INTRODUCTION
Cholesterol is an insoluble lipid molecule that provides stability to membrane bilayers and is essential for their function (Cohen, 2008). It is also a precursor for steroid hormones such as sex hormones and corticosteroids (Wang et al., 2017). Despite the gastrointestinal tract’s ability to absorb substantial amounts of cholesterol, almost all cells can generate their own requirement. Only the liver’s hepatocytes, however, are capable of removing huge amounts of cholesterol (Cohen, 2008).
Cholesterol is transferred in the bloodstream by lipoprotein particles. Low-density lipoprotein (LDL), high-density lipoprotein (HDL), and the two main cholesterol-carrying blood lipoproteins were identified by physiologists in the 1950s and 1960s. Epidemiologists revealed that high LDL levels increase the risk of heart attacks, while high HDL levels protect against heart disease (Goldstein and Brown, 2009). Cholesterol usually forms solid lumps in the walls of arteries when LDL levels are abnormally high, a state known as atherosclerosis, which is primarily responsible for coronary heart disease and other kinds of cardiovascular disease (Hrydziuszko et al., 2014).
The main cause of morbidity and mortality worldwide is atherosclerotic cardiovascular disease (ASCVD) and its clinical manifestations, such as myocardial infarction and ischemic stroke (Ference et al., 2017). The preservation and accumulation of cholesterol-rich apoB-containing lipoproteins within the arterial intima at plaque-prone sites are key pathophysiological mechanisms in the establishment of ASCVD (Ference et al., 2017). Over 200 studies including more than 2 million individuals and over 150,000 cardiovascular events demonstrated a dose-dependent, log-linear relationship between LDL level and ASCVD threat (Ference et al., 2017; Lewington et al., 2007; Nordestgaard et al., 2013).
Statins are commercially available drugs used to lower cholesterol level. They are well known for inhibiting 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase), the enzyme that catalyzes the rate-limiting step in cholesterol biosynthesis. The therapeutic efficacy of statins for individuals with coronary artery disease and cardiovascular disease has been proven (Jiang et al., 2018). They are not, however, without adverse effects, which are usually dose-related and include elevated liver enzymes and muscle pain (Grundy, 2005). Also, despite their ability to lower plasma LDL, statins were not always effective at preventing cardiovascular disease (Tavazzi et al., 2008). Therefore, new drugs with less side effects and better therapeutic outcomes are needed.
Isorhamnetin is a naturally occurring methylated derivative of quercetin. Isorhamnetin is present in Hippophae rhamnoides L., Ginkgo biloba L., and Brassica juncea (Yokozawa et al., 2002). It had a wide spectrum of pharmacological effects, including hypoglycemic (Yokozawa et al., 2002), cardioprotective (Zhao and Liu, 2008), neuroprotective (Zhao et al., 2016), anti-inflammation (Chi et al., 2016), antitumor (Wu et al., 2019), and antioxidant effects (Dong et al., 2015). In an in vivo study, isorhamnetin lowered serum cholesterol of rats fed high-cholesterol diet (Igarashi and Ohmuma, 1995). However, its mechanism of action was not studied. This work was designed to investigate the effect of isorhamnetin on HMG-CoA reductase and LDLR gene and protein expression in HepG2 hepatoma cell line. Furthermore, the antioxidant activity of isorhamnetin in HepG2 was investigated.
MATERIAL AND METHODS
Drugs
Isorhamnetin stock solution was prepared by dissolving it in 10% DMSO. Stock solution was diluted by Dulbecco’s Modified Eagles Medium (DMEM) media to obtain the final concentrations 25, 50, or 100 μM used in different studies.
Cells and culture media
Human HepG2 hepatocarcinoma (ECACC/UK) cells were cultured as monolayer using complete DMEM (Euroclone, Italy). DMEM contains four times the amino acid and vitamin concentration of original Eagle’s Minimal Essential Medium. Additional supplements include 4 mM L-glutamine, 4,500 mg/l glucose, 1 mM sodium pyruvate, and 1,500 mg/l sodium bicarbonate. To DMEM medium, 10% fetal bovine serum and penicillin/streptomycin (100 U/ml) were added and incubated in a humidified 5% CO2 atmosphere at 37°C.
Cytotoxicity assay
The toxicity of isorhamnetin was investigated using 3-(4,5-dimethylthiazolyl)-2,5-diphenyl-tetrazolium bromide (MTT) assay (Promega, USA). The test was performed according to the manufacturer’s directions as described earlier (Abbas et al., 2020).
The MTT test (Promega, USA) is based on the ability of mitochondrial dehydrogenase to convert MTT to a purple formazan product. The antiproliferative activity of isorhamnetin (Biosynth Carbosynth, UK) (200, 100, 50, 25, 12.5, 6.25, and 3.125 g/ml) was evaluated using this method. The cells were suspended, collected, and counted to 104/100 μl before being seeded in a 96-well plate and incubated for 24 hours at 37°C, 5% CO2. The test was carried out in accordance with the manufacturer’s instructions: in each well, 15 μl of MTT dye solution was added and incubated for 4 hours. The MTT-formazan product was then stopped by adding 100 μl of MTT stop solution to each well. The absorbance was then measured at 590 nm with a microplate reader (Biotech, USA); a decrease in absorbance compared to untreated control cells is a measure of cell viability. Each test was performed in triplicate and HepG2 cells without treatment were as negative control. The final concentration of DMSO in all solutions was <0.05%.
Cell viability percentage was calculated using the formula:
% Cell viability = (ODSample − ODBlank)/(ODVehicle − ODBlank) × 100%.
OD is the optical density.
Quantitative real-time polymerase chain reaction (PCR)
HepG2 cell line (106 cells) was cultured in DMEM medium and seeded in 24 cell plates. Then, HepG2 cells were treated with less than 1% DMSO (vehicle) or isorhamnetin (25, 50, or 100 μM) for 24 hours. At the end of incubation, cells were washed with PBS and then trypsinized. Total RNA was extracted using Direct-zol RNA MiniPrep Kit (Zymo, USA Cat# R2050) according to the manufacturer’s instructions. RNA (1 μg) was extracted from HepG2 cells treated with vehicle or isorhamnetin and used to prepare cDNA and to run real-time PCR using GoTaq® 1-Step RT-qPCR (Promega, Cat# A6020, USA). RT-PCR was performed using iCycler Thermal Cycler System apparatus (Thermo Fisher, USA) under the following conditions: 90°C for 10 seconds followed by 60°C for 30 seconds, and 72°C for 30 seconds for 40 cycles. At the end of RT-PCR melting curve step at 55°C–95°C was done. Primers of the genes: HMGCR that encodes HMG-CoA reductase enzyme, LDLR that encodes LDLR, and ACTB (housekeeping gene) that encodes β-actin were purchased from Alpha DNA (Canada). Sequence of sense and antisense primers are shown in Table 1. Expression data were normalized using β-actin ACTB used as internal control for each sample.
The results of quantitative PCR were calculated using 2-Delta Delta C(T) method to analyze the relative changes in gene expression. This experiment was repeated twice in triplicate manner to insure that the results are reproducible.
Protein extraction
After treatment with vehicle or isorhamnetin for 24 hours, HepG2 cells (106 cells) of each group were washed twice with PBS and incubated with lysis buffer (iNtRON biotechnology Cat. #17081, Korea) for 20 minutes at 4°C and finally centrifuged at 15,000 rpm for 5 minutes. Protein concentration was measured using Pierce BCA Protein Assay Kit (Thermo Scientific, USA). The supernatant was stored at −20°C for analysis of superoxide dismutase (SOD), catalase activity, western blot, and cellular enzyme linked immunosorbent assay (ELISA).
 | Table 1. Sequence of primers. [Click here to view] |
Western blot
Protein samples (30 μg) were separated by electrophoresis on a 10% sodium dodecyl sulfate–polyacrylamide gel. At the end of the run, separated proteins were transferred to a nitrocellulose membrane (ThermoFisher, USA). 5% bovine serum albumin (BSA) (Bio-Techne, UK) in Tris-buffered saline (ChemCruz, USA) was used for blocking the membranes at 4°C overnight. In the next day, the membranes were incubated with 1:1000 rabbit monoclonal antibodies specific for HMG-CoA reductase (Abcam, US) for 1 hour at room temperature followed by washing 3 times for 10 minutes each using Tris-buffered saline with Tween-20. The membranes were then incubated for 1 hour with anti-rabbit secondary antibody conjugated with horseradish peroxidase (Bio-Techne, Minneapolis, MN).
Indirect ELISA for the detection of LDLR
HepG2 cells were cultured with vehicle or with different concentrations (25, 50, 100 μM) of isorhamnetin. ELISA plate was coated with 100 μl/well of cell lysate (10 μg/ml protein extract) in triplicates overnight at 4°C. At the end of the incubation period, the plate was blocked with 200 μl of 5% BSA (Bio-Techne, UK) in PBS for 2 hours at room temperature. After washing, anti-LDLR primary antibody (Bio-Techne, UK) (1:1,000 dilution) was added at room temperature for 1 hour. At the end of the incubation period, washing three times with 0.05% PBS-Tween was performed. One hundred microliters of 1:1,000 HRP-conjugated goat anti-mouse immunoglobulin G (Promega, USA) were added and incubated at room temperature for 1 hour. At the end of the incubation period, washing three times with 0.05% PBS-Tween was done. Then, 100 μl of TMB substrate solution (Biotech, USA) was added to each well and incubated for 15 minutes at room temperature before adding the stopping solution. The plate was finally read with ELISA plate reader (Biotech, USA) at 450 nm.
Cellular ELISA was also performed using 104 cells monolayer cultured for 24 hours in 96-well plate and treated with vehicle or with different concentrations (25, 50, 100 μM) of isorhamnetin in triplicates. Then, anti-LDLR primary antibody (Bio-Techne, UK) (1:1,000) dilution was added at room temperature, for 1 hour to bind with the target receptor on HepG2 cells. At the end of the incubation period, washing three times with 0.05% PBS-Tween was performed. The cells were then fixed with 4% paraformaldehyde and blocked with 200 μl of 5% BSA. HRP-conjugated goat anti-mouse immunoglobulin G (Promega, USA) (1:1,000 dilution) was added and incubated at room temperature for 1 hour. Then, 100 μl of TMB substrate was added to each well and incubated for 15 minutes at room temperature before the stop solution was added. Finally, the plate was read with ELISA plate reader (Biotech, USA) at 450 nm.
SOD activity assay
SOD activity was measured using Abcam SOD assay kit (Abcam, US, Cat.# ab65354). The test was performed according to the manufacturer’s directions. Percentage change in SOD activity was calculated using the formula:
% SOD activity = (SODSample − SOD Control) / SOD Control × 100%.
Catalase activity assay
Catalase assay test (Abcam, USA, Cat.# ab83464) was performed according to the manufacturer’s directions.
Statistical analysis
In all tests, GraphPad Prism version 8 was used to perform one-way analysis of variance followed by Tukey’s post-hoc test. p < 0.05 was regarded as significant.
RESULTS AND DISCUSSION
Cell cytotoxicity assay
In HepG2 cells, isorhamnetin exerted cytotoxic action on the HepG2 cell line with a half-maximal inhibitory concentration (IC50) value of 100 μM after 24 hours incubation, 53.3 μM after 48 hours, and 40.02 μM after 72 hours, respectively (Fig. 1). In a previous study, the IC50 of isorhamnetin in HepG2 cells was reported to be 50 μM after 48 hours (Runhuan and Haiyan, 2018). This value is very close to the IC50 obtained in our study. Xue et al. (2021) found that the IC50 value of isorhamnetin was 174 μM after 72 hours of treatment in HepG2 cells. In a study performed by Gao et al. (2021), isorhamnetin showed proliferation inhibition on HepG2 cells with an IC50 of 54.32 μM after 24 hours of treatment. Also, in another study, it was found that with the increase in isorhamnetin concentration, cell viability gradually reduced. A study of bioactivities of seven flavonoids from Osmanthus fragrans “Jinqiu” was done by Zhou et al. (2018) on seven flavonoid compounds. Isorhamnetin cell proliferation inhibition on HepG2 cells indicated IC50 of 50.15 μg/ml (158 μM) after 72 hours.
HMG-CoA reductase gene and protein expression
Significant downregulation of HMG-CoA reductase gene expression was produced by 25, 50, and 100 μM isorhamnetin by 48.0%, 90.0%, and 65.3%, respectively (Fig. 2). On the other hand, isorhamnetin (100 μM) decreased HMG-CoA reductase compared to vehicle-treated control (Fig. 3).
HMG-CoA reductase, the time-limiting enzyme in cholesterol biosynthesis, is a main contributor in cholesterol biosynthesis and regulation (Goldstein and Brown, 1990). Currently, HMG-CoA reductase inhibitors, such as statins, are commonly used to lower blood cholesterol level (Reiner, 2010). In our study, isorhamnetin decreased HMG-CoA reductase gene expression in HepG2 cells. Our results agree with earlier reports that isorhamnetin extracted from sea buckthorn decreased HMG-CoA reductase gene expression in a dose-dependent manner in HL7702 human normal liver cell line (Xiao et al., 2021). Also, a decrease in HMG-CoA reductase gene expression was reported in high-fat diet-induced nonalcoholic fatty liver disease model in rats (Zhang et al., 2013). Furthermore, it was demonstrated that isorhamnetin was able to activate AMP-activated protein kinase (AMPK) in HepG2 cells (Dong et al., 2014). This enzyme phosphorylates and inhibits SREBP-2, a transcription factor that binds to the promoter region of HMG-CoA reductase to regulate its gene expression (Li et al., 2011).
 | Figure 1. Cell cytotoxicity after treatment with isorhamnetin at 24, 48, and 72 hours. [Click here to view] |
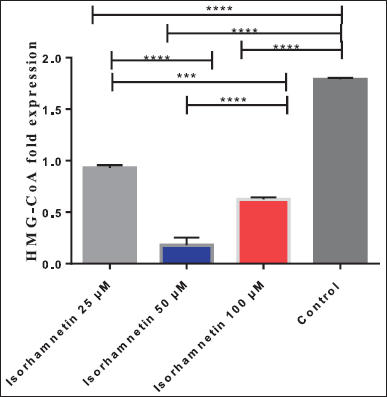 | Figure 2. Gene expression of HMGCR reductase enzyme in vehicle-treated and isorhamnetin-treated HepG2 cells. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. [Click here to view] |
 | Figure 3. Immunoblot of HMG-CoA reductase enzyme. Control: Extract of HepG2 treated with DMSO. Sample 1: extract of HepG2 treated with isorhamnetin 25 μM. Sample 2: extract of HepG2 treated with isorhamnetin 100 μM. [Click here to view] |
LDLR gene expression and protein expression in tissue homogenate and on cell surface
Cellular ELISA was used in this work to measure the surface expression of the receptor while ELISA for the whole cell extract was used to measure the total concentration of the receptor either that was expressed on the surface or present in the cytoplasm (Pandey et al., 2019). Isorhamnetin downregulated LDLR expression by 47.9%, 80.6%, and 82.7% using 25, 50, and 100 μM doses, respectively (Fig. 4). In tissue homogenate, isorhamnetin produced an increase in total LDLR protein expression at all studied concentrations (Fig. 5a). On the other hand, isorhamnetin produced no change in membranous LDLR protein expression on cell surface at all studied doses (Fig. 5b).
 | Figure 4. Gene expression of LDLR in vehicle-treated, and isorhamnetin-treated HepG2 cells. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. [Click here to view] |
LDL cholesterol clearance from cells to the liver is crucial to avoid the formation of atherosclerotic lesions. In fact, about 75% of the circulating cholesterol is removed by LDLR endocytic circulation (Yang et al., 2020). It is well established that statins—the most commonly used antihypercholesterolemic drugs—upregulate LDLR (Vogel, 2012). In the present study, LDLR gene expression decreased after treatment of HepG2 cells with isorhamnetin. Similar results were obtained in a previous study on normal human liver cells (HL7702) where isorhamnetin—extracted from sea buckthorn—downregulated LDLR and SREBP-2 gene expression (Gu et al., 2022). Membranous LDLR was not affected by isorhamnetin treatment after 24 hours in the present investigation. On the other hand, total LDLR, including the intracellular LDLR, was higher compared to the control. One possible explanation is that isorhamnetin might decrease the degradation of LDLR. Detailed investigation of the effect of isorhamnetin on inducible degrader of the LDLR (IDOL) and proprotein convertase subtilisin/kexin type 9 pathways, involved in LDLR degradation, in HepG2 is needed. It is worth mentioning that isorhamnetin downregulated liver X receptor (LXR-?) gene expression in 3T3 cell line (Lee et al., 2009). LXR is a transcription factor that induces the expression of IDOL, a protein that triggers ubiquitination of the receptor and targets it for degradation (Zelcer et al., 2009). If LXR was downregulated, it is expected that LDLR will increase.
Another suggested mechanism for the effect of isorhamnetin on LDLR accumulation inside the cells is through the activation of AMPK, the enzyme that phosphorylates and inhibits SREBP-2 (Dong et al., 2014). It is well established that LDLR gene is regulated by the nuclear form of nSREBP-2 (Attie and Seidah, 2005). Therefore, investigating the signaling pathway AMPK/SREBP-2/LDLR is required.
Antioxidant activity
Isorhamnetin 25 and 50 μM, but not 100 μM, increased SOD activity compared to the vehicle-treated cells (control) significantly by 75.66% and 115.69%, respectively (Fig. 6a). Similarly, isorhamnetin 25, 50, and 100 μM reduced H2O2 level significantly by 46.44%, 72.30%, and 66.67%, respectively (Fig. 6b). This study demonstrated that isorhamnetin decreased oxidative stress in HepG2 cell line. SOD activity increased by 2.16 folds by 50 μM isorhamnetin treatment. Similarly, catalase activity showed a decrease in H2O2 level (by 3.42 folds using 50 μM isorhamnetin). In HepG2, isorhamnetin containing extract of Tamarindus indica leaf increased the activity of SOD and catalase (Razali et al., 2015). Also, isorhamnetin enhanced antioxidant effect in different tissues including heart, brain, and testis. Isorhamnetin exerted cardioprotective effect against myocardial ischemia-reperfusion injury in isolated rat heart and increased SOD and catalase activity (Xu et al., 2020). In a study of the effect of isorhamnetin in brain, isorhamnetin improved cognition and memory, presumably via improving the antioxidant defense system (SOD and catalase activity), cholinergic signaling, and synaptic plasticity. Pretreatment of mice with isorhamnetin before scopolamine produced dose dependency and significant increase in SOD activity in the hippocampus (2.95 folds) and prefrontal cortex (2.26 folds) compared with vehicle-scopolamine-treated group. Similarly, pretreatment of mice with isorhamnetin produced dose dependency and a significant increase in catalase activity in the hippocampus (2.41 folds) and prefrontal cortex (2.63 folds) when compared with vehicle-scopolamine-treated group (Ishola et al., 2019). Mustafa et al. (2022) studied the effect of isorhamnetin in doxorubicin-induced testicular injury model in male rats. Doxorubicin-induced oxidative stress was significantly reduced by isorhamnetin treatment due to the increase in SOD (by 2.39 folds) and catalase (by 1.72 folds) activities compared to doxorubicin-treated control (Mustafa et al., 2022).
 | Figure 5. Detection of LDLR in HepG2 cell homogenate (a) or expressed in the cell membrane by cellular ELISA (b) after treatment with vehicle or different doses of isorhamnetin. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. [Click here to view] |
 | Figure 6. (a) SOD activity after isorhamnetin treatment. (b) H2O2 reduction after isorhamnetin treatment which reflects catalase activity. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. [Click here to view] |
CONCLUSION
Isorhamnetin lowered the expression of HMG-CoA reductase. Also, it increased LDLR expression in HepG2 cells. Furthermore, this flavonoid exerted antioxidant effects by increasing the activity of SOD and catalase. Future preclinical and clinical studies are needed to investigate thoroughly the effect of this flavonoid on cholesterol and lipid metabolism and to study its toxicity in vivo.
Limitations of the study
To follow up gene and protein expression precisely, frequent testing is required at different time intervals. The time for performing each experiment was based on pilot studies. Frequent sampling was not possible.
AUTHORS’ CONTRIBUTIONS
This is a part of Randa Al-Rayyes’s Msc. thesis; she contributed to the practical work. Manal M. Abbas contributed with study design, writing the manuscript, and statistical analysis. Razan Obeidat performed PCR, ELISA, and Immunoblot experiments.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTERESTS
The authors declare that they have no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbas MM, Kandil Y?, Abbas MA. R-(-)-carvone attenuated doxorubicin induced cardiotoxicity in vivo and potentiated its anticancer toxicity in vitro. Balkan Med J, 2020; 37:98.
Attie AD, Seidah NG. Dual regulation of the LDL receptor—some clarity and new questions. Cell Metab, 2005; 1:290–2.
Chi G, Zhong W, Liu Y, Lu G, Lü H, Wang D, Sun F. Isorhamnetin protects mice from lipopolysaccharide-induced acute lung injury via the inhibition of inflammatory responses. Inflamm Res, 2016; 65:33–41.
Cohen DE. Balancing cholesterol synthesis and absorption in the gastrointestinal tract. J Clin Lipidol, 2008; 2:S1–3.
Dong G-Z, Lee J-H, Ki SH, Yang JH, Cho IJ, Kang SH, Zhao RJ, Kim SC, Kim YW. AMPK activation by isorhamnetin protects hepatocytes against oxidative stress and mitochondrial dysfunction. Eur J Pharmacol, 2014; 740:634–40.
Dong X, Sun G, Luo Y. Protective effect of isorhamnetin on oxidative stress induced by H2O2 in H9C2 cells. Chin Pharmacol Bull, 2015; 31:853–60.
Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen MR, Tokgözoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J, 2017; 38:2459–72.
Gao X, Yanan J, Santhanam RK, Wang Y, Lu Y, Zhang M, Chen H. Garlic flavonoids alleviate H2O2 induced oxidative damage in L02 cells and induced apoptosis in HepG2 cells by Bcl-2/Caspase pathway. J Food Sci, 2021; 86(2):366–75.
Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature, 1990; 343:425–30.
Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol, 2009; 29:431–8.
Grundy SM. The issue of statin safety: where do we stand? Circulation, 2005; 111:3016–9.
Gu Y, Wang X, Liu F, Zhang J, Zhang X, Liu J, Li S, Wang D, Guan H, Hou D. Total flavonoids of sea buckthorn (Hippophae rhamnoides L.) improve MC903-induced atopic dermatitis-like lesions. J Ethnopharmacol, 2022; 292:115195.
Hrydziuszko O, Wrona A, Balbus J, Kubica K. Mathematical two-compartment model of human cholesterol transport in application to high blood cholesterol diagnosis and treatment. Electron Notes Theor Comput Sci, 2014; 306:19–30.
Igarashi K, Ohmuma M. Effects of isorhamnetin, rhamnetin, and quercetin on the concentrations of cholesterol and lipoperoxide in the serum and liver and on the blood and liver antioxidative enzyme activities of rats. Biosci Biotechnol Biochem, 1995; 59:595–601.
Ishola IO, Osele MO, Chijioke MC, Adeyemi OO. Isorhamnetin enhanced cortico-hippocampal learning and memory capability in mice with scopolamine-induced amnesia: role of antioxidant defense, cholinergic and BDNF signaling. Brain Res, 2019; 1712:188–96.
Jiang S-Y, Li H, Tang J-J, Wang J, Luo J, Liu B, Wang JK, Shi XJ, Cui HW, Tang J, Yang F. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat Commun, 2018; 9:5138.
Lee J, Jung E, Lee J, Kim S, Huh S, Kim Y, Kim Y, Byun SY, Kim YS, Park D. Isorhamnetin represses adipogenesis in 3T3-L1 cells. Obesity, 2009; 17:226–32.
Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet, 2007; 370:1829–39.
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab, 2011; 13:376–88.
Mustafa S, Ijaz MU, ul Ain Q, Afsar T, Almajwal A, Shafique H, Razak S. Isorhamnetin: a flavonoid, attenuated doxorubicin-induced testicular injury via regulation of steroidogenic enzymes and apoptotic signaling gene expression in male rats. Toxicol Res, 2022; 11(3):475–85.
Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J, 2013; 34:3478–90a.
Pandey S, Roy D, Shukla AK. Measuring surface expression and endocytosis of GPCRs using whole-cell ELISA. Methods Cell Biol, 2019; 149:131–40.
Razali N, Aziz AA, Lim CY, Junit SM. Investigation into the effects of antioxidant-rich extract of Tamarindus indica leaf on antioxidant enzyme activities, oxidative stress and gene expression profiles in HepG2 cells. Peer J, 2015; 3:e1292.
Reiner Ž. Combined therapy in the treatment of dyslipidemia. Fundam Clin Pharmacol, 2010; 24:19–28.
Runhuan F, Haiyan W. Quantitative proteomic analysis of Isorhamnetin treatment in human liver cancer cells. J Med Plant Res, 2018; 12:77–88.
Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, Gissi-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet, 2008; 372:1231–9.
Vogel RA. PCSK9 inhibition: the next statin? J Am Coll Cardiol, 2012; 59:2354–5.
Wang HH, Garruti G, Liu M, Portincasa P, Wang DQH. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol, 2017; 16:S27–42.
Wu MS, Chien CC, Chang J, Chen YC. Pro-apoptotic effect of haem oxygenase-1 in human colorectal carcinoma cells via endoplasmic reticular stress. J Cell Mol Med, 2019; 23:5692–704.
Xiao PT, Liu SY, Kuang YJ, Jiang ZM, Lin Y, Xie ZS, Liu EH. Network pharmacology analysis and experimental validation to explore the mechanism of sea buckthorn flavonoids on hyperlipidemia. J Ethnopharmacol, 2021; 264:113380.
Xu Y, Tang C, Tan S, Duan J, Tian H, Yang Y. Cardioprotective effect of isorhamnetin against myocardial ischemia reperfusion (I/R) injury in isolated rat heart through attenuation of apoptosis. J Cell Mol Med, 2020; 24(11):6253–62.
Xue Z, Song W, Gao X, Yu W, Hou X, Kou X. Structure identification and evaluation of chemical components from the flos sophorae immaturus for inhibitory effects against HepG2. Curr Top Nutraceutical Res, 2021; 19(4):452.
Yang HX, Zhang M, Long SY, Tuo QH, Tian Y, Chen JX, Zhang CP, Liao DF. Cholesterol in LDL receptor recycling and degradation. Clin Chim Acta, 2020; 500:81–6.
Yokozawa T, Kim HY, Cho EJ, Choi JS, Chung HY. Antioxidant effects of isorhamnetin 3, 7-di-O-β-D-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J Agric Food Chem, 2002; 50:5490–5.
Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science, 2009; 325:100–4.
Zhang S, Zheng L, Dong D, Xu L, Yin L, Qi Y, Han X, Lin Y, Liu K, Peng J. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem, 2013; 141:2108–16.
Zhao Z, Liu Y. Cardiovascular protective effect of isorhamnetin. Med Recapitulate, 2008; 14:2321–3.
Zhao J-J, Song J-Q, Pan S-Y, Wang K. Treatment with isorhamnetin protects the brain against ischemic injury in mice. Neurochemi Res, 2016; 41:1939–48.
Zhou JL, Fang XY, Wang JQ, Zhao LG, Li Y, Tang F, Yue YD. Structures and bioactivities of seven flavonoids from Osmanthus fragrans ‘Jinqiu’essential oil extraction residues. Nat Prod Res, 2018; 32(5):588–91.