INTRODUCTION
Epilepsy is a chronic non-communicable disease of the brain that affects people of all ages. People afflicted with epilepsy have three times higher risk for death than the general population (World Health Organization, 2022). The World Health Organization (WHO) is concerned with the low access to timely therapy for the patients with epilepsy in low- and middle-income countries (LMICs) and the social stigma posed by society to the families and patients.
In 2016, 24 million patients with idiopathic epilepsy and 45.9 million patients with all-active epilepsy have been registered in the world. This makes epilepsy one of the most common neurological disorders globally. Worldwide prevalence of active epilepsy increased with age. The global age-standardized mortality rate of idiopathic epilepsy is about 1.74 per 100,000. During 1990–2016, a non-significant change in the age-standardized prevalence of idiopathic epilepsy has been observed, but there was a significant decrease in age-standardized mortality rates (Beghi et al., 2019).
The disability-adjusted life years (DALYs) for patients with epilepsy raised by 13.8%, while age-standardized DALY rate decreased significantly from 1990 to 2017. The epilepsy burden was concentrated in males mainly in developing countries. Epilepsy poses higher burden among elderly people due to the worsening in quality of life and comorbidities (Bunschoten et al., 2020).
Treatment of epilepsy is well standardized and newer antiepileptic medicines have been introduced, although not many of them have added value, especially for patients that are resistant to therapy (Schmidt and Schachter, 2014). According to the estimations, around 70% of the patients could live seizure-free if diagnosed and treated properly. Therefore, studying the utilization and prices of antiepileptic medicines can help in access improvement and trend comparison. The total annual cost of epilepsy treatment is quite high (Melkamu et al., 2021), so the overall health care and social services costs lead to a high rate of health expenditures (Ostendorf and Gedela, 2017). A systematic review found that indirect costs for treatment of epilepsy ranged between 12% and 85% of the total annual costs (Strzelczyk et al., 2008). A study exploring affordability and prices of antiepileptic medicines in 46 countries found that in LMICs, availability and affordability of antiepileptic products are insufficient and create a barrier for timely patient therapy (Cameron et al., 2012).
The aim of our study is to test the hypothesis if the entrance of generics affects medicines utilization and price variations analyzing generics availability, prices, and utilization of antiepileptic medicines in Bulgarian market.
MATERIALS AND METHODS
Design of the study
We performed a three-step retrospective, observational analysis of antiepileptic medicines reimbursed by the National Health Insurance Fund (NHIF) in Bulgaria during the period 2014–2020. The study focuses on 16 international nonproprietary names (INNs) included in positive drug list (PDL) and belonged to anatomical therapeutic chemical codes N03AB, N03AE, N03AD, N03AF, N03AG, and N03AX.
In its first part, we compare the number of reimbursed generics and trademarks aiming to find a potential relationship between generics availability, trademarks variations, and their impact on prices and utilization.
The second part of the study examines the differences in reference price per defined daily dose (DDD) of each individual INN. The reference price per DDD (lowest one) has been extracted from the official public register of the National Council on Pricing and Reimbursement of Medicinal Products (NCPRMP) in Bulgaria twice a year (in January and in December). The aim of the second part is to find whether the price variations have been affected by the availability of new generic medicinal products and/or trademarks.
Finally, the medicines utilization per INN as per defined daily doses per 1,000 inhabitants per day (DDD/1,000 inh/day) has been calculated. The calculations are based on a formula proposed by WHO (2017):
The changes in utilization were tested with Mann–Whitney test for statistically significant differences between 2014 and 2020. p-values less than 0.05 have been determined as statistically significant.
Data sources
The number of reimbursed and dispensed packages of observed INNs has been extracted by the NHIF official register (NHIF database, 2014–2020). Non-published data for the period 2014–2017 have been provided by NHIF for study purposes after a written request for public access. All other data are publicly accessible and there is no need of individual permission. There is also no need of ethical approval because the data are not provided per patient but per INN of medicines.
The reference price per DDD (also defined as the lowest reimbursed price paid by NHIF) and the number of generics have been extracted from Annex 1 of PDL “Medicinal Products for Outpatient Use” officially published on the website of the NCPRMP (NCPRMP database, 2014–2020).
The official database of the National Statistical Institute (NSI) (NSI database, 2014–2021) has been used to define the number of the inhabitants of the country. According to the data, the total population in Bulgaria was as follows: 7,202,198 in 2014; 7,153,784 in 2015, 7,101,859 in 2016; 7,050,034 in 2017; 7,000,039 in 2018; 6,951,482 in 2019; and 6,924,882 in 2020.
RESULTS
Availability of antiepileptic generic medicines
The number of reimbursed generics, trademarks, and pharmaceutical forms considering each individual INN has been examined and illustrated at the beginning and at the end of the observed period (Table 1).
During the study period, four INNs have been excluded from the PDL (tiagabine, retigabine, phenytoin, and clonazepam), while one new INN is included (brivaracetam). Overall number of trademarks declined significantly from 107 to 81. New generics in three INNs (eslicarbazepine, pregabalin, and lacosamide) entered the market after expired patent protection. After the patent expiry, the dynamic of inclusion of new generic products in PDL is significant in INN pregabalin where the number of trademarks rises to 16, and in INN lacosamide the number increases to 12.
Reference price per DDD
The reference price per DDD is based on the lowest retail price of the medicinal product within INN grouped by active substance and pharmaceutical form, according to Bulgarian legislation. It is used as a basis for calculation of reimbursed value paid by NHIF for each individual trade name. The difference between reference prices per DDD has been examined twice a year in order to establish the main changes during the observed period (Table 2). Four of the examined INNs reimbursed in Bulgaria have been presented in both liquid and solid dosage forms. Variations in the reference price per DDD have been examined separately for the different pharmaceutical forms belonging to the same INN as the way they are grouped in PDL according to the legislation.
Comparison among INNs available in 2014 and 2020 reveals that a total of 11 INNs have changed their reference prices. The decreasing rate is found for nine INNs, whereas the increasing is registered in two of them (Fig. 1).
The study findings reveal the highest decreasing rate in pregabalin (92%), followed by eslicarbazepine (70%) and lacosamide (64%). The number of newly approved generics and brand names reimbursed for outpatient use is the highest within the group of pregabalin and lacosamide which resulted in significant reference price declining.
Utilization of antiepileptic medicines
A decreasing consumption was found in most of the INNs during 2014–2020 (Table 3). The utilization of oxcarbazepine, levetiracetam, pregabalin, and lacosamide slightly increases.
Valproic acid is the INN with the highest utilization (2.4492 DDD/1,000 inh/day in 2014 and 1.1438 DDD/1,000 inh/day in 2020), followed by oxcarbazepine (1.0539 DDD/1,000 inh/day in 2014 and 0.8981 DDD/1,000 inh/day in 2020). According to pharmacotherapeutic recommendations approved in Bulgaria, both products are considered as a first line monotherapy for generalized or focal epilepsies. The other INNs recommended also as a first line therapy include carbamazepine (focal epilepsy), ethosuximide (generalized epilepsy), lamotrigine, levetiracetam, and topiramate (generalized or focal epilepsies). The results reveal that carbamazepine (0.6046 DDD/1,000 inh/day in 2014 and 0.4911 DDD/1,000 inh/day in 2020), levetiracetam (0.4846 DDD/1,000 inh/day in 2014 and 0.619 DDD/1,000 inh/day in 2020), lamotrigine (0.1745 DDD/1,000 inh/day in 2014 and 0.1688 DDD/1,000 inh/day in 2020), and topiramate (0.1201 DDD/1,000 inh/day in 2014 and 0.099 DDD/1,000 inh/day in 2020) are also widely used in Bulgaria during the study period.
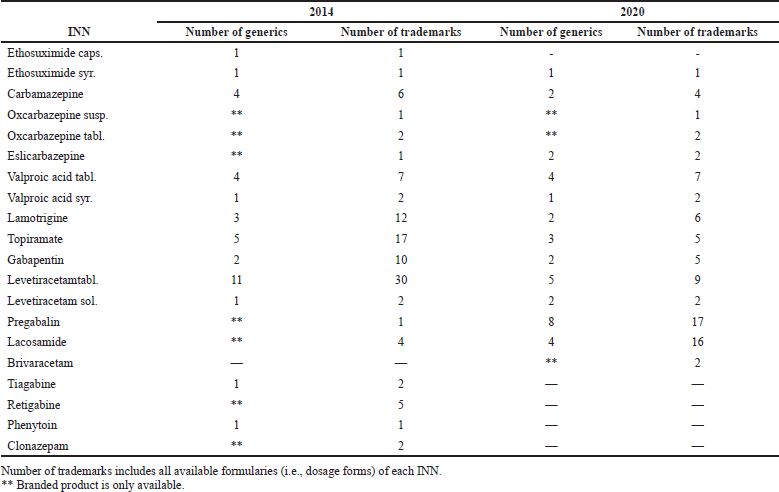 | Table 1. Number of generics and trademarks included in PDL in 2014 and in 2020. [Click here to view] |
The applied Mann–Whitney test shows that there are no statistically significant differences (p ? 0.05) in utilization between 2014 and 2020.
DISCUSSION
Generics medicines approval and their market entry could affect prescription habits, market share, and patients’ access to therapy. The impact of the generic medicines’ availability on the utilization and price changes of antiepileptic medicines is rarely studied, and according to our knowledge, no similar study has been performed in Bulgaria. The results reveal that the overall number of trademarks paid by NHIF has decreased with 26, whereas the number of INNs has decreased with 4 during 2014–2020. At the same time, total utilization has decreased from 5.33 to 3.93 DDD/1,000 inh/day. We also found that generics entrance within the group of pregabalin, lacosamide, and eslicarbazepine leads to a decrease of the reference price per DDD (with 90%, 64%, 70%, respectively) and to increasing utilization (from 0.04 to 0.2211 DDD/1,000 inh/day, from 0.03 to 0.05 DDD/1,000 inh/day, and to 0.0125 DDD/1,000 inh/day, respectively). The declining number of generics resulted in lower medicines consumption, despite the value of reference price per DDD decreasing. This might be commented as a risk for the irrational prescribing and utilization.
A similar study exploring utilization of antiepileptics in Morocco reveals a decrease of the average cost per DDD and an increase of the consumption during 2008–2018. This fact is mainly due to inclusion of several new molecules, declining of medicines prices during 2014, and the generic drugs policy. Finally, the consumption has increased with 45%, while price decrease is about 30% (Cherkaoui et al., 2022).
The utilization of antiepileptic medicines depends on prescribing patterns, pharmacotherapeutic recommendations, and disease epidemiology. The most often prescribed medicines in Bulgaria are valproic acid (2.4869 DDD/1,000 inh/day), oxcarbazepine (1.053 DDD/1,000 inh/day), and carbamazepine (0.604 DDD/1,000 inh/day) in 2014 and valproic acid (1.1748 DDD/1,000 inh/day), oxcarbazepine (0.8981 DDD/1,000 inh/day), and levetiracetam (0.6619 DDD/1,000 inh/day) in 2020. The highest rate is also found for lamotrigine (0.1745 DDD/1,000 inh/day in 2014 and 0.1688 DDD/1,000 inh/day in 2020) and topiramate (0.1201 DDD/1,000 inh/day in 2014 and 0.099 DDD/1,000 inh/day in 2020). These results could be explained by pharmacotherapeutic recommendations as the aforementioned products are considered as a first line therapy in focal and generalized epilepsy. The results confirm the compliance of clinical practice with the official recommendations approved in Bulgaria.
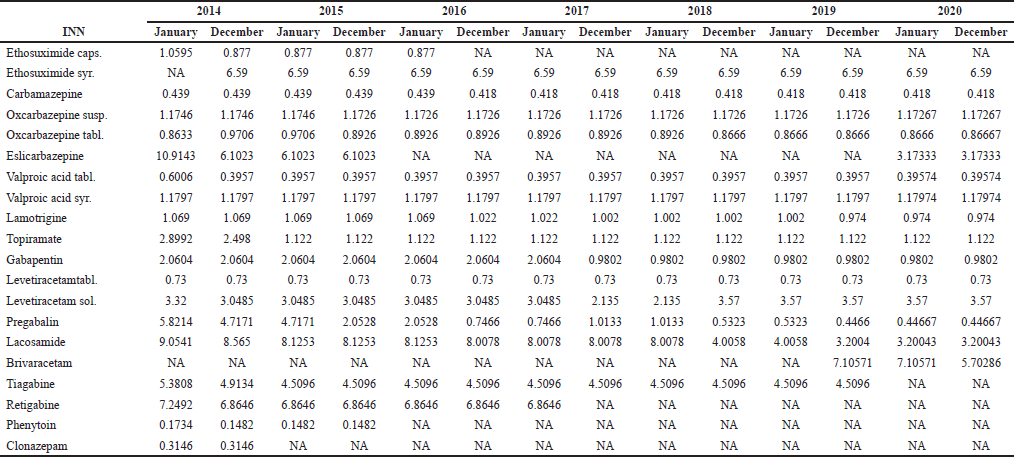 | Table 2. Reference price per DDD of antiepileptics during 2014–2020. [Click here to view] |
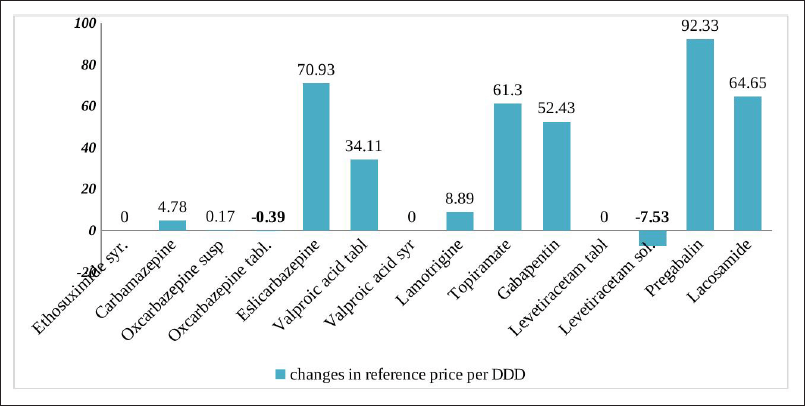 | Figure 1. Changes in reference price per DDD (%) compared in 2014 and in 2020. [Click here to view] |
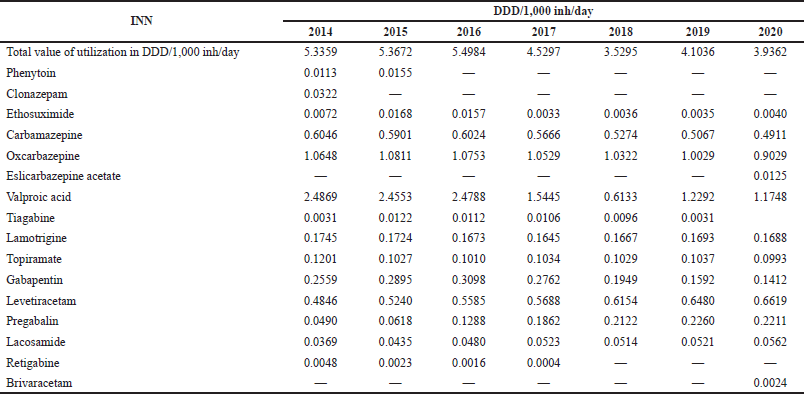 | Table 3. Utilization of antiepileptic in DDD/1,000 inh/day during 2014–2020. [Click here to view] |
The Australian report reveals a similar trend as those found in our study. Valproic acid was the most commonly dispensed antiepileptic medicine (24% of all antiepileptic prescriptions filled), followed by levetiracetam (22%) in 2019–2020 (Australian Institute of Health and Welfare, 2022). The conventional AEDs remained a main treatment choice in Israel with relatively high use of older medicines during 2010–2014 (Berman et al., 2016).
Valproic acid (28.7%), levetiracetam (19.1%), and phenytoin (16.9%) are the most often used monotherapies in Taiwan during 2016 (Liang et al., 2022). There were similar results to ours published in Belgium whereas, as a first choice of therapy, valproate and carbamazepine were preferred. The newer generation medicines such as lamotrigine, levetiracetam, topiramate, and oxcarbazepine were used most often as second line choices for epilepsy treatment. In the absence of reimbursement restrictions, lamotrigine and oxcarbazepine would be more frequently prescribed (Boon et al., 2008).
The main barriers leading to poor access to AEDs in Europe include high medicine prices, application of reimbursement restrictions, and regulatory approval. It resulted in restricted access, particularly to new medicines, and also it led to different availability across European countries. As the reimbursement policies ranged from full reimbursement to complete lack of similar policy, the availability of antiepileptics is higher in countries with public reimbursement policy (Baftiu et al., 2015).
The antiepileptic medicines are fully reimbursed in the following European countries: Bulgaria, Czech Republic, Latvia, Slovenia, and Estonia. Therefore, we might assume that there is a better affordability of these medicines for patients in such countries with high reimbursement rate. There are also existing reimbursement restrictions in some countries based on a patient’s age, brand, dosage of a particular medication, etc. Generic prescription of antiepileptics is introduced in most countries by developing generic policy (J?drzejczak et al., 2013) which could also increase the generics consumption and market share of antiepileptic medicines (Braoudaki et al., 2017). Prescription of generic antiepileptics could provide reduction in public expenditures and a great number of patients would successfully switch to generic antiepileptic formulations (Shaw and Hartman, 2010). The requirement imposed by Bulgarian legislation for reimbursement of the lowest price per DDD is a form of generics-oriented policy and could be considered such that the generic policy is also valid for the country.
In Croatia, the share of generic drugs compared to the total drug utilization has decreased by 32% measured by DDD/1,000/day (N03AE-AX) during 2001–2010. The study finds 6.49 DDD/1,000 inh/day overall consumption in 2010 with prevalence rate of generics. The total cost for psychopharmaceuticals has increased by 20.1%, as the number is more visible for branded medicines than the generics (32.7% vs. 7.4%). The authors have concluded that generic policy needs further development (Poli?-Vižintin et al., 2014).
In Bulgaria, the number of reimbursed generics decreased substantially during the observational 7-year period. A direct relationship between medicines availability, prices, and utilization could not be concluded. The study found decreasing and stable prices of antiepileptic medicines during the observed period due to the applied mechanism for external reference pricing in Bulgaria (Ministry of Health, 2021). The price decrease was accompanied by the reduced use and withdrawal procedures. The main reason could be explained such that medicines utilization depends on other factors such as deviations in number of patients, dosage, and type of prescribed medicines. A longer market observation is needed in order to explore the tendencies and main factors affecting them.
The main limitation of this study is the lack of epidemiological data about the total number of patients. Information is available only for the patients and antiepileptic medicines subject to reimbursement. An official register for patients with epilepsy does not exist in Bulgaria which makes comparison and summary of medicines utilization quite difficult.
CONCLUSION
The 7-year review of antiepileptic medicines placed on the market in Bulgaria showed a tendency of decrease in the number of generics and consumption despite the reduction in reference prices per DDD. This fact indicated that different factors and the country’s environment also played an important role, and have affected the overall medicines market. A comprehensive generic policy should be developed in the country to ensure timely patient access to treatment and stable level of medicines utilization.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design of the work; acquisition, analysis, and interpretation of data; and drafting the manuscript and revising it critically, and they have approved the final version of it.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Australian Institute of Health and Welfare. Epilepsy in Australia. Australian Government 2022. Available via https://www.aihw.gov.au/reports/chronic-disease/epilepsy-in-australia/contents/treatment-and-management-of-epilepsy
Baftiu A, Johannessen Landmark C, Nikaj V, Neslein IL, Johannessen SI, Perucca E. Availability of antiepileptic drugs across Europe. Epilepsia, 2015; 56(12):e191–7; doi:10.1111/epi.13210.
Beghi E, Giussani G, Nichols E, Abd-Allah F, Abdela J, Abdelalim A, Beghi E, Giussani G, Abd-Allah F, Abdela J, Abdelalim A, Abraha HN, Adib MG, Agrawal S, Alandab F, Awasthi A, Ayele Y, Barboza MA, Belachew AB, Biadgo B, Bijani A, Bitew H, Carvalho F, Chaiah Y, Daryani A, Huyen Phuc Do HPD, Dubey M, Endries AYY, Eskandarieh S, Faro A, Farzadfar F, Fereshtehnejad S-M, Fernandes E, Fijabi DO, Filip I, Fischer F, Gebre AK, Tsadik AG, Gebremichael TG, Gezae KE, Ghasemi-Kasman M, Weldegwergs KG, Degefa MG, Gnedovskaya Elena V, Hagos TB, Haj-Mirzaian A, Haj-Mirzaian A, Hassen HY, Hay Simon I, Jakovljevic M, Kasaeian A, Kassa TD, Khader YS, Khalil I, Khan EA, Khubchandani J, Kisa A, Krohn KJ, Kulkarni C, Nirayo YL, Mackay MT, Majdan M, Majeed A, Manhertz T, Mehndiratta MM, Mekonen T, Meles HG, Mengistu G, Mohammed S, Naghavi M, Mokdad AH, Mustafa G, Irvani SSN, Long Hoang Nguyen LHN, Nichols E, Nixon MR, Ogbo FA, Olagunju AT, Olagunju TO, Owolabi MO, Philips MR, Pinilla-Monsalve GD, Qorbani M, Radfar A, Rafay A, Rahimi-Movaghar V, Reinig N, Sachdev PS, Safari H, Safari S, Safiri S, Sahraian MA, Samy AM, Sarvi S, Sawhney M, Shaikh MA, Sharif M, Singh G, Smith M, Szoeke Cassandra E, I, Tabares-Seisdedos R, Temsah M-H, Temsah O, Tortajada-Girbes M, Bach Xuan Tran BXT, Tsegay AAT, Ullah I, Venketasubramanian N, Westerman R, Winkler AS, Yimer EM, Yonemoto N, Feigin VL, Vos T, Murray CJL. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the global burden of disease study. Lancet Neurol, 2019; 18(4):357–75; doi: 10.1016/S1474-4422(18)30454-X
Berman E, Marom E, Ekstein D, Blatt I, Eyal S. Utilization of antiepileptic drugs in Israel. Epilepsy Behav, 2016; 61:82–5; doi: 10.1016/j.yebeh.2016.05.004.
Boon P, Dejonghe P, Legros B, Sadzot B, van Rijckevorsel K, Schmedding E. Impact of reimbursement restrictions on the choice of antiepileptic drugs: Belgian Study on Epilepsy Treatment (BESET). Seizure, 2008; 17(4):350–7; doi: 10.1016/j.seizure.2007.11.005.
Braoudaki E, Naoum V, Karampli E, Athanasakis K, Kyriopoulos J. Generics policies: a systematic review of their effectiveness. Value Health, 2017; 20(9):A662; doi:10.1016/j.jval.2017.08.1595.
Bunschoten JW, van der Palen J, Sander JW, Thijs RD. Medication burden in epilepsy: exploring the impact of non-epilepsy concomitant drugs load. Seizure, 2020; 81:104–10; doi:10.1016/j.seizure.2020.07.017.
Cameron A, Bansal A, Dua T, Hill SR, Moshe SL, Mantel-Teeuwisse AK, Saxena S. Mapping the availability, price, and affordability of antiepileptic drugs in 46 countries. Epilepsia, 2012; 53(6):962–9; doi: 10.1111/j.1528-1167.2012.03446.x.
Cherkaoui G, Cheikh A, Razine R, Bnouhanna W, Regragui W, Benomar A, Cherrah Y. Trends in the consumption and cost of antiepileptics in Morocco between 2008 and 2018. Rev Epidemiol Sante Publique, 2022; 70(2):75–81; doi: 10.1016/j.respe.2022.01.127.
J?drzejczak J, Marusic P, Haldre S, Majkowska-Zwoli?ska B, Bojinova-Tchamova V, Mameniskiene R, Mindruta I, Ravnik IM, Szupera Z, Sykora P, Verzbickis A, Daniluk J. Current status of epilepsy health care for adult patients from central and eastern European Union countries: a survey of members of the Central Europe Epilepsy Experts Working Group. Seizure, 2013; 22(6):452–6; doi: 10.1016/j.seizure.2013.03.001.
Liang CY, Chiang KL, Hsieh LP, Chien LN. Prescription patterns and dosages of antiepileptic drugs in prevalent patients with epilepsy in Taiwan: a nationwide retrospective cross-sectional study. Epilepsy Behav, 2022; 126:108450; doi: 10.1016/j.yebeh.2021.108450.
Melkamu P, Animut Y, Minyihun A, Atnafu A, Yitayal M. Cost of illness of epilepsy and associated factors in patients attending adult outpatient department of University of Gondar Referral Hospital, Northwest Ethiopia. Risk Manag Healthc Policy, 2021; 4(14):2385–94; doi: 10.2147/RMHP.S289113
Ministry of Health. Ordinance on the terms, rules and procedure for regulation and registration of prices for medicinal products. Effective as from 30 April 2013; amended and supplemented SG N. 28 of 6 April 2021.
National Health Insurance Fund. Reports on number of patients and reimbursement amount. Available via https://www.nhif.bg/page/218 (Accessed August 2022).
National Council on Prices and Reimbursement of Medicinal Products. Registry archive during 2014–2020. Available via https://portal.ncpr.bg/registers/pages/register/archive.xhtml
National Statistical Institute of Bulgaria. Population by districts, place of residence and gender. Available via https://www.nsi.bg/bg/content/2975/.
Ostendorf AP, Gedela S. Effect of epilepsy on families, communities, and society. Semin Pediatr Neurol, 2017; 24:340–7; doi: 10.1016/j.spen.2017.10.007.
Poli?-Vižintin M, Štimac D, Šostar Z, Ingrid Tripkovi?. Distribution and trends in outpatient utilization of generic versus brand name psycho-pharmaceuticals during a ten-year period in Croatia. BMC Health Serv Res, 2014; 14:343; doi: 10.1186/1472-6963-14-343.
Schmidt D, Schachter SC. Drug treatment of epilepsy in adults. BMJ, 2014; 348:g254; doi: 10.1136/bmj.g254.
Shaw SJ, Hartman AL. The controversy over generic antiepileptic drugs. J Pediatr Pharmacol Ther, 2010; 15(2):81–93.
Strzelczyk A, Reese JP, Dodel R, Hamer HM. Cost of epilepsy: a systematic review. Pharmacoeconomics, 2008; 26(6):463–76; doi: 10.2165/00019053-200826060-00002.
WHO. Collaborating Centre for Drug Statistics Methodology Guidelines for ATC classification and DDD assignment 2018. World Health Organization, Oslo, Norway, 2017. Available via https://www.whocc.no/atc_ddd_index_and_guidelines/guidelines/ (Accessed August 2022).
World Health Organization. Epilepsy. Key facts. 2022. Available via https://www.who.int/news-room/fact-sheets/detail/epilepsy (Accessed June 2022).