INTRODUCTION
Creams are semisolid dosage forms prepared for external use in microbes, plants, animals, and humans. They are usually applied to the skin and also mucous membranes such as the rectum or vagina. They are usually semisolid emulsions or viscous liquids with opaque appearances. Their rheological character and consistency depend on the emulsion type, oil-in-water (o/w) or water-in-oil (w/o) type, and on the type of the solids in the dispersed phase (Idson and Lazarus, 2009; Rai et al., 2019) and work at critical micellar concentrations. They are topical preparations that are usually used for cosmetic purposes or therapeutic use. Topical delivery is the skin application of formulations that contain active pharmaceutical ingredients to directly treat disorders of the skin or the skin manifestations of a general disease (e.g., psoriasis) (Chauhan and Gupta, 2020). Various medicaments have been added to creams to achieve therapeutic or prophylactic effects, and they include antimicrobials (Deepika and Singh, 2017; Okafo et al., 2021; Pal et al., 2013), antibacterials/anti-inflammatories (Sekar and Jalil, 2017), analgesics/anti-inflammatories (Bolla et al., 2020), antiaging medicaments (Moldovan et al., 2017), and antioxidants (Hartiadi and Sahamastuti, 2020). The incorporated medicaments may be of synthetic or herbal origin.
Medicinal plants are of great use in traditional or herbal medicine and as a precursor of many modern medicines (Emencheta et al., 2019; Odeh and Tor-Anyiin, 2014). This is because of the presence of many secondary metabolites in medicinal plants. Plant parts such as leaves, fruits, seeds, stems, roots, and barks or whole plants are used for healing purposes to cure different human or other animal diseases or as prophylactics (Mba-Omeje et al., 2017). Recently, there has been a rise in the usage of herbal medicines. This is because of the belief that herbal medicines have little or no side effects, unlike orthodox medicines. They are readily available and usually affordable. Different plant species have been screened to substantiate the traditional claim of their effectiveness as therapeutic agents.
Pterocarpus santalinoides L’Herit. ex DC, family Leguminosae, is a tree that can grow up to a height of 9–12 m and a diameter of 1 m with low, straggling branches (Emencheta et al., 2019). It provides a shade tree that grows along the riverine forests of Africa and tropical South America (Emencheta et al., 2019; Odeh and Tor-Anyiin, 2014). In Nigeria, the plant is used for its medicinal and food values. Soups are prepared from tender and fresh leaves (Ogbonna and Idumah, 2018). The leaves, seeds, fruits, and stem bark are used in treating different ailments. The plant is used in treating gastrointestinal diseases, skin diseases, malaria, diabetes, etc. (Emencheta et al., 2019; Nworu et al., 2009; Odeh and Tor-Anyiin, 2014). The antimicrobial (Emencheta et al., 2019; Odeh and Tor-Anyiin, 2014) and antioxidant (Akaniro-Ejim et al., 2018) activities of extracts from several plant parts have been studied. Escherichia coli, Salmonella typhi, Staphylococcus aureus, Proteus mirabilis, and Candida albicans were susceptible to the antibacterial properties of butanol, ethanol, and the aqueous extracts of P. santalinoides, but Streptococcus pyogenes and Pseudomonas aeruginosa were resistant (Odeh and Tor-Anyiin, 2014). Emencheta et al. (2019) reported the antibacterial potentials of the different plant parts against S. typhi and E. coli but negligible antifungal activities. Most of the research works on the antimicrobial potentials of P. santalinoides were conducted using the leaves, barks, and stems, but very little has been done using the seeds.
This study was carried out to formulate the methanol extract of P. santalinoides seeds into creams and to determine the physicochemical and antimicrobial potentials of the prepared creams.
MATERIALS AND METHODS
Materials
The materials used were as follows: methanol (JHD, Guangdong Guanghua Chemical Factory Co. Ltd., China), liquid paraffin (Niram Chemicals, India), nutrient agar, Sabourand dextrose agar (Titan Biotech, India), Mueller-Hinton agar (MHA) (Titan Biotech, India), nutrient broth, propylparaben (Kermel, India), methylparaben (Central Drug House, India), olive oil (Goya), Tween 80 (Guangdong Guanghua Sci-Tech Co. Ltd., Guanghua, China), Span 80 (Guangdong Guanghua Sci-Tech Co. Ltd., Guanghua, China), cetostearyl alcohol, glycerin (Merck Schuchardt OHG, Hohenbrunn, Germany), ketoconazole, and gentamicin.
Organisms
Staphylococcus aureus, P. aeruginosa, Bacillus subtilis, E. coli, Aspergillus niger, and C. albicans were collected from the Department of Pharmaceutical Microbiology and Biotechnology’s laboratory, Delta State University, Abraka.
Collection and identification of plant material
Pterocarpus santalinoides seeds were obtained from a farm in Enugu, Nigeria. The plant was identified by Mr. Felix Nwafor, a taxonomist in the Department of Pharmacognosy and Environmental Medicine, University of Nigeria, Nsukka, Nigeria. It was issued a voucher number, PCG/UNN/0036.
Extraction of P. santalinoides seeds
Pterocarpus santalinoides seeds were dried under shade and ground to a powder. A 200 g powder was macerated for 48 hours in 400 ml of methanol. It was filtered using a Whatman filter paper, and the filtrate was concentrated to semisolid form at 60°C for 6 hours using a rotary evaporator (Rotavapor RII, Büchi Labortechnik AG, Flawil, Switzerland). It was concentrated until a constant weight to ensure complete evaporation of methanol.
Physicochemical properties of the extract
The extract’s organoleptic properties such as color and odor were observed. The extract’s pH was also measured.
Phytochemical screening
The extract was examined for the absence or presence of phytochemicals such as alkaloids, saponins, flavonoids, terpenoids, and phenols using standard methods as stated in the literature (Biswas et al., 2013; Mba-Omeje et al., 2017; Onyekere et al., 2019; Trease and Evans, 2002. Phytochemicals were also determined quantitatively using the methods of Ezeonu and Ejikeme (2016) and Khalifa et al. (2017).
Antimicrobial assay of the methanol extract of P. santalinoides seeds
This was conducted using the agar well diffusion technique. MHA was prepared following the manufacturer’s specifications. It was autoclaved, and 20 ml of it was aseptically transferred into each Petri dish. These were then allowed to solidify. The extract was two-fold serially diluted to obtain the following concentrations: 100, 50, 25, 12.5, 6.25, and 3.125 mg/ml. The bacteria were spread on the agar in the Petri dishes to inoculate them. Wells were bored into each agar plate using a sterile cork borer. The different concentrations of the extract were transferred into the respective wells and labeled appropriately. Gentamicin was used as the positive control (40 mg/ml) and was transferred to the well at the center. The plates were incubated for 24 hours at a temperature of 37°C. The inhibition zone diameters (IZDs) were measured in millimeters. The process was repeated using SDA and fungal isolates. The incubation was done for 48 hours at room temperature. Ketoconazole (20 mg/ml) was used as the positive control. The IZDs were measured in millimeters.
Determination of minimum inhibition concentration (MIC)
The method of Okafo et al. (2020) was used. The two-fold serial dilutions (1 ml) of the extract were transferred into respective labeled Petri dishes. A 19 ml quantity each of molten MHA for test bacteria and molten SDA for test fungi were transferred into the labeled Petri dishes. The agars in the Petri dishes were allowed to solidify, and the respective test organisms were streaked on them. The Petri dishes containing the bacteria were incubated for 24 hours at 37°C, and those containing the fungi were incubated for 48 hours at room temperature. The MIC of the extract for the test organisms was determined.
Preparation of creams
Pterocarpus santalinoides creams were prepared using the method of Hartiadi and Sahamastuti (2020) according to the formula shown in Table 1. About 7.15 g of Tween 80 was mixed with 10 g of glycerin in a beaker using a magnetic stirrer with a hot plate. Then, 0.54 g of methylparaben and 0.06 g of propylparaben were added to the Tween 80/glycerin mixture and stirred properly at 70°C using the magnetic stirrer with a hot plate. A 2.5 g quantity of the P. santalinoides methanol extract was dissolved in 58.9 g of distilled water and was added to the Tween 80/glycerin mixture. The mixture was stirred using the magnetic stirrer with a hot plate at 70°C to form the aqueous phase. The oil phase was produced by melting 8 g of cetostearyl alcohol in a water bath at 70°C. A 10 g quantity of olive oil or liquid paraffin and a 2.85 g quantity of Span 80 were added and stirred properly with a glass rod. The oil phase was added gradually to the aqueous phase and stirred using a magnetic stirrer at a temperature of 70°C for 5 minutes. The stirring continued until the mixture cooled. The cream was transferred into a container and was stored in a cool place. This was repeated for all the formulations using the formula in Table 1.
Evaluation of creams
Physical appearance
The organoleptic properties like color, odor, and phase separation were noted.
pH
The pH of the creams was determined using a pH meter (Hanna Instruments, India).
Spreadability
The method of Kaushik et al. (2020) was used with slight modifications. Spreadability was evaluated by placing 0.5 g of the cream within a premarked circle of a 1 cm diameter on a glass plate. A second glass plate was placed over the first plate, and a 300 g weight was kept on the upper glass plate for 5 minutes. The weight caused the cream to spread, and this resulted in an increase in the diameter of the circle. The increase was measured in centimeters, and the results were taken as comparative values for spreadability.
Homogeneity
The creams were viewed under a microscope to examine their homogeneity (Hartiadi and Sahamastuti, 2020). Homogeneity was also examined by visual inspection (Okafo et al., 2019; Ubaid and Murtaza, 2016) and by pressing the cream between the thumb and index finger (Chen et al., 2016).
Ease of removal
This was evaluated by washing off the cream applied to a specific part of the body with flowing tap water (Ashish et al., 2013; Sekar and Jalil, 2017).
Viscosity
The viscosity of the creams was evaluated with a Brookfield viscometer at a temperature of 28°C. Spindle 4 was inserted in the creams and was rotated at 6, 12, 30, and 60 rpm, respectively. The displayed readings were recorded.
Centrifugation test
A 2 g quantity of the sample was put in the centrifuge (Remi Elektrotechnik Ltd., Vasai, India) and was rotated for 30 minutes at 3,000 rpm at room temperature. The creams were evaluated for phase separation at the end of the centrifugation (Chandrasekar et al., 2018).
Extrudability
Extrudability was based upon the quantity in the percentage of cream extruded from the tube on the application of a certain load. Collapsible lacquered aluminum tubes with 10 mm openings were filled with the respective formulations. A 1 kg load was placed on each tube, and the quantity of the formulation that was extruded 60 seconds after each of the tubes was opened was recorded (Fauzee and Walker, 2020; Ilievska et al., 2016; Khan et al., 2020; Ugandar and Deivi, 2013). The extrudability of the formulations was calculated using the following equation (Asija et al., 2015):
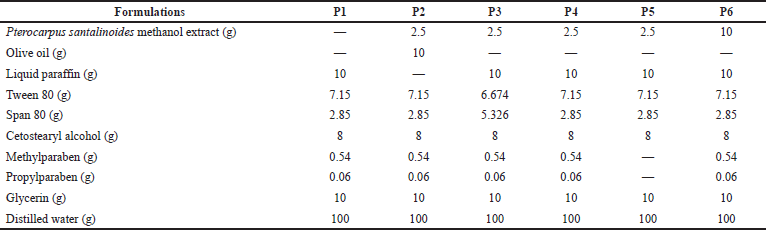 | Table 1. Composition of P. santalinoides creams. [Click here to view] |
1
Determination of cream type
This was done using a dye test (Ordu et al., 2018; Swabrick et al., 2005) and dilution tests (Swabrick et al., 2005). A scarlet red dye was mixed with the creams. A drop of the cream was placed on a microscope slide, covered with a cover slip, and then examined under a microscope. Observations of the color of the dispersed globules and the background were made. The dilution test was conducted by adding 10 ml of distilled water to 1 g of the cream in a beaker. It was stirred very well and was observed for separation.
Antimicrobial susceptibility test of the creams
The antimicrobial activity of the creams was evaluated using the disc diffusion method. MHA and SDA were prepared, sterilized, and transferred into Petri dishes, where they were allowed to solidify. They were inoculated with the test bacteria and fungi, respectively, using sterile cotton swabs. Sterile blank discs were impregnated individually with the respective cream formulations and were placed onto the inoculated Petri dishes. The Petri dishes containing bacteria were inverted and incubated for 24 hours at 37°C while those that contained fungi were incubated at 25°C for 48 hours. The antimicrobial activity was evaluated by measuring the IZDs to the nearest millimeter (Sekar and Jalil, 2017).
Skin irritation test
The Research and Ethical Committee of the Faculty of Basic Medical Sciences of Delta State University, Abraka, Nigeria, issued an ethical clearance (Resolution No. REC/FBMS/DELSU/21/98) for this study before it was carried out. Male Wistar rats were used for this study. The Wistar rats were divided into four groups, namely, control, standard, test group 1, and test group 2. The hairs in the dorsal region of the rats were removed a day prior to the start of the study. Formalin (0.8%), a standard irritant, was applied to the standard group after 24 hours of shaving the rat’s skin. Formulations P3 and P6 were applied to test groups 1 and 2, respectively. Distilled water was applied to the control group. The rats were observed for any irritation such as erythema or edema at the end of 24 hours (Khullar et al., 2012; Shaik et al., 2019).
Accelerated stability testing
The method of Hartiadi and Sahamastuti (2020) was used. The creams were subjected to three cycles of the freezing and thawing test. The freezing was carried out in a refrigerator at 4°C for 24 hours, and the thawing was carried out in an oven set at 40°C for 24 hours to complete a cycle. This was done for three cycles, after which the creams were reexamined for organoleptic properties, pH, homogeneity, centrifugation, and viscosity.
Statistical analysis
All experiments were carried out in triplicate for the validity of the statistical analysis and the results were expressed as mean ± SD. The statistical analysis was done using Microsoft Excel and IBM SPSS 23 software. Differences between means were determined with a one-way analysis of variance (ANOVA) at a significance level of p < 0.05.
RESULTS
Extraction and phytochemical screening
The percentage yield of the methanol extract was 10.1% w/w. The extract was dark brown in color with a honey-like odor. The pH of the extract was 5.1. Phytochemical screening of the plant extract was done to ascertain the presence of alkaloids, saponins, terpenoids, flavonoids, and phenols (Table 2). This was evident by the color change when the reagents were added. The quantitative analysis results (Table 2) also indicated the concentrations of the different phytochemical constituents contained in the extract.
Antimicrobial screening of methanol extract of P. santalinoides
The result of the antimicrobial susceptibility test (Table 3) revealed that the methanol extract of P. santalinoides at a 100 mg/ml concentration and above had antimicrobial activity against all the microorganisms studied.
Physicochemical evaluation of methanol extract of P. santalinoides seeds creams
The creams’ physicochemical properties are shown in Table 4. Creams were of varying colors: white (P1), light brown (P2), cream (P3, P4, and P5), and brown (P6). Their pH was between 5.0 ± 0.29 and 5.5 ± 0.17. All the creams had good homogeneity on visual and microscopic inspection. Palpable masses were absent when the creams were felt between the index finger and the thumb. All the creams were easily washed off from the applied skin surface by flowing tap water. The creams were of the o/w type. Upon centrifugation, formulations P1 and P3 were noted to be stable but other formulated creams separated. Formulation P2 cracked and could not reform to a normal cream upon stirring, indicating permanent instability. Formulations P4, P5, and P6 when stirred returned to normal creams; therefore, their instability was temporary (creaming). The formulated creams had good spreadability, though formulations P1 and P4 were less spreadable than the rest. The formulated creams were easily extruded from collapsible tubes (% extrudability of 66.33 ± 0.70 to 90.95 ± 1.46%). All the creams showed pseudoplastic flow (Fig. 1). They displayed shear-thinning behavior; the viscosity decreased as shear stress (spindle speed) increased. The creams’ viscosity was between 1,240 and 48,950 mPas. There was skin irritation among the rats in the control group but none among those rats in the standard and test groups (Fig. 2).
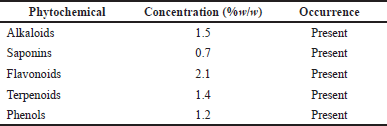 | Table 2. Phytochemical constituents of P. santalinoides methanol extract. [Click here to view] |
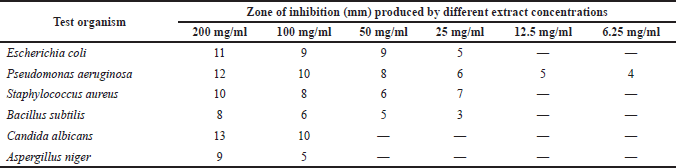 | Table 3. Antimicrobial susceptibility of test organisms to methanol extract of P. santalinoides. [Click here to view] |
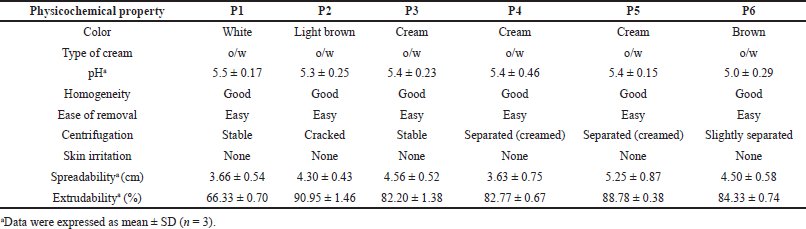 | Table 4. Physicochemical parameters of formulated creams. [Click here to view] |
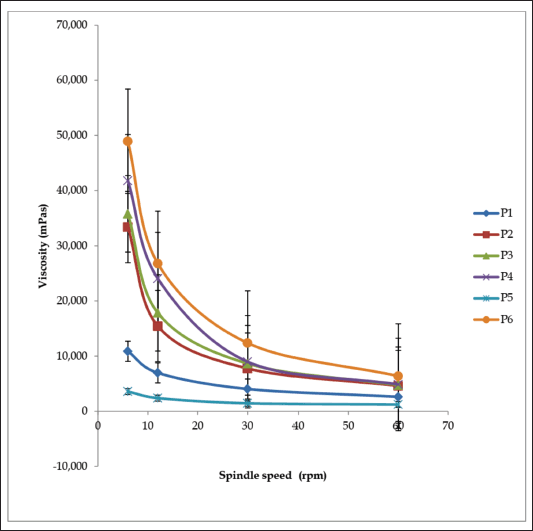 | Figure 1. Viscosity curves of cream formulations P1–P6. [Click here to view] |
Antimicrobial susceptibility of the microorganisms to cream formulations P1–P6
The antimicrobial property of the extract was retained by the creams on all the bacteria studied (Table 5). Formulation P1 which contained no extract exhibited some activity against the bacteria, probably due to the presence of a preservative (methyl-and propylparaben) in its formula. Formulations P1, P2, and P3 had no antifungal effect on the fungi used. The fungi were susceptible to formulations P4, P5, and P6.
Accelerated stability testing
After three freeze-thaw cycles, all the creams maintained good homogeneity and ease of removal. There was a minor decrease in cream pH values (Fig. 3a), but they were not appreciably different (p < 0.05) except for formulation P5. There was an increase in spreadability for formulations P1, P3, P4, and P5 but a decrease in P2 and P6 (Fig. 3b); however, the change was not significant for formulations P2, P3, and P5 (p < 0.05). The shear-thinning property of the creams was retained after the freeze-thaw cycles (Fig. 4a and b), but there was a decrease in viscosity for P1, P2, P4, and P6 and a rise in viscosity for P5. The viscosity of formulation P3 was relatively stable throughout the freeze-thaw cycles. The change in viscosity was significant (p < 0.05) for all the creams except formulation P3.
DISCUSSION
Extraction and phytochemical screening
The yield of the methanol extract of P. santalinoides (10.1% w/w) in this work was higher than the 1.97% reported by Emencheta et al. (2019). This could be because of environmental or geographical differences in the plant sources, which may result in different quantities and types of phytochemicals present. The process and efficiency of the extraction method could be because of the causes of the difference in percentage yield. The existence of saponins, flavonoids, alkaloids, and phenols in the extract was confirmed, as reported by Ogbonna and Idumah (2018). Odeh and Tor-Anyiin (2014) reported the presence of terpenoids and saponins-glycosides, which were also observed in this study.
Antimicrobial screening of methanol extract of P. santalinoides
Pseudomonas aeruginosa, a Gram negative bacterium, was susceptible even at an extract concentration of 6.25 mg/ml. It had a 4–12 mm IZD for the concentrations of 6.25–200 mg/ml used in the research work. The IZDs of the extract at the 25–200 mg/ml concentrations for E. coli were 5–11 mm and 7–10 mm for S. aureus. The fungi were susceptible to higher concentrations (≥100 mg/ml) of the extract; C. albicans had IZD of 10–13 mm while A. niger had IZD of 5–9 mm. This study agrees with the work of Odeh and Tor-Anyiin (2014) that reported sensitivity of S. aureus, E. coli, and C. albicans to ethanol, butanol, and the aqueous extract of the P. santalinoides leaf; however, unlike this study, they reported that P. aeruginosa was resistant. This study was in disagreement with Emencheta et al. (2019), who reported that C. albicans and A. niger were resistant to 100 mg/ml of the P. santalinoides seeds crude methanol extract, butanol, ethyl acetate, and n-hexane fractions. It is however in agreement with their report on the sensitivity of E. coli to the crude methanol extract.
Physicochemical evaluation of methanol extract of P. santalinoides seeds creams
The creams possessed various colors due to their varied content of ingredients. Formulation P1 which did not contain the methanol extract remained white in color. Formulation P2 which contained 6.25 mg of the extract and olive oil was light brown while formulations P3, P4, and P5 which contained 6.25 mg of the extract and liquid paraffin were milk or cream colored. Formulation P6 which contained 10 g of the extract and liquid paraffin was brown in color. The brown color was because of the large quantity of extract (dark brown) in the formulation. The color of the creams did not change after the freeze-thaw cycles. Color changes in a cream on storage may signify instability.
The creams’ pH (5.0 ± 0.29–5.5 ± 0.17) was in the normal skin pH range (4–6) (Chen et al., 2016); therefore, there would be a very minute risk of skin irritation (Bolla et al., 2020), and this was corroborated by the results of the skin irritation test, which showed that there was no skin irritation by any of the formulations used. After the three freeze-thaw cycles, the pH values of the creams decreased slightly, though they were still within the normal skin pH range. Marked change in pH for creams is an indication of instability of the formulation. This may arise due to microbial degradation or incongruity between the ingredients in the formula. All the creams possessed good homogeneity on visual and microscopic inspection before and after the accelerated stability study. When the creams were felt between the index finger and the thumb, there were no palpable masses. The creams were washed off easily from the applied skin parts before and after the freeze-thaw cycles, and this showed that there was no phase change from o/w to w/o creams. The globules of the creams were observed to be stained red whereas the dispersion phase was not stained when observed under the microscope after the creams were stained with the scarlet red dye, a fat-soluble dye. This showed that oil was the dispersed phase while water was the dispersion phase. This confirms the creams to be of the o/w type. Upon centrifugation, formulations P1 and P3 were stable but other creams separated (creamed). Formulation P2 cracked and could not reform to a normal cream upon stirring, showing signs of permanent instability. Formulations P4, P5, and P6 when stirred returned to normal creams; therefore, their instability was temporary (creaming).
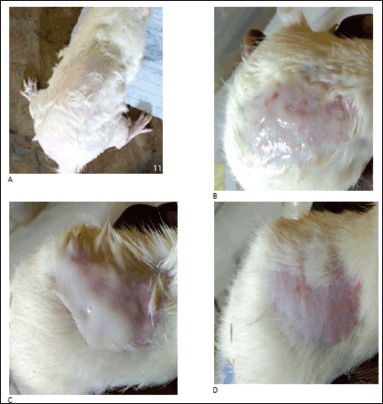 | Figure 2. Skin irritation studies 24 hours after application of (A) formalin, (B) distilled water, (C) formulation P3, and (D) formulation P6. [Click here to view] |
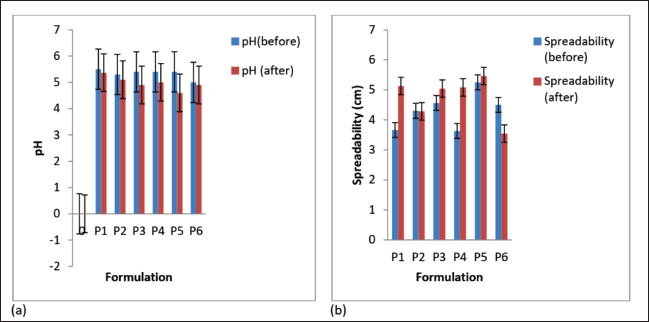 | Figure 3. (a) pH and (b) spreadability of the formulated creams before and after three freeze-thaw cycles. Values are expressed as the mean ± SD (n = 3). [Click here to view] |
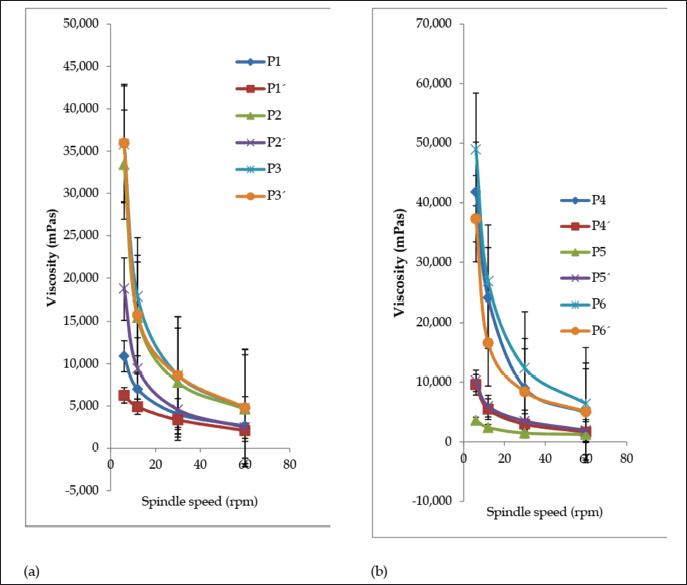 | Figure 4. Viscosity curves of formulated creams (a) P1–P3 and (b) P4–P6 before (P) and after (P’) three sets of freeze-thaw cycles. Values are expressed as the mean ± SD (n = 3). [Click here to view] |
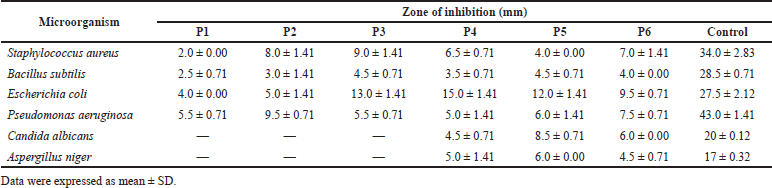 | Table 5. Antimicrobial susceptibility of the test organisms to formulated creams. [Click here to view] |
Cream spreadability is its ability to spread evenly on the surface of the skin, and this plays a vital role in its standard dose administration to the parts of the skin and its efficacy as a topical treatment (Chen et al., 2016). It shows the level at which a cream will readily spread when a small amount of shear is used on the skin. The formulated creams had good spreadability, though formulations P1 and P4 were less spreadable than the rest.
The determination of tube extrudability is very important in the evaluation of topical preparations such as creams, emulgels, and ointments. Extrudability is the force that is applied to remove the formulation from the tube. The larger the quantity that is extruded, the better the extrudability (Fauzee and Walker, 2020; Ilievska et al., 2016). Easy extrusion of the preparation from the tube enhances patient compliance. Preparations that are highly viscous may be difficult to extrude, while low viscous ones may be extruded quickly (Obanewa and Oyeniran, 2019).
Viscosity as a parameter is salient in the evaluation of topical preparations since extrudability as well as drug release depends on it (Obanewa and Oyeniran, 2019). The viscosity of all the creams was 3,600–48,950 mPas before and 1,240–6,390 mPas after the accelerated stability study. The creams exhibited pseudoplastic flow. They displayed shear-thinning behavior because the viscosity decreases as shear stress (spindle speed) increases. Formulation P3 maintained stable viscosity before (35,800 mPas) and after (35,950 mPas) the freeze-thaw cycle. Formulations such as P1, P2, and P4 recorded a significant decrease in viscosity, P6 recorded a slight decrease, while P5 recorded an increase. Marked change in viscosity of a cream on storage is an indication of instability.
Antimicrobial susceptibility of the microorganisms to the creams
The creams retained the activity of the methanol extract against the bacteria used in this study (Table 5). The IZDs recorded by formulation P1 (2–5.5 mm) were low compared to the other formulations (P2–P6) that contained the methanol extract (3–15 mm). The IZDs displayed by the creams against the microorganisms were very much less than those recorded for the control (gentamicin). This may be because the extract was still in the crude form and required further purification and fractionation to isolate the pure active constituent. Formulation P6 (formulated with 100 mg/ml of the extract) exhibited IZD of 6 mm for C. albicans and 4.5 mm for A. niger, which was comparable to those produced by the methanol extract alone (IZD of 10 mm for C. albicans and 5 mm for A. niger). However, formulations P4 and P5 that contained 25 mg/ml of the methanol extract showed IZD of 4.5–8.5 mm against C. albicans and IZD of 5–6 mm against A. niger, respectively. This activity against the fungi by formulations P4 and P5 could not be easily explained because they were formulated with the 25 mg/ml extract concentration and the extract alone at that concentration did not show any inhibitory activity against the fungi. Formulation P2, which had no inhibitory activity against the fungi used in the study, was formulated with the same ingredients as formulation P4 except that olive oil instead of liquid paraffin was used in its formulation. Formulations P1, P2, and P3 were inactive against the test fungi.
CONCLUSION
This study showed that the methanol extract of P. santalinoides has antibacterial activity at a concentration of 25 mg/ml and antifungal activity at 100 mg/ml. The creams had antibacterial activity against the test bacteria. The cream formulated with 100 mg/ml of the extract (P6) exhibited antifungal activity against C. albicans and A. niger. Some of the creams containing 25 mg/ml (P4 and P5) displayed antifungal activity against the test fungi while the rest did not. The creams all exhibited good physicochemical properties: good homogeneity, ease of removal, type of cream, and spreadability. The pH of the creams was within the normal pH range for the skin. The creams had a good shear-thinning property. Formulations P1 and P3 were stable, formulations P4 and P6 were slightly unstable but reformed to good creams when agitated, while formulations P2 and P5 were completely unstable.
Formulation P3 is chosen as the optimized antibacterial formulation because it exhibited good physicochemical properties and was stable after accelerated stability testing, though it lacked antifungal activity against the test fungi. Formulation P6 displayed good antimicrobial activity against all test bacteria and fungi but was slightly unstable after accelerated stability testing. The other formulations were either completely unstable or lacked good antimicrobial activity.
ACKNOWLEDGMENTS
The authors are grateful to the Department of Pharmaceutics and Industrial Pharmacy and the Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmacy, Delta State University, Abraka, Nigeria, for providing their laboratory for the research. Mr. Michael Oghenejobo’s contribution to microbiological analysis is highly appreciated.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest.
FUNDING
There is no funding to report.
AUTHORS’ CONTRIBUTIONS
Concept/design and supervision were done by S. E. Okafo, C. O. Anie, and C. A. Alalor. Data acquisition, data analysis, and interpretation were carried out by S. E. Okafo, C. O. Anie, C. A. Alalor, and L. U. Nwankwo. Drafting of the manuscript was done by S. E. Okafo. Critical revision of the manuscript, funding, and final approval were done by S. E. Okafo, C. O. Anie, C. A. Alalor, and L. U. Nwankwo. Statistical analysis, admin, technical, and material support were provided by S. E. Okafo, C. O. Anie, and L. U. Nwankwo.
ETHICAL APPROVALS
The Research and Ethical Committee of the Faculty of Basic Medical Sciences of Delta State University, Abraka, Nigeria, issued an ethical clearance (Resolution No. REC/FBMS/ DELSU/21/98).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akaniro-Ejim EN, Ibe MI, Engwa GA. Polyphenol content and in vitro antioxidant activity of aqueous-ethanol extracts of Pterocarpus soyauxii and Pterocarpus santalinoides. Glob J Med Res (B), 2018; 18(2):1–8.
Ashish A, Mohini K, Abhiram R. Preparation and evaluation of polyherbal cosmetics cream. Pharm Lett, 2013; 5(1):83–8.
Asija R, Dhaker PC, Nama N. Formulation & evaluation of voriconazole ointment for topical delivery. J Drug Discov Ther, 2015; 3(26):07–14.
Biswas B, Rogers K, Mclaughlin F, Daniels D, Yadav A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int J Microbiol, 2013; 2013:1–7.
Bolla PK, Clark BA, Juluri A, Cheruvu HS, Renukuntla J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using strat-m® membrane. Pharmaceutics, 2020; 12:151.
Chandrasekar R, Priyanka K, Sakhira K, Sreeprada K, Harshita K, Haripriya B, NiranjanBabu M. Formulation and stability evaluation of natural preservatives in poly-herbal skin care cream. Int J Res Dev Pharm L Sci 2018; 7(3):2999–3005.
Chauhan L, Gupta S. Creams: a review on classification, preparation methods, evaluation and its applications. J Drug Deliv Ther, 2020; 10(5–s):281–9.
Chen MX, Alexander KS, Baki G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J Pharm 2016; http://dx.doi.org/10.1155/2016/5754349
Deepika P, Singh RK. Formulation and characterization of an antibacterial cream from Lantana camara leaf extract. Pharm Chem J, 2017; 4(5):137–42.
Emencheta SC, Enweani IB, Oli AN, Okezie UM, Attama AA. Evaluation of antimicrobial activities of fractions of plant parts of Pterocarpus santalinoides. Biotechnol J Int, 2019; 23(3):1–11.
Ezeonu CS, Ejikeme CM. Qualitative and quantitative determination of phytochemical contents of Indigenous Nigerian softwoods. New J Sci, 2016; 2016:9.
Fauzee AFB, Walker RB. The impact of formulation variables on the optimization of pilot scale clobetasol 17-propionate creams. Cogent Eng, 2020; 7:1804713.
Hartiadi LY, Sahamastuti AAT. Protective effect of Caesalpinia sappan L. extract against H2O2-induced oxidative stress on HaCaT and its formulation as antioxidant cream. J Res Pharm, 2020; 24(4):508–17.
Idson B, Lazarus J. Semisolids. In: Lachman L, Lieberman HA (eds.). The theory and practice of industrial pharmacy, CBS Publishers & Distributors Pvt. Ltd., New Delhi, India, pp.534–63, 2009.
Ilievska B, Loftsson T, Hjalmarsdottir MA, Asgrimsdottir GM. Topical formulation comprising fatty acid extract from cod liver oil: development, evaluation and stability studies. Mar Drugs 2016; 14:105
Kaushik K, Sharma RB, Sharma A, Agarwal S. Evaluation of antifungal activity of crude methanolic extract of leaves of Ipomoea carnea and its formulation as a gel. J Res Pharm, 2020; 24(3):368–79.
Khalifa AA, Hanan SA, Wesam AK, Fouzy A, Salem ME. Qualitative and quantitative phytochemical analysis and antimicrobial activity of “Retama” extract grown in Zliten Libya. Int J Med Sci Clin Invent 2017; 4(4):2861–6.
Khan S, Mulla G, Bhise K. Development and characterization of topical nanoparticulate antipsoriatic polyherbal cream. Int J App Pharm, 2020; 12(3):67–73.
Khullar R, Kumar D, Seth N, Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm J, 2012; 20:63–7.
Mba-Omeje KN, Ugwu CC, Ezugwu RI, Iloputaife EJ. Antimicrobial and phytochemical studies of Carica papaya leaves against Escherichia coli and Staphylococcus aureus. World J Pharm Pharm Sci, 2017; 7(1):180–9.
Moldovan M, Lahmar A, Bogdan C, Parauan S, Tomuta I, Crisan M. Formulation and evaluation of a water-in-oil cream containing herbal active ingredients and ferulic acid. Clujul Med, 2017; 90(2):212–9.
Nworu CS, Akah PA, Nwachukwu JO, Asogwa C. Antidiarrhoeal activity of Pterocarpus santalinoides L’Herit ex DC leaf extract. J Compl Integr Med. 2009; 6(1):1553-60.
Obanewa OA, Oyeniran OT. Development and estimation of anti-inflammatory activity of topical etoricoxib emulgel by carrageenan induced paw oedema method. Uni J Pharm Res 2019; 4(3):22–358.
Odeh IC, Tor-Anyiin TA. Phytochemical and antimicrobial evaluation of leaf-extracts of Pterocarpus santalinoides. Eur J Med Plants, 2014; 4(1):105–15.
Ogbonna PC, Idumah MC. Phytochemical and mineral content in leaves, stem and bark of Pterocarpus santalinoides (Nturukpa) from Afikpo, Ebonyi State, Nigeria. J Appl Sci Environ Manage, 2018; 22(8):1147–50.
Okafo SE, Akpo CO, Okafor CC. Formulation and evaluation of antimicrobial herbal creams from aqueous extract of Moringa oleifera Lam seeds. Nig J Sci Environ, 2020; 18(1):50–7.
Okafo SE, Anie CO, Nwanua MC. Formulation and evaluation of antimicrobial topical creams from ethanolic extract of Vernonia ambigua Leaves. Nig J Pharm Res, 2019; 15(2):249–55
Okafo SE, Enwa FO, Amusile O. Formulation and evaluation of antimicrobial properties of Psidium guajava ethanol leaf extract creams. Trop J Nat Prod Res, 2021; 5(12):2144–8.
Onyekere PF, Enebechi CK, Nnamani DO, Peculiar-Onyekere CO, Okonta EO. Phytochemical analysis and antimicrobial activity of methanol extract of the leaves of Hippocratea welwitschii Oliv (celastraceae). Afr J Pharm Res Dev, 2019; 11(2):106–15.
Ordu JI, Sunday BR, Okafo SE. Evaluation of the activity of Garcinia kola seed oil and honey on skin cream formulation. Pharm Innov J, 2018; 7(5):675–81.
Pal TK, Dutta D, Banerjee R, Maity S. Formulation and evaluation of antimicrobial topical semisolid dosage form containing whole plant extract of Biophytum sensitivum. An Int J, 2013; 1(7):641–6.
Rai R, Poudyl AP, Das S. Pharmaceutical creams and their use in wound healing: a review. J Drug Deliv Ther, 2019; 9(3–s):907–12.
Sekar M, Jalil NSA. Formulation and evaluation of novel antibacterial and anti-inflammatory cream containing Muntingia calabura leaves extract. Asian J Pharm Clin Res, 2017; 10(12):376–9.
Shaik NB, Gera S, Kukati L, Goverdhanam H. Formulation and evaluation of emulgel of flurbiprofen. Int Res J Pharm, 2019; 10(8):68–76.
Swabrick J, Rubino JT, Rubino OP. Coarse dispersion. In: Remington. The science and practice of pharmacy. 21st edition, Pharmaceutical Press, London, UK, pp 319–37, 2005.
Trease GE, Evans WC. Textbook of pharmacognosy. 14th edition, Balliere Tindall, London, UK, 2002.
Ubaid M, Ilyas S, Mir S, Khan AK, Rashid R, Khan MZU, Kanwal ZG, Nawaz A, Shah A, Murtaza G. Formulation and in vitro evaluation of carbopol 934-based modified clotrimazole gel for topical application. An Acad Bras Ciên 2016; 88(4): 2303-17.
Ugandar RE, Deivi KS. Formulation and evaluation of natural palm oil based vnishing cream. Int J Pharm Sci Res, 2013; 4(9):3375–80.