INTRODUCTION
The human body is home to diverse microorganisms which are referred to as the microbiota. Although these microorganisms are largely present in the skin and mucosal layers lining oral-, nasal-, pulmonary-, and vaginal cavities, they are found in extraordinary numbers in the gastrointestinal (GI) tract, therefore, recent studies on the gut microbiota and its role in governing the body’s vital functions have gained interest. The gut microorganisms are responsible for normal bodily processes, such as vitamin synthesis, mineral absorption, breakdown of toxins, etc. It has been reported that the microbiome of the gut interacts with the brain through biological pathways (Westfall et al., 2019) and it has recently been demonstrated that the microbiome in the GI tract and the central nervous system has two-way communication and is responsible for the healthy functioning of endocrine signaling, intestinal permeability, and immune activation, development, and maintenance of immune tissues (Belizário and Faintuch, 2018; Lynch and Pedersen, 2016; Silva et al., 2020; Westfall et al., 2018). A disparity in the microbial constitution of the gut could lead to a number of conditions ranging from GI disorders to respiratory, neurological, metabolic, and cardiovascular disorders inducing clinical symptoms and the onset of long-term diseases, such as diabetes, irritable bowel syndrome, and cancer (Belizário and Faintuch, 2018; Grochowska et al., 2019).
Probiotics are living microorganisms that when taken in adequate quantities elicits beneficial effect on the host system. The probable mode of action of these beneficial micro-organisms includes regulation of the immune functions of the intestine, suppression of pathogens, and differentiation and maintenance of the integrity of the intestinal barrier. The most commonly studied probiotic groups of bacteria include Lactobacillus species and Bifidobacterium species. While the administration of individual probiotics is beneficial, evidence indicates that the efficacy of single probiotic therapies with multi strains formulations showed 75% of multi-strain combinations to be better than their individual components (Chapman et al., 2011). Belenguer et al. (2006) studied the effects of xylooligosaccharides alone, and in amalgamation with Bifidobacterium lactis in adults. Xylooligosaccharides showed advantageous effects on GI health, plasma lipid profile, and inflammation (Belenguer et al., 2006).
Lactobacillus fermentum is a gram-positive probiotic bacteria known to produce ferulic acid (FA) or trans FA which are copious phytochemicals present in the form of phenolic acid in fruit and vegetables and function as a robust antioxidant. FA ethyl ester is a hydrophobic form of FA that was recently found to be protective of neuronal cell culture and synaptosomal membrane against radical oxidation (Pluta et al., 2020).
Prebiotics are used as substances that augment the growth of probiotics in turn for the bacterial species to exhibit their potential. Prebiotics are indigestible substances that have a beneficial role in the host by selectively inducing the proliferation and functions of beneficial microbes. It has been defined as “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the GI microflora that confers benefits upon host well-being and health” (Roberfroid, 2007). Triphala (TFLA), a polyherbal formula having equal concentrations of Emblica officinalis, Terminalia bellirica, and Terminalia chebula. It is a traditional Ayurvedic plant known to have a favorable effect on digestive distress and is also reported to have anti-inflammatory, anti-oxidant, analgesic, hypoglycemic, and wound-healing effects. In the past few years, there has been a trend to combine probiotics with specific prebiotics called synbiotes which can increase their beneficial activity. Studies reveal that a combination of both prebiotic and post-biotic therapy had 75% effectiveness (Westfall et al., 2018).
Para probiotics are also known as non-viable or inactivated probiotics which when delivered in sufficient amount renders beneficial effects. Although there is no clear explanation for the mechanism underlying the action of para probiotics, it is believed that the molecules present on the surface of the cells offer interaction with the host. It has also been proved that para probiotics can mediate anti-inflammatory and immune responses (Siciliano et al., 2021).
Drosophila melanogaster was chosen as the model organism as it is ideal for studying human biology reflecting similar specificity and reduced complexity which makes it feasible to manipulate effectively. In spite of its small size, it has complex biological processes and is also a host for a wide range of microbiota. The aim and objectives of this study are to compare and study the effect of probiotics, symbiotic, and heat-inactivated L. fermentum on aged flies and analyze its effects based on the changes in the biochemical and behavioral assays. The study was performed with the intent to investigate if the prepared symbiotic formulations can reverse enzyme levels that are generally insufficient in aged flies and also positively impact the behavior of the flies. The three treatment groups of probiotics, synbiotic, and heat-killed L. fermentum were studied in a comparative manner by including them in the fly diet and by performing various physiological and biochemical assays.
MATERIALS AND METHODS
Chemicals
Agar-agar type I (HiMedia), sodium phosphate dibasic (HiMedia), D-glucose Sisco Research Laboratories Pvt. Ltd. (SRL), potassium chloride (SRL), sodium phosphate monobasic (HiMedia), nitro blue tetrazolium (NBT) chloride (SRL), yeast extract, tetrasodium pyrophosphate decahydrate (SRL), β-Nicotinamide Adenine Dinucleotide Disodium Salt (Reduced) (NADH), phenazolium methosulfate. Propionic acid (Merck), Orthophosphoric acid (Thermo Fischer Scientific), methyl para hydroxy benzoate (Rankem) Lactobacillus Man-Rogosa-Sharpe (MRS) broth (SRL). The chemicals used were of analytical grade. Triphala powder was procured from a local vendor in Chennai, Tamil Nadu, India.
Culturing of probiotic strain
Lactobacillus fermentum (MCC2760) was procured from National Centre for Microbial Resource, Pune. Bacterial culture was maintained in Lactobacillus MRS media, at 37°C on liquid media or agar plates. The concentration of the probiotic culture was maintained at 1.5 × 108 CFU/ml throughout the experimental period. The synbiotic formulation and the heat-killed formulation had Triphala in 0.5% w/v (Westfall et al., 2019).
Rearing of D. melanogaster
Male and female Wild Type Oregon-K flies were reared on cornmeal agar media at 25°C with 60% relative humidity. The media consists of corn flour, D-glucose, sugar, agar-agar type 1, and yeast extract powder mixed in water. The prepared media was autoclaved and anti-fungal agents such as Tego (methyl parahydroxy benzoate in ethanol), propionic acid, and ortho-phosphoric acid were added to the cornmeal diet at 55°C to prevent fungal growth.
Experimental setup
An overnight culture of the probiotic strain was prepared. The culture was added to already cooled semi-solid media at 1.5 × 108 CFU/ml. Treatment groups were divided into control, probiotic, synbiotic, and heat-killed, depending on the assays approximately 20–30 adult male/female flies were taken. Three doses of infection i.e., 20, 30, and 40 μl were added to the fly media in triplicates. The treatment period for the adult male flies was one month and the flies were changed every 3–5 days into vials containing fresh media.
Crawling assay
Nine third instar larvae were taken and washed in milliQ water then transferred to a petri plate containing 2% agarose. The Drosophila larvae were permitted to move on the smooth agar surface for 1 minute and recorded. A graph sheet was used to calculate the distance crawled by the larvae (Sundararajan et al., 2019).
Negative geotaxis assay
The adult male flies post-exposure were shifted to vials having a 3 cm mark. The vials were tapped and a video was recorded for 1 minute. The number of flies that climbed above the 3 cm mark was counted (Sundararajan et al., 2019).
Acetylcholine esterase activity
Acetylcholinesterase (AChE) activity in adult flies was assessed as per Ellman’s method, with a few modifications. Briefly, 20 adult male flies post-treatment were homogenized and 200 μl of fly homogenate was added to the reaction mixture having the following components – sodium phosphate buffer,5,5′-dithiobis-2-nitrobenzoic acid, and acetylthiocholine iodide. Change in absorbance/min was recorded at 412 nm (Ellman et al., 1961).
Superoxide dismutase (SOD) activity
The SOD activity was estimated according to the method of Ahamed et al. (2010) with few modifications. About 25 adult flies were homogenized and the reaction was started at room temperature by adding 200 μl of 780 μM NADH, 1 ml of 0.052 M sodium pyrophosphate buffer (pH8), 0.1 ml of 186 μM phenazine methosulphate, 300 μl of NBT, 0.2 ml of milliQ water, and 0.2 ml of supernatant. The reaction was stopped and the absorbance was recorded at 560 nm (Ahamed et al., 2010).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 to compare the significance of treatment groups with respect to the control. One-way analysis of variances (ANOVA) was used to determine the statistical difference between the control and the treatment groups with significance set to p < 0.05. Each group is an average of triplicate groups +/− SEM.
RESULTS AND DISCUSSION
The gut hosts a wide range of microorganisms that coexist and play a crucial role in regulating the host’s physiological mechanisms. However, factors like stress, improper diet, sleep pattern alterations, antibiotic exposure, and infections cause an imbalance in the gut microbiota leading to intestinal dysbiosis. Lactobacillus and Bifidobacterium species, both of which are gram-positive bacteria have a beneficial effect on host health and metabolism. These species have been used, in the form of dietary supplements, to deal with the effect of harmful bacteria (Ambrosini et al., 2019). This study aims to analyze the influence of probiotics, synbiotics, and heat-killed probiotic strains on Drosophila behavioral and biochemical parameters.
Crawling assay
The crawling pattern of third instar larvae was assessed to identify any deficit in their locomotor function and subsequent behavioral changes. Figure 1(a)–(c) shows the optimization of probiotic, synbiotic, and heat-killed treatment doses at 20, 30, and 40 μl and their subsequent effect on larval crawling behavior. The results indicated that L. fermentum, in general, did not induce any adverse effect on larval crawling patterns. However, at varying doses of treatment exposure, the crawling pattern of the larvae showed a significant enhancement, especially larvae exposed to 30 μl dose of probiotic, synbiotic, and heat-killed treatment respectively. The optimized bacterial dose was used for further studies. Figure 1(d), shows the comparison where the flies were exposed to the optimized bacterial load for a period of 1 month. The data show elevated larval crawling activity when compared to the control, however, after statistical analysis, it was noted that probiotic treatment alone does not indicate any significant surge in crawling activity as compared to the control, whereas, heat-killed and synbiotic show significant enhancement. Also, the treatments were not observed to show any negative influence on locomotion activity as compared to the control.
Negative geotaxis assay
Also termed a climbing assay, it is done to test the effects of the probiotic, heat-killed, and synbiotic treatments on motility using the negative geotaxis assay apparatus to assess the behavioral parameters such as the climbing pattern in aging male flies. Figure 2(a)–(c) show the climbing assay done in separate treatments with the three doses. It was observed that after 1 month of treatment, in each of the treatment groups, 30 μl of infection showed significantly higher motility than the other two doses, when compared to the control. Once the amount of infection was optimized, the three treatment groups were compared to each other with respect to the control.Figure 2(d) indicates that amongst the three treatments, although all the treatments show an increase in motility, synbiotic treatment shows significantly high motility in adult flies.
AChE activity
Figure 3(a) shows the AChE activity recorded in Oregon-K flies that were treated for 1 month. After 1 month of treatment, an increase in the levels of AChE has been observed in the heat-killed and synbiotic treatments. The AChE activity is expected to reduce with age. It can be observed in the given graph that at the same age (1 month), the flies treated with the respective treatments have a slightly elevated AChE activity when compared to the control. However, the observation that heats killed and synbiotic treatments shows a higher activity level of AChE, among the three treatments, is suggestive of the better effect of this formulation on AChE activity in aging flies.
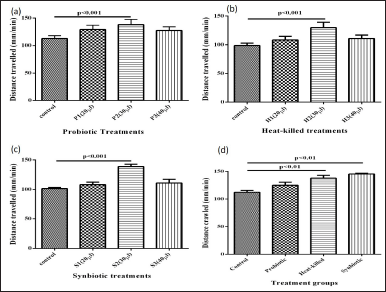 | Figure 1. (a), (b), (c): Crawling assay for optimizing the dose in probiotic, synbiotic, and heat-killed treatment in larvae. (d) Optimized crawling assay. [Click here to view] |
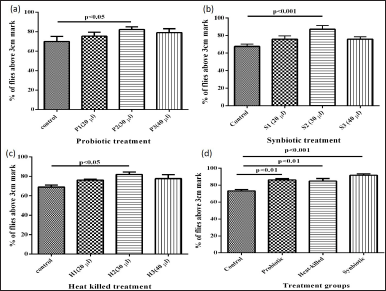 | Figure 2. (a), (b), (c): Negative geotaxis assay for optimizing the dose in probiotic, synbiotic, and heat-killed treatment when fed orally for 1 month to adult male flies. (d) Optimized negative geotaxis assay after feeding for 1 month. [Click here to view] |
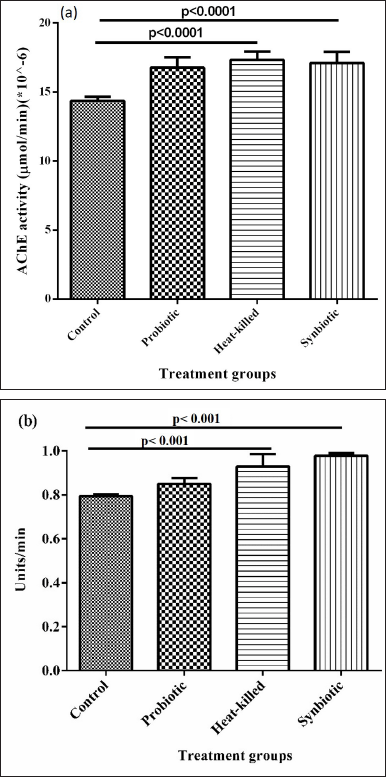 | Figure 3. (a): The effects of the treatments on AChE activity in 1-month-old Oregon-K flies. Each group is an average of n = 9 individual groups +/− SEM. (b) SOD activity of adult flies after 1-month treatment. Analyses are done on Graphpad Prism version 5 using one-way ANOVA. The significance of the analysis is set at p < 0.05. [Click here to view] |
SOD activity
Literature confirms that oxidative stress accumulates with the process of aging and this accumulation of oxidative stress causes the bulk production of superoxide radicals that in turn cause a negative impact on homeostasis. Figure 3(b) shows the comparison of SOD activity of 1-month-old Oregon-K flies. The graph shows that though there is an increase in SOD activity with all treatments, heat-killed, and synbiotic have a significant increase. This suggests that these two treatments have a better effect on SOD activity in aged flies.
CONCLUSION
In conclusion, aging is an inevitable process and this study indicates that probiotics on their own or in formulation with prebiotics (synbiotics) or just the metabolites (heat-killed) have a significant influence on Drosophila behavior and biochemical parameters. This study further emphasizes the major influence of synbiotics on various behavioral parameters that involve complex neuronal mechanisms in aged fruit flies. The cellular antioxidant markers and AChE levels in synbiotic-treated aged flies further highlight the influence of probiotic and prebiotic combinations. Overall, the results signify the favorable effects of symbiotic formulations on the tested behavioral and biochemical parameters, in general, are noticed to be subsided in aged flies. Previous studies have shown that synbiotics have the ability to activate metabolic, anti-oxidative, anti-inflammatory, and regulatory axes of the mitochondria, while probiotics have the potential to reduce pathological markers for Alzheimer’s disease. Therefore, further studies such as gene expression analysis, biochemical enzyme activity, and survivability studies are warranted to explore the role of probiotics and synbiotics in neurodegenerative disorders and age-related factors using transgenic fly models.
FUNDING
This manuscript received financial support from the National Symposium on “Emerging Innovations in Applied Biological Sciences (EIABS)” conducted by the Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur, 603 203.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVALS
No animal or human subjects are used in this research that requires ethical clearance.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahamed M, Posgai R, Gorey TJ, Nielsen M, Hussain SM, Rowe JJ. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol Appl Pharmacol, 2010; 242:263–9; doi:10.1016/j.taap.2009.10.016 CrossRef
Ambrosini YM, Borcherding D, Kanthasamy A, Kim HJ, Willette AA, Jergens A, Allenspach K, Mochel JP. The gut-brain axis in neurodegenerative diseases and relevance of the canine model: a review. Front Aging Neurosci, 2019; 11:1–14; doi:10.3389/fnagi.2019.00130 CrossRef
Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ. Two routes of metabolic cross-feeding between bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut downloaded from the human gut. Appl Environ Microbiol, 2006; 72:3593–9; doi:10.1128/AEM.72.5.3593-3599.2006 CrossRef
Belizário JE, Faintuch J. Microbiome and gut dysbiosis. Exp Suppl, 2018; 109:459–76; doi:10.1007/978-3-319-74932-7_13 CrossRef
Chapman CMC, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr, 2011; 50:1–17; doi:10.1007/s00394-010-0166-z CrossRef
Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol, 1961; 7:88–95; doi:10.1016/0006-2952(61)90145-9 CrossRef
Grochowska M, Laskus T, Radkowski M. Gut microbiota in neurological disorders. Arch Immunol Ther Exp (Warsz), 2019; 67:375–83; doi:10.1007/s00005-019-00561-6 CrossRef
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med, 2016; 375:2369–79; doi:10.1056/NEJMRA1600266 CrossRef
Pluta R, Kozio? MU, Januszewski S, Czuczwar SJ. Gut microbiota and pro / prebiotics in Alzheimer’s disease. Aging, 2020; 12:5539–50. CrossRef
Roberfroid M. Prebiotics: the concept revisited. J Nutr, 2007; 137; doi:10.1093/JN/137.3.830S CrossRef
Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol, 2020; 1:25; doi:10.3389/fendo.2020.00025. CrossRef
Siciliano RA, Reale A, Fiorella Mazzeo M, Morandi S, Silvetti T, Brasca M. Paraprobiotics: a new perspective for functional foods and nutraceuticals. Nutrients, 2021; 13(4):1225; doi:10.3390/nu13041225 CrossRef
Sundararajan V, Dan P, Kumar A, Venkatasubbu GD, Ichihara S, Ichihara G, Mohideen SS. Drosophila melanogaster as an in vivo model to study the potential toxicity of cerium oxide nanoparticles. Appl Surf Sci, 2019; 490:70–80; doi:10.1016/j.apsusc.2019.06.017 CrossRef
Westfall S, Lomis N, Prakash S. A novel synbiotic delays Alzheimer’s disease onset via combinatorial gut-brain-axis signaling in Drosophila melanogaster. PLoS One, 2019; 14:1–24; doi:10.1371/journal.pone.0214985 CrossRef
Westfall S, Lomis N, Prakash S. A novel polyphenolic prebiotic and probiotic formulation have synergistic effects on the gut microbiota influencing Drosophila melanogaster physiology. Artif Cells Nanomed Biotechnol, 2018; 46:441–55; doi:10.1080/21691401.2018.1458731 CrossRef