INTRODUCTION
Pharmaceuticals tend to deteriorate to degrade with time. The degradation process is a decrease in distinct characteristics over time. A drug’s shelf life is estimated by analyzing two independent properties after it has been stressed to degrade. First, determine how much active ingredient is lost. Second, identify the degradation products that result from the aging process. Degradants need to be predicted and assessed at concentrations greater than 0.1% of the parent molecule in this test, which can be challenging. Trace analysis is required for this part of the assay, which demands the establishment of a stability-indicating assay method (SIAM) for the drug and its dosage forms. Physical, chemical, microbiological, and environmental degradation determine the quality and purity of a drug product. Over time, an active pharmaceutical ingredient (API) or excipients may transit from a metastable state to a more thermodynamically stable state. A drug molecule can undergo chemical degradation due to several reactions such as oxidation, isomerization, hydrolysis, racemization, elimination, and UV degradation. In addition, there may be interactions with excipients and other pharmaceuticals (Connors et al., 1986). When water interacts with parental formulations, it leads to hydrolytic breakdown (Waterman et al., 2002). The most frequent cause of pharmaceutical degradation is oxidative degradation, which can take one of three paths: i) peroxide-accelerated, which involves exposing the drug to dilute peroxide solutions; ii) photochemically stimulated; and iii) radically simulated, which involves reversal loss of electrons (Bajaj et al., 2012; Cosa, 2004; Deidda et al., 2018; Quadri et al., 2014; Sahu et al., 2018).
Cefepime (CFP) was developed in 1994 as a fourth-generation cephalosporin. Microorganisms such as Pseudomonas aeruginosa, Enterobacteriaceae, Staphylococcus aureus, and multidrug-resistant Streptococcus pneumoniae are susceptible to CFP. The chemical name of CFP (Fig. 1) is (6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-[(1-methylpyrrolidinium-1-yl) methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate. It is a semi-synthetic drug having an aminothiazolyl group and a Z-methoxyimine moiety at C-7, expanding its spectrum and enhancing its β-lactamase stability. At the C-3 position, the presence of the quaternary N-methylpyrrolidine group aids in Gram-negative bacteria penetration. It works by preventing cell wall formation by inhibiting protein synthesis. It is used to treat urinary tract infections, septicemia, staphylococcal infections, bronchitis, intra-abdominal infections, skin and its structures infections, pneumonia infections, and in febrile neutropenic patients (Glish and Burinsky, 2008; Lemke et al., 2002). CFP stability was estimated using several methods using chromatography (Abd El Aziz Shama et al., 2021; Al Kamaly, 2022; Bjergum et al., 2021; De Borba et al., 2008; Dos Anjos et al., 2022; El-Beltagy et al., 2019; El-Dars et al., 2019; Fage et al., 2021; Isla et al., 2005; Jagadeesh Kumar et al., 2010; Jiang et al., 2010; Jiménez Palacios et al., 2005; Kalyani et al., 2018; Kommana et al., 2014; Liu et al., 2018; Mameli et al., 2019; Moorthy et al., 2020; Nemutlu et al., 2009, Ocaña González et al., 2004; Ohmori et al., 2011; Patil et al., 2018; Rehm and Rentsch, 2020; Rodrigues et al., 2016; Seraisso et al., 2022; Shrestha et al., 2014; Siddiqui et al., 2010; Sun et al., 2022; Sundara Raj et al., 2013; Sunitha et al., 2013; Van Vooren and Verstraete, 2021; Zander et al., 2015) but these methods had some disadvantages as complex mobile phase composition, increased matrix effect, decreased sensitivity, and inability to identify degradants using trace analysis in addition to fragmentation (Niessen, 2010; Niessen and Ricardo, 2017), less environmentally friendly, and time-consuming. However, there have been no previous analytical procedures of trace analysis reported on CFP stability studies and its degradants using ultra-performance liquid chromatography-mass spectrometry (UPLC-MS)/MS (Jabeen and Suma, 2022). The aim was to develop an eco-friendly, selective, sensitive, and accurate method for the analysis of the SIAM for CFP by using UPLC-ESI-QQQ-MS.
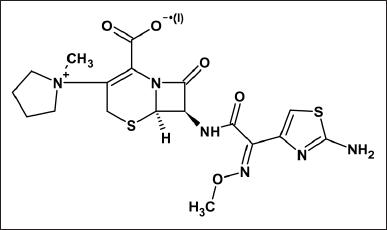 | Figure 1. Structure of Cefepime. [Click here to view] |
MATERIALS AND METHODS
Chemicals and reagents
CFP standard (99.8%) was acquired as samples from Kshetra Analyticals. The liquid chromatography grade water, formic acid, dimethyl sulfoxide (DMSO), acetonitrile (ACN), methanol, and all other reagents were procured from SD fine chemicals limited (Hyderabad, India).
Instrumentation and chromatographic conditions
The chromatographic separations were performed on a UPLC system (Acquity C18 BEH, 50 × 2.1 mm, 1.7 m) with MS/MS (UPLC-MS/MS—All Waters, USA). Waters Quattro premier mass spectrophotometer was utilized in tandem MS/MS using a triple quadrupole mass analyzer with an electrospray ionization (+ESI) probe. The system data were collected using the masslynx version 4.1 software. The isocratic elution with a mobile phase composed of 20:80 v/v—0.2% formic acid in water and ACN was used. A flow rate of 0.15 ml/minute, with an injection volume of 5 μl, and a run time of 3 minutes were maintained. The column temperature was maintained at 30°C.
Preparations of standard stock solution
Standard stock solutions
For CFP, a 1,000 μg/ml standard stock solution was prepared using DMSO solvent (Stock Solution A). All of the dilutions of standard stock solution A were performed with 50% methanol (diluent solvent).
Standard working solution
To obtain stock solution B of 200 μg/ml, 2 ml of CFP stock solution A was diluted to 10 ml using a diluent (50% methanol) solvent. To make stock solution C, 1 ml of stock solution B was diluted to 10 ml with a diluent solvent to yield a concentration of 20 μg/ml. The entire standard solution was properly sonicated and filtered using 0.45 μm membrane filters before the analysis. Aluminum foil was used to protect stock solution C from light and it was kept in the refrigerator. For stability testing, the normal stock solution C was employed.
Preparation of the mobile phase
A mixture of 0.2% formic acid in water and ACN 20:80 v/v was used as the mobile phase.
Validation procedure
According to the International Conference on Harmonization of Technical Requirement for Registration of Pharmaceuticals for Human Use (ICH) guidelines, the proposed UPLC-MS/MS methodology for CFP was validated (ICH, 2003).
Forced degradation studies of CFP
Standard or control
About 0.5 ml of 20 μg/ml CFP solution was diluted with 4.5 ml of diluent to make a standard working solution (2,000 ng/ml), which was then injected into the UPLC-MS/MS.
Stress conditions
Forced degradation studies were studied per ICH Q1 guidelines using different stress conditions such as acidic, basic, neutral, peroxide, and thermal degradation. The solutions were analyzed regularly at 6, 12, and 24 hours for a week using UPLC-MS/MS. Hydrolysis of CFP in the presence of water was checked because it is reconstituted using water for injection for intravenous or intramuscular purposes. Degradation of the drug under stress conditions is confirmed by the reduction in area in the chromatogram and drug quantity.
RESULTS AND DISCUSSION
Method development and validation
Using ESI in positive ion mode MS/MS detector optimum settings was investigated to acquire transition for each molecule. Full scan spectra were recorded to find the most prevalent m/z value to optimize the cone voltage with the precursor ions as [M+H]+ ions. The most frequent ions were found to have the most sensitive transition using collision energies. MS/MS transition for quantification and confirmation for CFP was observed at 481.3 → 166.85. Typical optimized operating conditions were at capillary voltage: 3.00 and 2.94 kV, cone voltage: 25 and 28 V, desolvation temperature: 400°C and 388°C, source temperature: 120°C, desolvation gas flow: 850 and 848 l/hour, cone gas flow: 100 and 97 l/hour, ion energy: 10.5, multiplier: 550–547 V, syringe pump flow: 100 μl/minutes, and collision gas flow: 0.15 and 0.15 ml/minutes. The chromatogram of standard CFP (for n = 6) was found to have a retention time of 0.82 ± 0.02 minutes. The MS/MS spectrum of [M+H]+ of CFP is at m/z of 481.35 with a peak area of 57,736.916 (Fig. 2). The results for CFP validation are summarized in Table 1.
Forced degradation studies
CFP has degraded in all the stress conditions. A typical chromatogram of CFP and its separated degradants is shown in Figure 3. In acidic, neutral, oxidation, and thermal stress conditions, the presence of m/z 481.42 ± 0.07 [M+H]+ peak indicates partial degradation of CFP into CFP degradant 1 (CD1) and CFP degradant 2 (CD2). The [M+H]+ peak at m/z 179.15 and 241.33 ± 0.03 indicate the presence of CD1 and CD2 respectively. In the basic medium, the absence of m/z 481.42 ± 0.07 [M+H]+ peak indicates complete degradation of CFP, and the presence of [M+H]+ peak at m/z 179.15 indicates that CFP has completely degraded into CD1. The MS/MS spectra of CD1 and CD2 are given in Figures 4 and 5 respectively. A summary of the SIAM UPLC-MS/MS method for CFP has been given below in Table 2.
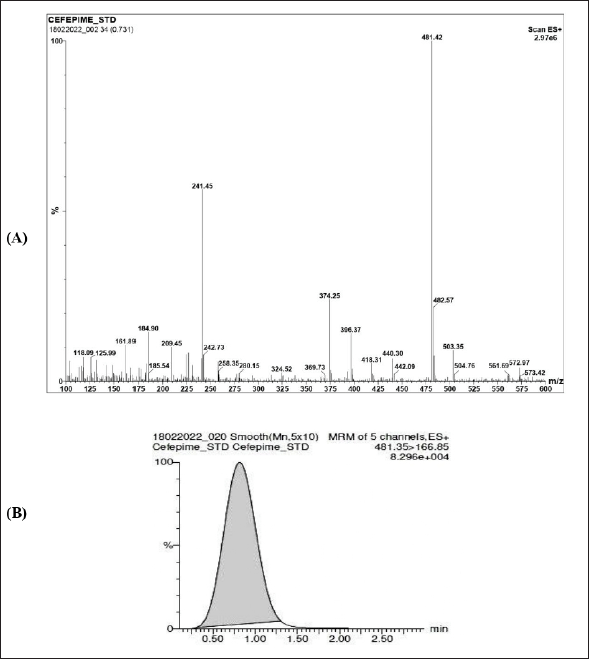 | Figure 2. Cefepime (A) MS/MS spectra. (B) Standard chromatogram. [Click here to view] |
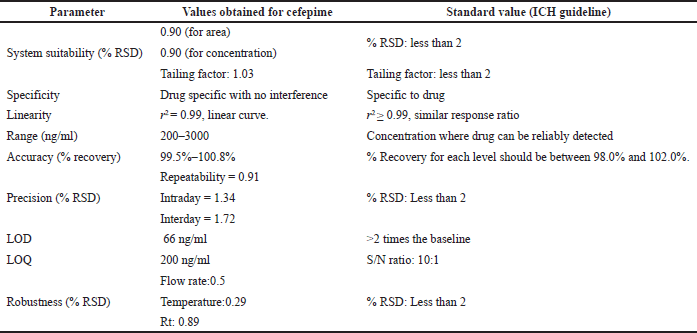 | Table 1. Summary of validation parameters for the proposed method of cefepime. [Click here to view] |
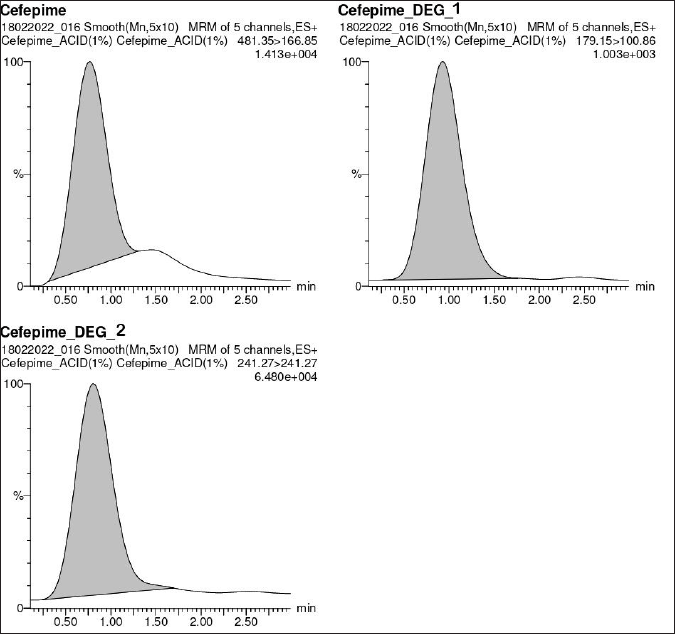 | Figure 3. Typical chromatogram of cefepime and its degradants. [Click here to view] |
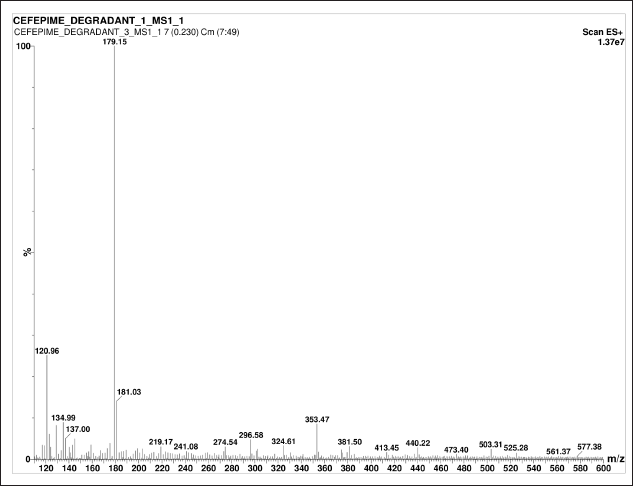 | Figure 4. MS/MS spectra of degradants CD1. [Click here to view] |
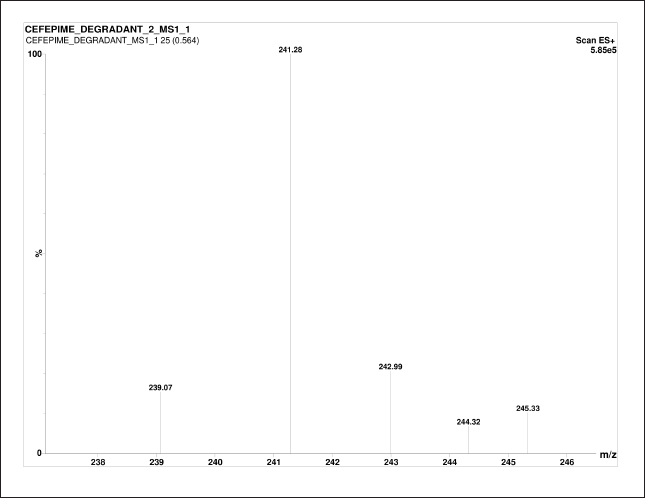 | Figure 5. MS/MS spectra of degradants CD2. [Click here to view] |
DISCUSSION
In this research, the retention time of the parent compound of CFP is 0.82 minute with MS/MS spectra of standard CFP obtained at an m/z ratio of 481.42. The retention time of degradant CD1 in different condition was 0.91 ±0.01 minute with MS/MS spectra of CD1 at m/z at 179.15. The retention time of degradant CD2 in different condition was 0.84 ± 0.1 minute with MS/MS spectra of CD2 at m/z at 241.33. The presence of these m/z spectra at 481.42 ± 0.09 in all stress conditions, except basic indicates that the CFP is unstable and has partially deteriorated. The peak in the chromatogram and MS/MS spectra of CFP in forced basic medium has been observed at m/z of 179.15 and Retention time at 0.91 ± 0.01 minute of degradant CD1 indicating CFP is more vulnerable to a basic condition resulting in complete degradation of the drug in basic medium. During the entire analysis of CFP, there was no interference observed in the solution. The fragmentation of standard CFP into daughter ion was observed at transition pair of 481.35→166.85. During the forced degradation studies, CFP and its stability solutions were analyzed by UPLC coupled to electrospray triple quadrupole MS (LC-ESI-QQQ-MS) to identify the possible degraded products under stress conditions. The general fragmentation pattern of CFP in UPLC-MS/MS represents the molecular weight and possible structures of fragments for CFP during long-term stability studies as shown in Figure 6. The molecular weight of degraded products with possible fragments was predicted based on the protonated molecular ions [M+H]+ and the fragmentation pathway of CFP was established from mass precision and fragmentation mass spectrum. All degradation products and possible fragmentations were characterized and the proposed structures are presented in Table 3.
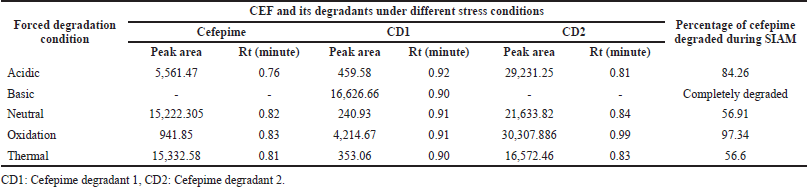 | Table 2. Forced degradation studies results. [Click here to view] |
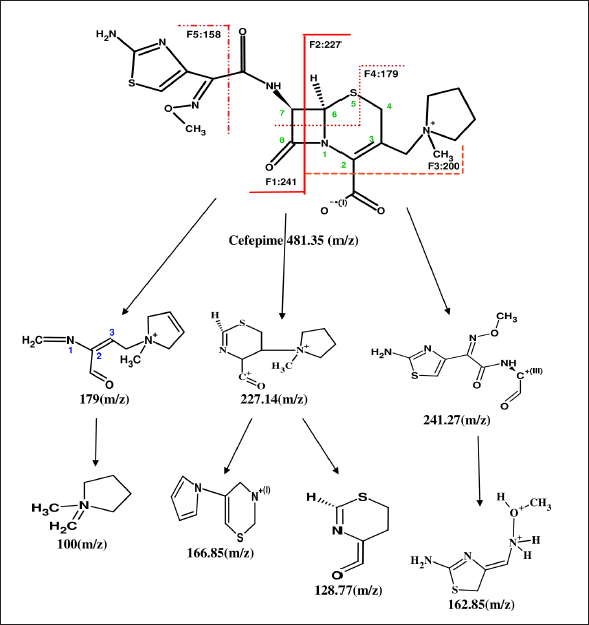 | Figure 6. Fragmentation pathway of cefepime. [Click here to view] |
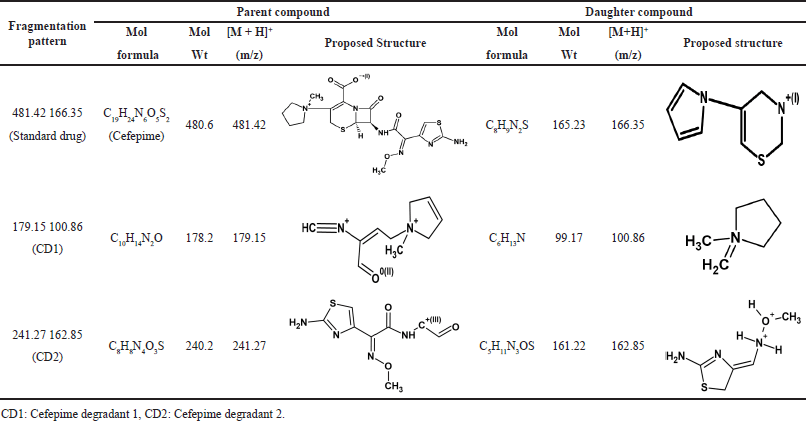 | Table 3. Degradation products and their proposed structures. [Click here to view] |
CONCLUSION
A novel stability-indicating UPLC-MS/MS method for the determination of stability in different stress conditions for CFP has been performed. Rt of the forced degradation studies solution was reported to be 0.82 minute for CFP, CD1 at 0.91 ± 0.01 minute, and CD2 at 0.84 ± 0.1 minute; this method has less Rt when compared to available assay methods found in the literature. Forced degradation studies were successfully developed as per ICH guidelines.
FUTURE PLAN OF WORK
The developed method can be applied to determine degradants of CFP in blood, cerebrospinal fluid, and urine samples by using this automated UPLC-MS/MS technique.
ACKNOWLEDGEMENT
The authors would like to thank Kshetra Analyticals, Hyderabad, Telangana, India, for providing the necessary facilities (chemicals and equipments) to carry out this research work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abd El Aziz Shama SA, Sharkawy AS El, Mobarez EA, Nassar SH. A simultaneous, validated RP-HPLC method for determination of eight cephalosporins in pharmaceutical formulations. Sys Rev Pharm, 2021; 12(3):646–53. Available via https://www.sysrevpharm.org/articles/a-simultaneous-validated-rphplc-method-for-determination-of-eight-cephalosporins-in-pharmaceutical-formulations.pdf
Al Kamaly O. Review on characterization, properties, and analytical methods of cefepime. Int J Anal Chem, 2022; 6909528; doi:10.1155/2022/6909528
Bajaj S, Singla D, Sakhuja, N. Stability testing of pharmaceutical products. J App Pharm Sci, 2012; 2(3):129–38; doi:10.73690952824/JAPS.2012.2322
Bjergum MW, Barreto EF, Scheetz MH, Rule AD, Jannetto PJ. Stability and validation of a high-throughput LC-MS/MS method for the quantification of cefepime, meropenem, and piperacillin and tazobactam in serum. J Appl Lab Med, 2021; 6(5):1202–12; doi:10.1093/jalm/jfab036
Connors KA, Amidon GL, Stella VJ. Chemical stability of pharmaceuticals. In: A handbook for pharmacists. 2nd edition, John Wiley & Sons, Inc, New York, NY, 1986.
Cosa G. Photodegradation and photosensitization in pharmaceutical products: assessing drug phototoxicity. Pure Appl Chem, 2004; 76(2):263–75; doi:10.1351/pac200476020263
De Borba B, Hurum DC, Rohrer JS. Determination of cefepime and related compounds using HPLC with UV detection. Application Note, 2008. Available via https://www.chromatographyonline.com/view/determination-cefepime-and-related compounds-using-hplc-uv-detection-1
Deidda R, Orlandini S, Hubert P, Hubert C. Risk-based approach for method development in pharmaceutical quality control context: a critical review. J Pharm Biomed Anal, 2018; 161:110–21; doi:10.1016/j.jpba.2018.07.050
Dos Anjos MV, Possa E, da Silva Fonseca G, Bergoza L, dos Santos PR, Moura E Silva S, Tasso L. Development and validation of an LC–ESI–QTOF–MS method to measure cefepime in the plasma and peritoneal fluid of rats using microdialysis: application in a pilot pharmacokinetic study. Biomed Chromatogr, 2022; 36(11):e5470; doi:10.1002/bmc.5470
El-Beltagy HE, Amin AS, El-Balkeny MN, Madkour SA. Simultaneous determination of cefepime, cefotaxime and ceftriaxone in pharmaceutical formulation by HPLC method. IOSR J Pharm Biol Sci, 2019; 14:81–7; doi:10.9790/3008-1401038187
El-Dars F, Elshater NS, Abd Elaziz SM. Analytical determination of cefepime residues in rabbit’ muscles, liver and kidney using HPLC. Curr Sci Int, 2019; 4:699-706; doi:10.36632/csi/2019.8.4.10
Fage D, Deprez G, Fontaine B, Wolff F, Cotton F. Simultaneous determination of 8 beta-lactams and linezolid by an ultra-performance liquid chromatography method with UV detection and cross-validation with a commercial immunoassay for the quantification of linezolid. Talanta, 2021; 221:121641.
Glish GL, Burinsky DJ. Hybrid mass spectrometers for tandem mass spectrometry. J Am Soc Mass Spectrom, 2008; 19(2):161–72.
ICH. ICH Q1A (R2) stability testing of new drug substances and products. In: International Conference on Harmonization, IFPMA, Geneva, Switzerland, 2003.
Isla A, Arzuaga A, Maynar J, Gascón AR, Solinís MA, Corral E, Pedraz JL. Determination of ceftazidime and cefepime in plasma and dialysate-ultrafiltrate from patients undergoing continuous veno-venous hemodiafiltration by HPLC. J Pharm Biomed Anal, 2005; 39(5): 996–1005.
Jabeen, Suma BV. Newly validated stability-indicating ultra-performance liquid chromatography-tandem mass spectrometry method for the estimation of ceftaroline fosamil by using a quadrupole mass detector. J Appl Pharm Sci, 2022; 12(6):215–23; doi:10.7324/JAPS.2022.120621
Jagadeesh Kumar V, Badarinadh Gupta P, Pavan Kumar KSR, Prasada Rao KVV, Rao KR, Prasanna SJ, Kumar Sharma H, Mukkanti K. Identification and characterization of new degradation products of cefepime dihydrochloride monohydrate drug substance during stress stability studies. Anal Sci, 2010; 26(10):1081–6; doi:10.2116/analsci.26.1081
Jiang M, Wang L, Ji R. Biotic and abiotic degradation of four cephalosporin antibiotics in a lake surface water and sediment. Chemosphere, 2010; 80(11), 1399–405.
Jiménez Palacios FJ, Callejón Mochón M, Jiménez Sánchez JC, Bello López MÁ, Guiráum Pérez A. Validation of an HPLC method for determination of cefepime (a fourth-generation cephalosporin). Determination in human serum, cerebrospinal fluid, and urine. Pharmacokinetic profiles. Chromatographia, 2005; 62, 355–61.
Kalyani L, Rao CVN. Stability indicating RP-HPLC method development and validation of cefepime and amikacin in pure and pharmaceutical dosage forms. Braz J Pharm Sci, 2018; 54(3):1–9; doi:10.1590/s2175-97902018000317258
Kommana R, Kannabattula G, Gurrala S, Yeradesi VR, Durga PA. Quantification and stress degradation studies of cefepime/Tazobactam in dry injection form by an RP-HPLC method. Braz J Pharm Sci, 2014; 50(4):895–902; doi:10.1590/S1984-82502014000400025
Lemke TL, Williams DA, Roche VF, Zito SW. Foye’s principles of medicinal chemistry. 6th edition, Lippincott Williams & Wilkins, Philadelphia, PA, 2002.
Liu Y, Zhang J, Hu C. Validated LC-MS/MS method for simultaneous analysis of 21 cephalosporins in zebrafish for a drug toxicity study. Anal Biochem, 2018; 558:28–34.
Mameli M, Vezzelli A, Verze’ S, Biondi S, Motta P, Greco A, Michi M, Breda M. Liquid chromatography–tandem mass spectrometry for the simultaneous quantitation of enmetazobactam and cefepime in human plasma. J Pharm Biomed Anal, 2019; 174:655–62; doi:10.1016/j.jpba.2019.06.041
Moorthy GS, Vedar C, Zane NR, Downes KJ, Prodell JL, DiLiberto MA, Zuppa AF. Development and validation of a volumetric absorptive microsampling- liquid chromatography mass spectrometry method for the analysis of cefepime in human whole blood: application to pediatric pharmacokinetic study. J Pharm Biomed Anal, 2020; 179:113002.
Nemutlu E, Kir S, Katlan D, Beksaç MS. Simultaneous multiresponse optimization of an HPLC method to separate seven cephalosporins in plasma and amniotic fluid: application to validation and quantification of cefepime, cefixime and cefoperazone. Talanta, 2009; 80(1):117–26.
Niessen WMA. Applications: small molecules. In: Liquid chromatography –mass chromatography. 3rd edition, Taylor & Francis, Boca Raton, London, NY, pp 385–6, 2010.
Niessen W, Ricardo CA. Fragmentation of antimicrobial compounds. In: Interpretation of MS—MS mass spectra of drugs and pesticide. 1st edition, John Wiley & Sons, Inc, Hoboken, NJ, pp 269–73, 2017.
Ocaña González JA, Jiménez Palacios FJ, Callejón Mochón M, Barragán De La Rosa FJ. Simultaneous determination of cefepime and grepafloxacin in human urine by high-performance liquid chromatography. J Pharm Biomed Anal, 2004; 36(1):117–23; doi:10.1016/j.jpba.2004.05.002
Ohmori T, Suzuki A, Niwa T, Ushikoshi H, Shirai K, Yoshida S, Ogura S, Itoh Y. Simultaneous determination of eight β-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci, 2011; 879(15–16), 1038–42.
Quadri SS, Sonwane LV, Poul BN, Kamshette SN. Review on stability indicating assay methods (SIAMs). PharmaTutor, 2014; 2(8):16–31. Available via http://www.pharmatutorjournal.com/index.php/pt/article/view/152
Rehm S, Rentsch KM. HILIC LC-MS/MS method for the quantification of cefepime, imipenem and meropenem. J Pharm Biomed Anal, 2020; 186:113289.
Rodrigues DF, Salgado HRN. Development and validation of a green analytical method of RP-HPLC for quantification of cefepime hydrochloride in pharmaceutical dosage form: simple, sensitive and economic. Curr Pharm Anal, 2016; 12(4):306–14.
Patil KR, Naik SK, Zope VS, Chavan RP, Yeole RD. Lc-Ms/Ms method for the simultaneous determination of cefepime and tazobactam in dog plasma. Drug Anal Res, 2018; 2(1):36–45; doi:10.22456/2527-2616.84357
Sahu PK, Ramisetti NR, Cecchi T, Swain S, Patro CS, Panda J. An overview of experimental designs in HPLC method development and validation. J Pharm Biomed Anal, 2018; 147:590–611; doi:10.1016/j.jpba.2017.05.006
Seraisso P, Lanot T, Baklouti S, Mané C, Ruiz S, Lavit M, De Riols P, Garrigues J-C, Gandia P. Evaluation of 4 quantification methods for monitoring 16 antibiotics and 1 beta-lactamase inhibitor in human serum by high-performance liquid chromatography with tandem mass spectrometry detection. J Pharm Biomed Anal, 2022; 219:114900; doi:10.1016/j.jpba.2022.114900
Shrestha B, Bhuyan NR, Sinha BN. Simultaneous determination of cefepime and tazobactam in injectables by ultra-high performance liquid chromatography method. Pharm Methods, 2014; 5(1):20–6; doi:10.5530/phm.2014.1.4
Siddiqui MR, Malick T, Reddy KD, Negi PS, Yadav JS, Bhatnagar A, Chaudhary M, Singh R. High performance liquid chromatographic method for simultaneous determination of cefepime and sulbactam in pharmaceutical formulation (supime) and biological samples. Int J Pharm, 2010; 6(3):271–7.
Sun H, Xing H, Tian X, Zhang X, Yang J, Wang P. UPLC-MS/MS method for simultaneous determination of 14 antimicrobials in human plasma and cerebrospinal fluid: application to therapeutic drug monitoring. J Anal Methods Chem, 2022; 2022:7048605; doi:10.1155/2022/7048605
Sundara Raj B, Punitha ISR, Krishnan S. Stability studies of cefepime hydrochloride by stability indicating RP-HPLC method. Int J Pharm Sci Nanotechnol, 2013; 6(3):2181–6; doi:10.37285/ijpsn.2013.6.3.10
Sunitha N, Sindhura L, Thangabalan B, Babu S. Development and validation of RP–HPLC method for simultaneous estimation of cefepime and tazobactam in injection formulation. Asian J Pharm Anal, 2013; 3(4):131–7.
Van Vooren S, Verstraete AG. A sensitive and high-throughput quantitative liquid chromatography high-resolution mass spectrometry method for therapeutic drug monitoring of 10 β-lactam antibiotics, linezolid and two β-lactamase inhibitors in human plasma. Biomedical Chromatogr, 2021; 35(7):e5092; doi:10.1002/bmc.5092
Waterman KC, Adami RC, Alsante KM, Antipas AS, Arenson DR, Carrier R, Hong J, Landis MS, Lombardo F, Shah JC, Shalaev E, Smith SW, Wang H. Hydrolysis in pharmaceutical formulations. Pharm Dev Technol, 2002; 7(2):113–46; doi:10.1081/PDT-120003494
Zander J, Maier B, Suhr A, Zoller M, Frey L, Teupser D, Vogeser M. Quantification of piperacillin, tazobactam, cefepime, meropenem, ciprofloxacin and linezolid in serum using an isotope dilution UHPLC-MS/MS method with semi-automated sample preparation. Clin Chem Lab Med, 2015; 53(5):781–91; doi:10.1515/cclm-2014-0746