29(4):830−3.
Tra NT, Son NT, Van Tuyen N, Cuong PV, Thu Ha NT, Anh LT, Huong DT, Ngan TB, Litaudon M. A new cytotoxic compound from the leaves of Styrax annamensis Guillaumin. Nat Prod Res, 2022; 36(6):1616−20.
Uzoigwe J, R Sauter E. SENP1 as a biomarker for the diagnosis of cancer: review of the science and published patents. Recent Pat Biomark, 2012; 2(1):29−35.
Vasconcelos FC, Gattas CR, Fernandes J, Rumjanek VM, Maia RC. Pomolic acid-induced apoptosis in cells from chronic myeloid leukemic patients is not affected by the MDR phenotype and status of disease. Blood, 2005; 106(11):4889−91.
Vasconcelos FC, Gattass CR, Rumjanek VM, Maia RC. Pomolic acid-induced apoptosis in cells from patients with chronic myeloid leukemia exhibiting different drug resistance profile. Invest New Drugs, 2007; 25(6):525−33.
Wei H, Guo J, Sun X, Gou W, Ning H, Shang H, Liu Q, Hou W, Li Y. Discovery of natural ursane-type SENP1 inhibitors and the platinum resistance reversal activity against human ovarian cancer cells: a structure–activity relationship study. J Nat Prod, 2022; 85(5):1248−55.
Wiemann J, Deckelmann AM, Csuk R. A remarkably simple and convergent partial synthesis of pomolic acid. Tetrahedron Lett, 2016; 57(35):3952−3.
Wo?niak ?, Sk?pska S, Marsza?ek K. Ursolic acid — a pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules, 2015; 20(11):20614−41.
Wu J, Zhang ZQ, Zhou XD, Yao QY, Chen ZL, Chu LL, Yu HH, Yang YP, Li B, Wang W. New terpenoids from Potentilla freyniana Bornm. and their cytotoxic activities. Molecules, 2022; 27(12):3665−77.
Yang G, Fen W, Xiao W, Sun H. Study on determination of pentacyclic triterpenoids in Chaenomeles by HPLC-ELSD. J Chromatogr Sci, 2009; 47(8):718−22.
Yang L, Guo Y, Hao Q, Li D, Qiao Z, Cui Y, Li J. Pomolic acid inhibits proliferation of human lung carcinoma cells via induction of apoptosis and suppression of cell migration and invasion. Trop J Pharm Res, 2022; 21(6):1201−7.
Yang ZG, Wen XF, Li YH, Matsuzaki K, Kitanaka S. Inhibitory effects of the constituents of Hippophae rhamnoides on 3T3-L1 cell differentiation and nitric oxide production in RAW 264.7 cells. Chem Pharm Bull, 2013; 61(3):279−85.
Yoo KH, Park JH, Lee DK, Fu YY, Baek NI, Chung IS. Pomolic acid induces apoptosis in SKOV-3 human ovarian adenocarcinoma cells through the mitochondrial-mediated intrinsic and death receptor-induced extrinsic pathways. Oncol Lett, 2013; 5(1):386−90.
Yoshida M, Fuchigami M, Nagao T, Okabe H, Matsunaga K, Takata J, Karube Y, Tsuchihashi R, Kinjo J, Mihashi K, Fujioka T. Antiproliferative constituents from Umbelliferae plants VII. Active triterpenes and rosmarinic acid from Centella asiatica. Biol Pharm Bull, 2005; 28(1):173−5.
Youn SH, Lee JS, Lee MS, Cha EY, Thuong PT, Kim JR, Chang ES. Anticancer properties of pomolic acid-induced AMP-activated protein kinase activation in MCF7 human breast cancer cells. Biol Pharm Bull, 2012; 35(1):105−10.