INTRODUCTION
The increased level of reactive oxygen species (ROS) appears to play a role in the pathogenesis of coronavirus disease 2019 (COVID-19) (Cecchini and Cecchini, 2020). ROS are small oxygen-derived molecules that can be in the form of oxidizing agents or also molecules that are easily converted into oxygen radicals (André-Lévigne et al., 2017). Increased production of ROS can lead to oxidative stress-induced molecular damage. Recent methodological advances have identified the enzymes responsible for producing and elevating ROS levels. Elevated ROS levels have been linked to a variety of diseases, including cardiovascular, immune, and nervous system disorders, as well as aging and cancer. According to a recent study, selectively targeting specific ROS levels enables the development of a futuristic refined redox medicine (Sammar et al., 2019; Sies and Jones, 2020). Production and elevation formation of ROS is correlated with almost every human disease. As a result, selectively targeting ROS-related proteins is an important step in the development of redox medicine. Network pharmacology and big data in drug discovery and development are modernized approaches in treating oxidative stress that has placed ROS as the basis of a number of diseases. However, studies on the regulation of ROS levels must be conducted in greater detail in order to elucidate the mechanism by which ROS causes disease and cellular damage (Casas et al., 2020).
Antioxidants have been extensively used as agents to lower ROS levels and treat oxidative stress. Antioxidant supplementation reduces COVID-19 severity due to its stress oxidative relieving effect (Delgado-Roche and Mesta, 2020; Pizzino et al., 2017).
The use of herbal medicine is becoming popular worldwide and well accepted and distributed as food composition and supplements that are safe as antioxidants (Ekor, 2014). Aloe vera is one of the plants known to exhibit great antioxidant activity. Hence, it has the potential to regulate ROS formation in the body. It contains a considerable number of metabolites, such as flavonoids, terpenoids, lectins, fatty acids, anthraquinones, pectins, hemicelluloses, glucomannan, tannins, campesterol, capreol, enzymes, salicylic acid, calcium, chromium, copper, iron, magnesium, manganese, potassium, phosphorus, sodium, zinc, retinol, ascorbic acid, alpha-tocopherol, β-carotene, B1–B3, B6, choline, B12, and folic acid (H?? et al., 2019).
The variety of metabolite makeup of a plant depends on several aspects, such as the type and conditions of cultivation, climate condition, harvesting time, position of the harvested leaves, species, variety, and method used for harvesting leaves (Giannakoudakis et al., 2018). We hypothesized that A. vera grown in North Sulawesi, Indonesia, contains different metabolites than those grown in other regions, owing to the different cultivation conditions and climates. Information on metabolite classes has been published. Specific metabolites from the peel and gel of A. vera collected from the Indonesian island of North Sulawesi, on the other hand, have never been previously reported.
There is a dearth of information regarding the composition of A. vera metabolites from North Sulawesi and their effect on ROS levels and COVID-19 patients. Hence, we used liquid chromatography-mass spectrometry (LC-MS) to determine the metabolite composition of the peel and gel of A. vera collected in North Sulawesi, Indonesia. Further docking of the compounds to the ROS-producing enzymes was performed to identify compounds that have the potential to inhibit the proteins’ function and stop their biological processes. In addition, to look at the direct inhibition potential against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), we also evaluated the inhibition potential of the metabolites against the receptor-binding domain (RBD) of the Omicron variant using the same method as against the ROS-producing enzymes. Since RBD plays an essential role in viral entry into human cells, targeting this domain can illustrate the role of the metabolites in halting the virus’s life cycle.
METHODS
Metabolite identification
The identification of the metabolites was carried out using an LC-MS instrument in accordance with the protocol described by Nur et al. (2021), with slight modifications to the run time, specifically for 25 minutes.
Molecular docking protocol
The identified metabolites were used as ligands. The structures were obtained from PubChem, their energy was minimized, and then PyRx 8.0 was used to dock them to the ROS-producing enzymes. The targets used in this protocol are RBD of SARS-CoV-2 Omicron variant (PDB code: 7T9L), inducible nitric oxide synthase (NOS) (PDB code: 4UX6), endothelial nitric oxide synthase (eNOS) (PDB code: 6CIF), neuronal nitric oxide synthase (nNOS) (PDB code: 6PMY), cyclooxygenase-1 (PDB code: 5U6X), cyclooxygenase-2 (PDB code: 6V3R), and 5-lipoxygenase (5-LOX) (PDB code: 6N2W). Vitamin C was used as a benchmark because it is an extremely powerful antioxidant. The docking result was ranked based on the docking scores (kcal/mol) and the number of hydrogen bonds due to the evaluation of the two of these aspects is important and may enhance the linearity with experimental data (Cheshire et al., 2011; Cinelli et al., 2020; Cingolani et al., 2017; Dallakyan and Olson, 2015; Gilbert et al., 2020; Li et al, 2018; Mousavi et al., 2021; Uddin et al., 2020; Tallei et al., 2020). The top-ranked ligands were further visualized using the BIOVIA Discovery Studio Visualizer.
RESULTS AND DISCUSSION
Metabolite identification
The previous study lacked data on the metabolites found in A. vera peel. Only the peel metabolites classes had been extensively identified, such as steroids, tannins, terpenoids, catechin, carotenoids, and anthraquinones (Bista et al., 2020; Dharajiya et al., 2017; Muthukumaran et al., 2018; Nalimu et al., 2021). However, the majority of the available research data did not differentiate between the peel and gel metabolite classes, so the localized major metabolites in each component of the plant are poorly known (Raad et al., 2020; Sánchez et al., 2020). We found that there are differences in the metabolites in the peel and gel of A. vera. The only available data on the metabolites in A. vera peel was the result of the study conducted by López et al. (2013), using samples collected from the Canary Islands of Spain. They identified sinapic acid, quercitroside, kaempferol, chamomile, protocatechuic, catechin, vanillic acid, epicatechin, cedar acid, 3-O-caffeoylquinic acid, gentisic acid, 3,4-dihydroxycinnamic acid, coumaric acid, 4-hydroxy-3-methoxycinnamic acid, rutin, myricetin, and quercetin. Our finding showed that A. vera collected from North Sulawesi of Indonesia contained different types of metabolites. As a result, it can be justified that plants’ metabolite content will vary according to their cultivation location (Kumar et al., 2017).
There are 13 metabolites identified in the peel and 12 metabolites in the gel of A. vera. Figures 1 and 2 depict the LC-MS chromatogram. The result showed that there are differences in metabolites in the peel and gel of A. vera. The metabolites detected in the peel include triacetyl-bromo-emodin, 6-O-(alpha-L-rhamnopyranosyl)-(1->6)-beta-D-glucopyranosyl)emodin, 8-demethyl-8-(alpha-L-rhamnosyl)tetracenomycin C, (10R)-1,8,10alpha-triacetoxy-3-acetoxymethyl-10-(2-O,3-O,4-O,6-O-tetraacetyl-beta-D-glucopyranosyl)anthrone, aloe-emodin dianthrone diglucoside, (9R)-9-[(9S)-2-carboxy-5-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-oxalooxyoxan-2-yl]oxy-4-hydroxy-10-oxo-9H-anthracen-9-yl]-4-hydroxy-10-oxo-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6(hydroxymethyl)oxan-2-yl]oxy-9H-anthracene-2-carboxylic acid, landomycin X, emodin-8-o-beta-gentiobioside, L-rhodinose-2-deoxy-L-fucose-2-deoxy-L-fucose-10-decarbomethoxy-epsilon-rhodomycinone, adxanthromycin A, landomycin B, and landomycin W.
The metabolites found in gel include aloin 8-O-alpha-D-glucopyranoside, aloe-emodin-d5, 1-hydroxyanthrone, 6-O-(alpha-L-rhamnopyranosyl)-(1->6)-beta-D-glucopyranosyl)emodin, 8-demethyl-8-(alpha-L-rhamnosyl)tetracenomycin C, 12-demethyl-elloramycin, (10R)-1,8,10alpha-triacetoxy-3-acetoxymethyl-10-(2-O,3-O,4-O,6-O-tetraacetyl-beta-D-glucopyranosyl)anthrone, aloe-emodin dianthrone diglucoside, (9R)-9-[(9S)-2-carboxy-5-[(2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-oxalooxyoxan-2-yl]oxy-4-hydroxy-10-oxo-9H-anthracen-9-yl]-4-hydroxy-10-oxo-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-9H-anthracene-2-carboxylic acid, landomycin X, emodin-8-o-beta-gentiobioside, landomycin A, and adxanthromycin A (Table 1). The Pubchem CIDs of the identified metabolites are listed in Supplementary Tables S1-S2
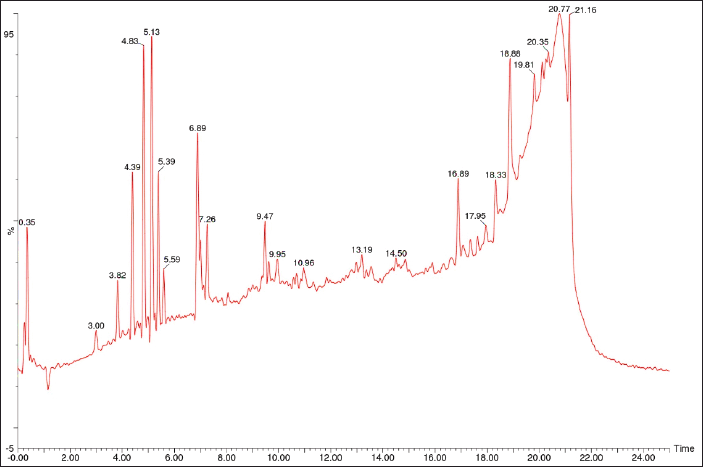 | Figure 1. LC-MS result of A. vera peel. [Click here to view] |
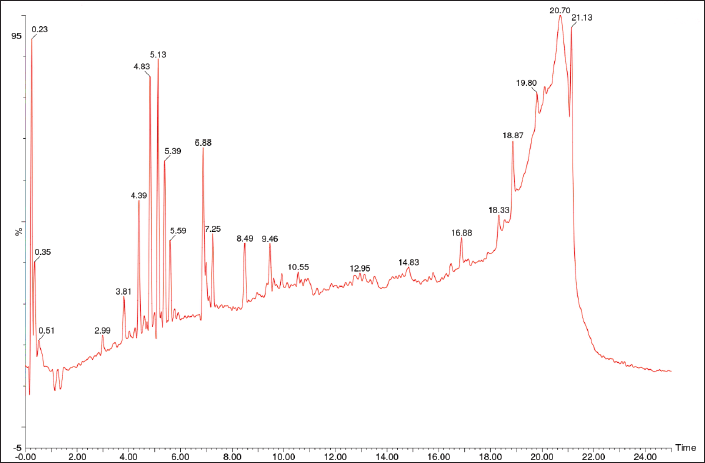 | Figure 2. LC-MS result of A. vera gel. [Click here to view] |
Molecular docking
The docking scores of vitamin C and A. vera metabolites docked to target the enzymes are shown in Table 2. The docking score, measured in kcal/mol, which is an energy per molecule unit, can represent the binding energy of a ligand against a particular protein target. The lower this score is, the higher the affinity is or the better the binding of the ligand-target complex is (Tallei et al., 2020). Due to all of the metabolites found in A. vera’s peel and gel having lower docking scores than vitamin C, they would highly likely form a better binding complex with the enzymes.
Figure 3 shows vitamin C interactions with all the target proteins, while Figures 4–10 show the visualization of the binding interactions. Numerous bond formations occur in the complexes of the compounds and all of the targeted enzymes, such as conventional hydrogen bond (dark green), carbon-hydrogen bond (light green), alkyl/pi-alkyl bond (light pink), pi-pi stacked bond (dark pink), pi-cation/anion bond (dark orange), pi-sulfur bond (light orange), and pi-sigma bond (purple), with hydrogen and alkyl bonds dominating the interaction.
In addition, the ligands also interact with the enzymes’ amino acids through van der Waals interactions. The interactions are also evaluated according to which amino acids in the metabolites bind to because each protein might have certain amino acids that are critical in the inhibition process (Kumar et al., 2021).
SARS-CoV-2 Omicron RBD inhibitor
There are 30 mutations that occur in the viral spike of the Omicron variant, among which eight mutations are situated in the spike RBD. These mutations invite the most interest from science and medical researchers due to their significant role as host recognition sites. D339, L371, P373, F375, N471, K440, S446, N477, K478, A484, R493, S496, R498, Y501, and H505 were the output residues of the Omicron variants. Landomycin A of A. vera, through hydrogen bond interactions, binds Ser484 and Ser493 in the mutation region of the Omicron RBD. It has been hypothesized that variants carrying multiple mutations in the RBD will probably exhibit an even higher stabilizing effect on the interaction between the viral spike and the host angiotensin-converting enzyme 2 (ACE2) receptor. Hence, landomycin A is predicted to alter this stabilizing effect of the Omicron RBD. Ortega et al. (2022) addressed several further analyses employing computational study which are needed to gain a novel perspective of the SARS-CoV-2 Omicron variant’s detailed structure, residue formation, and changes that can be potentially inhibited as a druggable binding domain (Ahmad, 2021; Mengist et al., 2021; Ortega et al., 2021, 2022).
The previous study also found that residues 301 and 430 in the main receptor-binding motif have a critical mechanism due to their role as the antibody recognition region of the viral spike. The antibody recognition site is critical for efficiency. However, the Omicron variant’s viral spike carries several mutations located in this region. Therefore, the current assumption is that the antibody will be less effective against the Omicron variant, and further study needs to be done to evaluate the immune response elicited by vaccines against recent emerging SARS-CoV-2 variants (Ewer et al., 2021; Harvey et al., 2021; Ravichandran et al., 2020).
Aloe vera metabolites show great docking scores in the Omicron RBD binding domain, with the value ranging from −6 kcal/mol to −10 kcal/mol. In terms of the binding affinity with ACE2, a significant influence of the Omicron variant RBD was found. Residues 468–473 were reported to play a role in this significant change (Kumar et al., 2021). In comparison with the wild-type RBD, the Omicron variant has a better ACE2 binding capacity. Residues T478K and N501Y are associated with increased ACE2 binding capacity (Omotuyi et al., 2022). Neither vitamin C nor landomycin A interacts with these residues. However, the B501Y mutation did not enhance Omicron infectivity and epidermal growth factor receptor (EGFR) binding due to the mutations of the charged to the uncharged polar side chain on its interface of interaction. Therefore, N501Y failed to deliver immune disruption and did not lower vaccine efficiency (Baek et al., 2021; Kazybay et al., 2022; Liu et al., 2021).
Nitric oxide synthase inhibitor
The release of ROS induces oxidative stress. In terms of improving the health conditions of COVID-19 patients, several clinical approaches have been used in order to lower ROS levels and production, as well as to repair the oxidative stress condition (Derouiche, 2020).
There are a number of studies showing that ROS plays a role in SARS-CoV-2 infections. The infections will generate a sustaining cycle of hematological damage, cytopathic hypoxia, and ROS production (Cecchini and Cecchini, 2020). During disease progression, patients with COVID-19 were shown to undergo hematological damage (Chen et al., 2020; Huang et al., 2020; Lippi and Mattiuzzi, 2020) and cytopathic hypoxia. Disseminated intravascular coagulopathy, sepsis, and decreased oxygen transport to the tissues are some of the main elements presented by COVID-19 patients. In the state of hypoxia, the body generates superoxide, such as hydrogen peroxide, that triggers inducible nitric oxide synthase (iNOS) activation resulting in an increased level of NO and peroxynitrite, which are toxic to mitochondria. Mitochondria impairment leads to cytopathic hypoxia. This creates a self-sustaining cycle and can progress to a more severe condition (Connors and Levy, 2020; Mantzarlis et al., 2017; Ottolenghi et al., 2020). In contrast with the NO-induced mitochondria impairment, the production of NO by the eNOS at the proper dose might intriguingly elevate the COVID-19 patient condition. Therefore, selectively targeting NOS is critical (Guan et al., 2020; Fang et al., 2021; Pieretti et al., 2021).
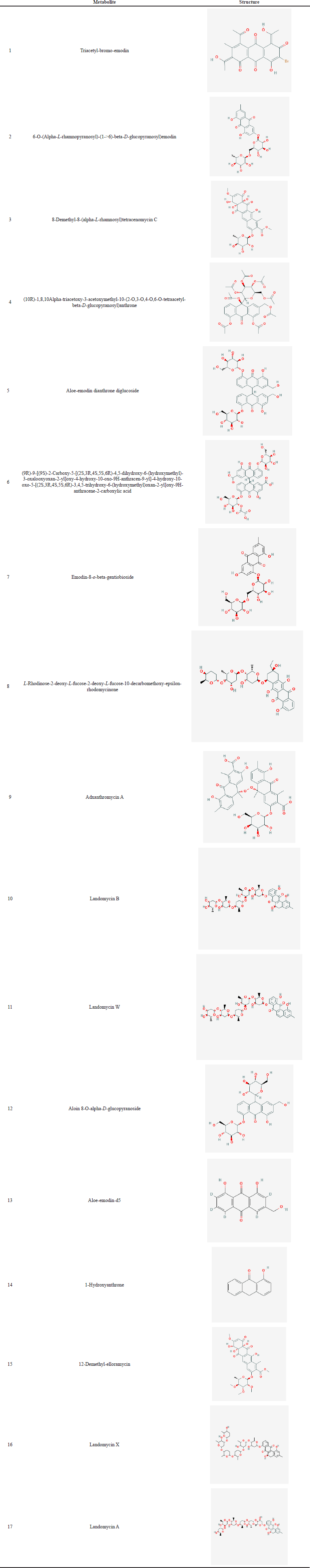 | Table 1. Chemical structure of A. vera peel and gel metabolites. [Click here to view] |
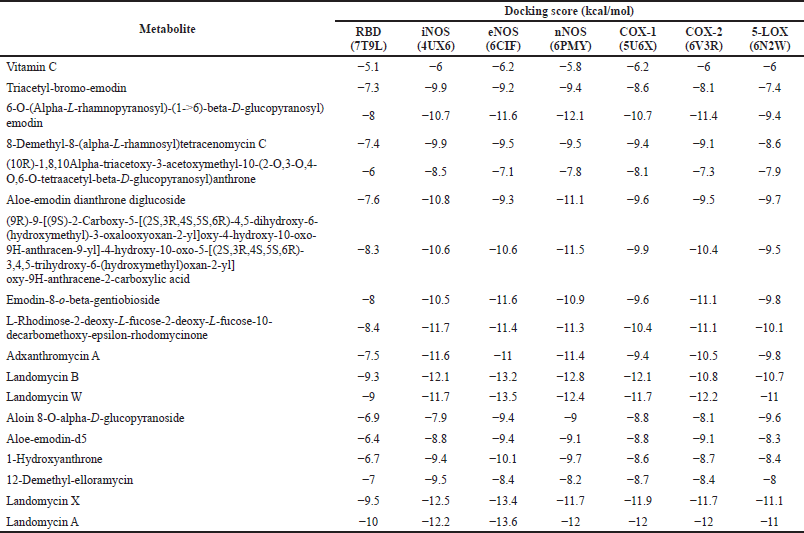 | Table 2. Molecular docking result of vitamin C and A. vera peel and gel metabolites against omicron RBD and ROS-producing enzymes. The lowest docking score values are bolded. [Click here to view] |
NOS, a homodimer nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) binding enzyme, is regulated by several modulatory interactions with the function of converting L-arginine to nitric oxide, one of the ROS. According to the localization or the regulation, NOS is available in three isoforms, namely, nNOS, iNOS, and eNOS. In the state of excessive NO production, this enzyme regulation may lead to cellular or tissue damage (Casas et al., 2020; Stuehr and Vasquez-Vivar, 2017).
The peel and gel of A. vera were shown to have metabolites that bind to NOS with a stable interaction due to the great docking scores and number of bond formations in the binding complex with landomycin X, landomycin A, and landomycin B. This indicates that these compounds can act as the most potent inhibitors. Our finding on the inhibition of NOS, the enzyme that can induce cellular damage in the state of excessive NO production, may explain the result of a study conducted by Moriyama et al. (2016), where they found that A. vera treatment increases cell number and wound healing.
Cyclooxygenase (COX) inhibitor
Viral infections have been associated with molecular processes that promote inflammation and oxidative damage during the infection (Komaravelli and Casola, 2014). COVID-19 pathogenesis is closely related to the development of oxidative stress (Chernyak et al., 2020). COxs are a part of ROS-producing enzymes (Liu et al., 2018). COX produces ROS in the intestinal mucosa. In the presence of free arachidonic acid, COX is activated in the pathway which will result in the production of IL-8 as an end-product and ROS as a byproduct (Alzoghaibi, 2013). The production of ROS leads to oxidative stress, one of the characteristics found in severe COVID-19 patients, that contributes to a complex cellular signaling process in COVID-19 disease progression.
In a severe condition, COVID-19 patients were also shown to have increased fatty acid levels along with inflammatory lipid storms. COX produced inflammatory lipids that were involved in these characteristics (Archambault et al., 2020). Activation of the COX pathway plays a pivotal role in the development of inflammation. It leads to the formation of prostaglandins like PGE2 and PGI2 which are involved in various aspects of inflammation, including the increase of IL-6 production, migration of leukocytes, and fever (McCarthy and Weinberg, 2012).
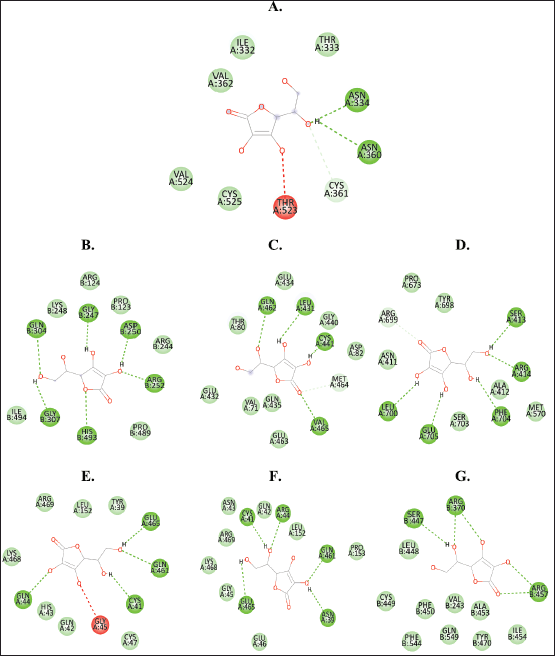 | Figure 3. Binding interaction of vitamin C with (A) Omicron RBD, (B) iNOS, (C) eNOS, (D) nNOS, (E) COX-1, (F) COX-2, and (G) 5-LOX. [Click here to view] |
Aloe vera metabolites can inhibit COX (COX-1 and COX-2) according to the molecular interactions formed in the complex in our finding. The most potential ligands as COX inhibitors are landomycin B and landomycin W. However, there is no clear line on whether COX-1 or COX-2 is more important in lowering ROS levels or in the progression of the viral infection. Therefore, the selectivity of the inhibition needs to be taken into consideration for further analysis.
5-Lipoxygenase inhibitor
The COVID-19 disease can also be characterized by alveolar and endothelial damage, thrombosis, pulmonary embolism, and inflammatory cell infiltration (Barton et al., 2020; Damiani et al., 2021; Huang et al., 2020; Zhang et al., 2020). The infiltrating inflammatory cells release ROS in order to clear the infection, leading to the production and accumulation of oxidized phospholipids (OxPLs), oxidized phospholipids, locally in the lungs. OxPLs, which were also detected in the lungs of SARS-CoV-2 patients, signal the activation and release of macrophages, TNF-α, and IL-1β (Merad and Martin, 2020; Poeckel and Funk, 2010). Respiratory symptoms are the hallmark manifestations of SARS-COV-2 infection (Terpos et al., 2020).
ROS, along with proinflammatory leukotrienes, are released via the lipoxygenase (LOX) pathway. The release can be derived from arachidonic acid or eicosapentaenoic acid or docosahexaenoic acid (Häfner et al., 2019; O’Flaherty et al., 2012). 5-LOX is one of the enzymes that mediate the LOX pathway. Other enzymes mediating the LOX pathway are 12-LOX, 15-LOX, and the epidermal LOX (Rådmark et al., 2015).
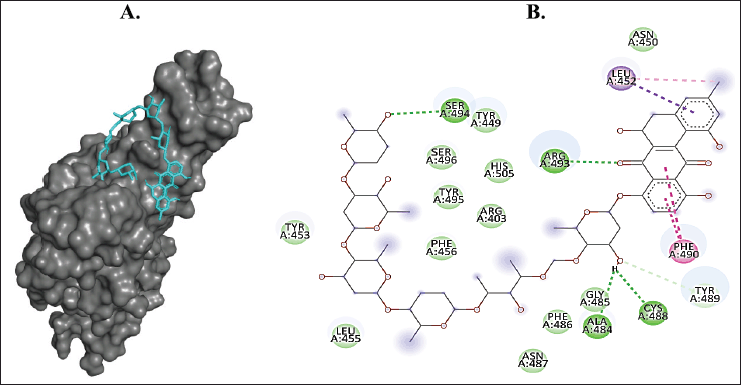 | Figure 4. Binding interaction of ligands with the lowest binding energy (landomycin A, PubChem CID: 9988748) against SARS-CoV-2 Omicron RBD. (A) The pose of interactions of the ligand (cyan) and the RBD (gray) in a 3D representation. (B) 2D representation of the pose shows the amino acids of the RBD that interact with the ligand. [Click here to view] |
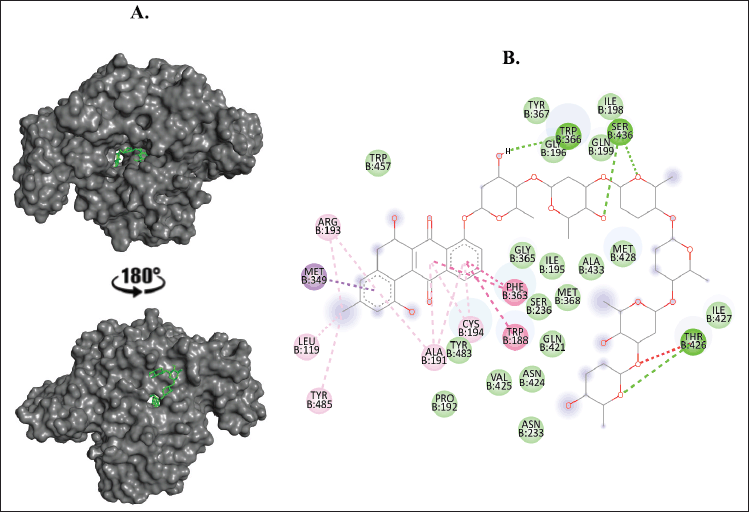 | Figure 5. Binding interaction of ligands with the lowest binding energy (landomycin X, PubChem CID: 156580396) against iNOS. (A) The pose of interactions of the ligand (green) and the iNOS (gray) in a 3D representation with 180° rotation views. (B) 2D representation of the pose shows the amino acids of the iNOS that interact with the ligand. [Click here to view] |
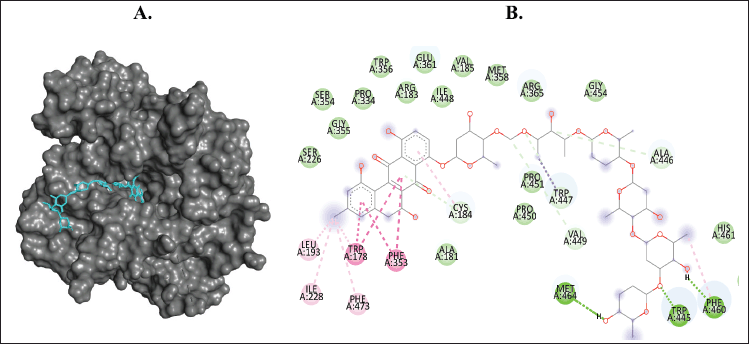 | Figure 6. Binding interaction of ligands with the lowest binding energy (landomycin A, PubChem CID: 9988748) against eNOS. (A) The pose of interactions of the ligand (cyan) and the eNOS (gray) in a 3D representation. (B) 2D representation of the pose shows the amino acids of the eNOS that interact with the ligand. [Click here to view] |
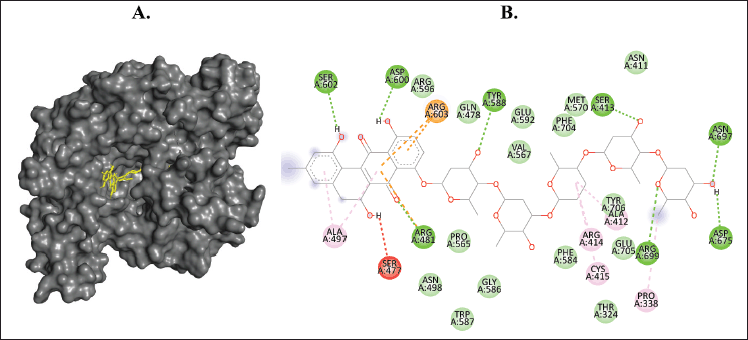 | Figure 7. Binding interaction of ligands with the lowest binding energy (landomycin B, PubChem CID: 53297405) against nNOS. (A) The pose of interactions of the ligand (yellow) and the nNOS (gray) in a 3D representation. (B) 2D representation of the pose shows the amino acids of the nNOS that interact with the ligand. [Click here to view] |
5-LOX, along with COX, can generate ROS as byproducts of its activation. In the presence of free arachidonic acid or NADH or NADPH, 5-LOX produces superoxide, one class of ROS. The activation of 5-LOX mediates the accumulation of intracellular superoxide (Alzoghaibi, 2013; Zhang et al., 2014). Landomycin W, a metabolite localized only in the peel of A. vera, is the most potential metabolite to be developed as a 5-LOX inhibitor due to the high number of bond formations and great docking scores. Landomycin W can possibly act as an inhibitor to stop 5-LOX activation. This inhibition is critical due to the activation of 5-LOX, and the release of leukotrienes may contribute to the severity of the disease (Ayola-Serrano et al., 2021). 5-LOX inhibitors can also stop the disease progression by preventing 5-LOX from inducing TNF-a activation and the translocation of NF-κB into the nucleus (Lin et al., 2014).
In severe disease progression, COVID-19 patients are characterized by robust levels of lipids produced by the COX and LOX pathways. Leukotrienes such as LTC4, LTB4, 20-COOH-LTB4, LTE4, and eoxin E4 are also increased (Archambault et al., 2020). A treatment targeting leukotrienes production has been previously offered to treat mild to severe COVID-19 infection (Funk and Ardakani, 2020; Halpin et al., 2020).
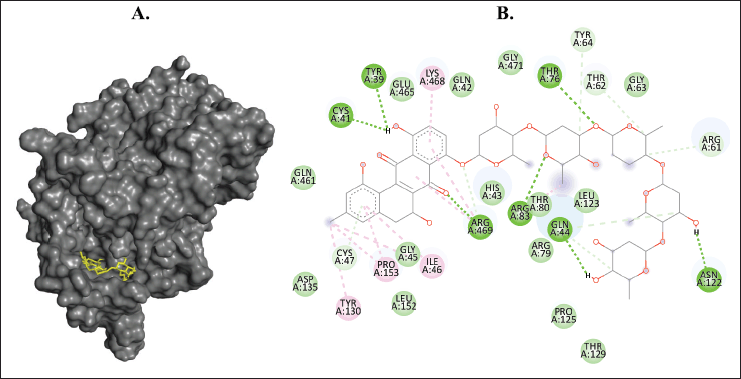 | Figure 8. Binding interaction of ligands with the lowest binding energy (landomycin B, PubChem CID: 53297405) against COX-1. (A) The pose of interactions of the ligand (yellow) and the COX-1 (gray) in a 3D representation. (B) 2D representation of the pose shows the amino acids of COX-1 that interact with the ligand. [Click here to view] |
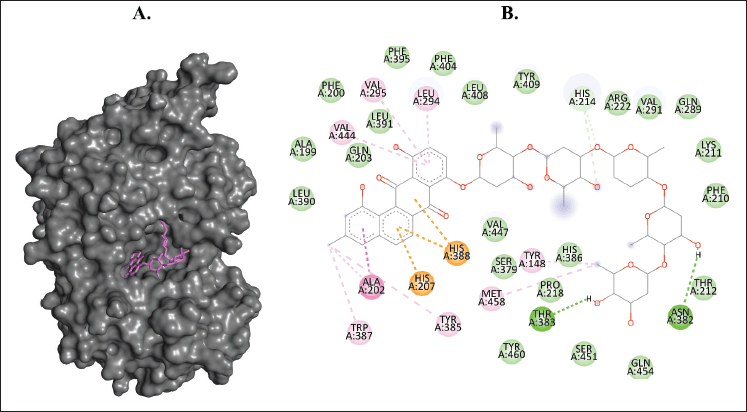 | Figure 9. Binding interaction of ligands with the lowest binding energy (landomycin W, PubChem CID: 50993663) against COX-2. (A) The pose of interactions of the ligand (violet) and the COX-2 (gray) in a 3D representation. (B) 2D representation of the pose shows the amino acids of COX-2 that interact with the ligand. [Click here to view] |
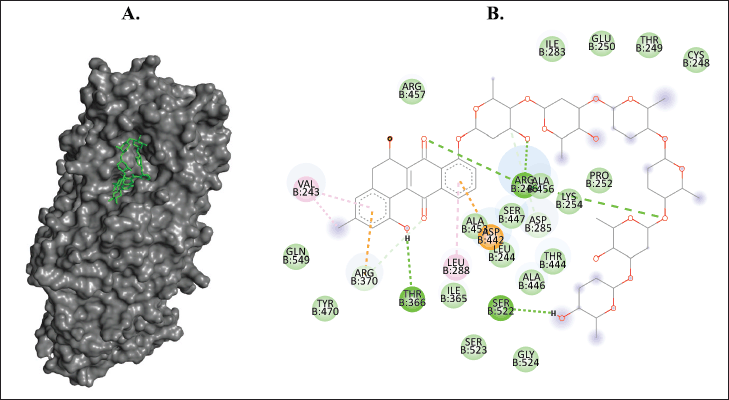 | Figure 10. Binding interaction of ligands with the lowest binding energy (landomycin X, PubChem CID: 53297405) against 5-LOX. (A) The pose of interactions of the ligand (green) and the 5-LOX (gray) in a 3D representation. (B) 2D representation of the pose shows the amino acids of 5-LOX that interact with the ligand. [Click here to view] |
CONCLUSION
In conclusion, there are differences in the metabolite composition of the peel and gel of A. vera. These metabolites of A. vera collected from North Sulawesi of Indonesia are found to be different from those collected from different cultivation areas. The docking score and bond formation evaluation of the metabolites in complex with Omicron RBD and ROS-producing enzymes suggest that A. vera can inactivate their function and alter the Omicron RBD-ACE2 complex formation and inhibit ROS production, which contributes to the pathogenesis and severity progression of COVID-19.
ACKNOWLEDGMENTS
The authors would like to thank Sam Ratulangi University for the support of this research through the UNSRAT Excellent Applied Research Scheme, which was funded by the PNBP UNSRAT fund with a Research Contract No. 821/UN12.13/LT/2021.
AUTHORS’ CONTRIBUTIONS
BJK and F designed the study. BJK and F carried out the laboratory work. BJK, MJK, and TET did the in silico work. BJK, MJK, and TET analyzed the data. BJK wrote the draft of the manuscript. BJK, MJK, TET, and F read and enriched the manuscript discussion. F and TET reviewed the final manuscript. All authors approved the final version of the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmad L. Implication of SARS-CoV-2 immune escape spike variants on secondary and vaccine breakthrough infections. Front Immunol, 2021; 12:742167; https://doi.org/10.3389/fimmu.2021.742167 CrossRef
Alzoghaibi MA. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J Gastroenterol, 2013; 19(39):6540–7; https://doi.org/10.3748/wjg.v19.i39.6540 CrossRef
André-Lévigne D, Modarressi A, Pepper MS, Pittet-Cuénod B. Reactive oxygen species and NOX enzymes are emerging as key players in cutaneous wound repair. Int J Mol Sci, 2017; 18(10):2149; https://doi.org/10.3390/ijms18102149 CrossRef
Archambault A-S, Zaid Y, Rakotoarivelo V, Doré E, Dubuc I, Martin C, Amar Y, Cheikh A, Fares H, El Hassan A, Tijani Y, Laviolette M, Boilard E, Flamand L, Flamand N. Lipid storm within the lungs of severe COVID-19 patients: extensive levels of cyclooxygenase and lipoxygenase-derived inflammatory metabolites. MedRxiv; https://doi.org/10.1101/2020.12.04.20242115 CrossRef
Ayola-Serrano NC, Roy N, Fathah Z, Anwar MM, Singh B, Ammar N, Sah R, Elba A, Utt RS, Pecho-Silva S, Rodriguez-Morales AJ, Dhama K, Quraishi S. The role of 5-lipoxygenase in the pathophysiology of COVID-19 and its therapeutic implications. Inflamm Res, 2021; 70(8):877–89; https://doi.org/10.1007/s00011-021-01473-y CrossRef
Baek M, DiMaio F, Anishchenko I, Dauparas J, Ovchinnikov S, Lee GR, Wang J, Cong Q, Kinch LN, Schaeffer RD, Millán C, Park H, Adams C, Glassman CR, DeGiovanni A, Pereira JH, Rodrigues AV, van Dijk AA, Ebrecht AC, Opperman DJ, Sagmeister T, Buhlheller C, Pavkov-Keller T, Rathinaswamy MK, Dalwadi U, Yip CK, Burke JE, Garcia KC, Grishin NV, Adams PD, Read RJ, Baker D. Accurate prediction of protein structures and interactions using a three-track neural network. Science, 2021; 373(6557):871–6; https://doi.org/10.1126/science.abj8754 CrossRef
Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol, 2020; 153(6):725–33; https://doi.org/10.1093/ajcp/aqaa062 CrossRef
Bista R, Ghimire A, Subedi S. Phytochemicals and antioxidant activities of Aloe Vera (Aloe Barbadensis). J Nut Sci Heal Diet, 2020; 1(1):25–36; https://doi.org/10.47890/JNSHD/2020/RBista/10243803 CrossRef
Casas AI, Nogales C, Mucke HAM, Petraina A, Cuadrado A, Rojo AI, Ghezzi P, Jaquet V, Augsburger F, Dufrasne F, Soubhye J, Deshwal S, Di Sante M, Kaludercic N, Di Lisa F, Schmidt HHHW. On the clinical pharmacology of reactive oxygen species. Pharmacol Rev, 2020; 72(4):801–28; https://doi.org/10.1124/pr.120.019422 CrossRef
Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses, 2020; 143:110102; https://doi.org/10.1016/j.mehy.2020.110102 CrossRef
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 2020; 395(10223):507–13; https://doi.org/10.1016/S0140-6736(20)30211-7 CrossRef
Chernyak BV, Popova EN, Prikhodko AS, Grebenchikov OA, Zinovkina LA, Zinovkin RA. COVID-19 and oxidative stress. Biochem, 2020; 85(12):1543–53; https://doi.org/10.1134/S0006297920120068 CrossRef
Cheshire DR, Åberg A, Andersson GMK, Andrews G, Beaton HG, Birkinshaw TN, Boughton-Smith N, Connolly S, Cook TR, Cooper A, Cooper SL, Cox D, Dixon J, Gensmantel N, Hamley PJ, Harrison R, Hartopp P, Käck H, Leeson PD, Luker T, Mete A, Millichip I, Nicholls DJ, Pimm AD, St-Gallay SA, Wallace AV. The discovery of novel, potent and highly selective inhibitors of inducible nitric oxide synthase (iNOS). Bioorg Med Chem Lett, 2011; 21(8):2468–71; https://doi.org/10.1016/j.bmcl.2011.02.061 CrossRef
Cinelli MA, Reidl CT, Li H, Chreifi G, Poulos TL, Silverman RB. First contact: 7-phenyl-2-aminoquinolines, potent and selective neuronal nitric oxide synthase inhibitors that target an isoform-specific aspartate. J Med Chem, 2020; 63(9):4528–54; https://doi.org/10.1021/acs.jmedchem.9b01573 CrossRef
Cingolani G, Panella A, Perrone MG, Vitale P, Di Mauro G, Fortuna CG, Armen RS, Ferorelli S, Smith WL, Scilimati A. Structural basis for selective inhibition of cyclooxygenase-1 (COX-1) by diarylisoxazoles mofezolac and 3-(5-chlorofuran-2-yl)-5-methyl-4-phenylisoxazole (P6). Eur J Med Chem, 2017; 138:661–8; https://doi.org/10.1016/j.ejmech.2017.06.045 CrossRef
Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood, 2020; 135(23):2033–40; https://doi.org/10.1182/blood.2020006000 CrossRef
Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol, 2015; 1263:243–50; https://doi.org/10.1007/978-1-4939-2269-7_19 CrossRef
Damiani S, Fiorentino M, De Palma A, Foschini MP, Lazzarotto T, Gabrielli L, Viale PL, Attard L, Riefolo M, D’Errico A. Pathological post-mortem findings in lungs infected with SARS-CoV-2. J Pathol, 2021; 253(1):31–40; https://doi.org/10.1002/path.5549 CrossRef
Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res, 2020; 51(5):384–7; https://doi.org/10.1016/j.arcmed.2020.04.019 CrossRef
Derouiche S. Oxidative stress associated with SARS-Cov-2 (COVID-19) increases the severity of the lung disease - a systematic review. J Infect Dis Epidemiol, 2020; 6:121; https://doi.org/10.23937/2474-3658/1510121 CrossRef
Dharajiya D, Pagi N, Jasani H, Patel P. Antimicrobial activity and phytochemical screening of Aloe vera (Aloe barbadensis Miller). Int J Curr Microbiol Appl Sci, 2017; 6(3):2152–62; https://doi.org/10.20546/ijcmas.2017.603.246 CrossRef
Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol, 2014; 4:177; https://doi.org/10.3389/fphar.2013.00177 CrossRef
Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, Flaxman A, Wright D, Bellamy D, Bittaye M, Dold C, Provine NM, Aboagye J, Fowler J, Silk SE, Alderson J, Aley PK, Angus B, Berrie E, Bibi S, Cicconi P, Clutterbuck EA, Chelysheva I, Folegatti PM, Fuskova M, Green CM, Jenkin D, Kerridge S, Lawrie A, Minassian AM, Moore M, Mujadidi Y, Plested E, Poulton I, Ramasamy NM, Robinson H, Song R, Snape MD, Tarrant R, Voysey M, Watson MEE, Douglas AD, Hill AVS, Gilbert SC, Pollard AJ, Lambe T, Oxford COVID Vaccine Trial Group. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med, 2021; 27(2):270–8; https://doi.org/10.1038/s41591-020-01194-5 CrossRef
Fang W, Jiang J, Su L, Shu T, Liu H, Lai S, Ghiladi RA, Wang J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic Biol Med, 2021; 163:153–62; https://doi.org/10.1016/j.freeradbiomed.2020.12.008 CrossRef
Funk CD, Ardakani A. A novel strategy to mitigate the hyperinflammatory response to COVID-19 by targeting leukotrienes. Front Pharmacol, 2020; 11:1214; https://doi.org/10.3389/fphar.2020.01214 CrossRef
Giannakoudakis DA, Hosseini-Bandegharaei A, Tsafrakidou P, Triantafyllidis KS, Kornaros M, Anastopoulos I. Aloe vera waste biomass-based adsorbents for the removal of aquatic pollutants: a review. J Environ Manage, 2018; 227:354–64; https://doi.org/10.1016/j.jenvman.2018.08.064 CrossRef
Gilbert NC, Gerstmeier J, Schexnaydre EE, Börner F, Garscha U, Neau DB, Werz O, Newcomer ME. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat Chem Biol, 2020; 16(7):783–90; https://doi.org/10.1038/s41589-020-0544-7 CrossRef
Guan SP, Seet RCS, Kennedy BK. Does eNOS derived nitric oxide protect the young from severe COVID-19 complications? Ageing Res Rev, 2020; 64:101201; https://doi.org/10.1016/j.arr.2020.101201 CrossRef
Häfner A-K, Kahnt AS, Steinhilber D. Beyond leukotriene formation—the noncanonical functions of 5-lipoxygenase. Prostaglandins Other Lipid Mediat, 2019; 142:24–32; https://doi.org/10.1016/j.prostaglandins.2019.03.003 CrossRef
Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, Agusti AA, Vogelmeier CF. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med, 2020; 203(1):24–36; https://doi.org/10.1164/rccm.202009-3533SO CrossRef
Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, COVID-19 Genomics UK (COG-UK) Consortium, Peacock SJ, Robertson DL. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol, 2021; 19(7):409–24; https://doi.org/10.1038/s41579-021-00573-0 CrossRef
H?? M, Dziedzic K, Górecka D, J?drusek-Goli?ska A, Gujska E. Aloe vera (L.) Webb.: natural sources of antioxidants - a review. Plant Foods Hum Nutr, 2019; 74(3):255–65; https://doi.org/10.1007/s11130-019-00747-5 CrossRef
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 2020; 395(10223):497–506; https://doi.org/10.1016/S0140-6736(20)30183-5
Kazybay B, Ahmad A, Mu C, Mengdesh D, Xie Y. Omicron N501Y mutation among SARS-CoV-2 lineages: insilico analysis of potent binding to tyrosine kinase and hypothetical repurposed medicine. Travel Med Infect Dis, 2022; 45:102242; https://doi.org/10.1016/j.tmaid.2021.102242 CrossRef
Komaravelli N, Casola A. Respiratory viral infections and subversion of cellular antioxidant defenses. J Pharmacogenomics Pharmacoproteomics, 2014; 5(4):1000141; https://doi.org/10.4172/2153-0645.1000141 CrossRef
Kumar S, Yadav M, Yadav A, Yadav JP. Impact of spatial and climatic conditions on phytochemical diversity and in vitro antioxidant activity of Indian Aloe vera (L.) Burm.f. South Afr J Bot, 2017; 111:50–9; https://doi.org/10.1016/j.sajb.2017.03.012 CrossRef
Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and delta variant of SARS-CoV-2: a comparative computational study of spike protein. J Med Virol, 2021; 1–9; https://doi.org/10.1002/jmv.27526 CrossRef
Li H, Evenson RJ, Chreifi G, Silverman RB, Poulos TL. Structural basis for isoform selective nitric oxide synthase inhibition by thiophene-2-carboximidamides. Biochemistry, 2018; 57(44):6319–25; https://doi.org/10.1021/acs.biochem.8b00895 CrossRef
Lin H-C, Lin T-H, Wu M-Y, Chiu Y-C, Tang C-H, Hour M-J, Liou H-C, Tu H-J, Yang R-S, Fu W-M. 5-Lipoxygenase inhibitors attenuate TNF-α-induced inflammation in human synovial fibroblasts. PLoS One, 2014; 9(9):e107890; https://doi.org/10.1371/journal.pone.0107890 CrossRef
Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther, 2020; 42(2):116–7; https://doi.org/10.1016/j.htct.2020.03.001 CrossRef
Liu Y, Liu J, Plante KS, Plante JA, Xie X, Zhang X, Ku Z, An Z, Scharton D, Schindewolf C, Widen SG, Menachery VD, Shi PY, Weaver SC. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature, 2021; https://doi.org/10.1038/s41586-021-04245-0 CrossRef
Liu Z, Ren Z, Zhang J, Chuang CC, Kandaswamy E, Zhou T, Zuo L. Role of ROS and nutritional antioxidants in human diseases. Front Physiol, 2018; 9:477; https://doi.org/10.3389/fphys.2018.00477 CrossRef
López A, de Tangil MS, Vega-Orellana O, Ramírez AS, Rico M. Phenolic constituents, antioxidant and preliminary antimycoplasmic activities of leaf skin and flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules, 2013; 18(5):4942–54; https://doi.org/10.3390/molecules18054942. CrossRef
Mantzarlis K, Tsolaki V, Zakynthinos E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid Med Cell Longev, 2017; 2017:5985209; https://doi.org/10.1155/2017/5985209 CrossRef
McCarthy MK, Weinberg JB. Eicosanoids and respiratory viral infection: coordinators of inflammation and potential therapeutic targets. Mediators Inflamm, 2012; 2012:236345; https://doi.org/10.1155/2012/236345 CrossRef
Mengist HM, Kombe Kombe AJ, Mekonnen D, Abebaw A, Getachew M, Jin T. Mutations of SARS-CoV-2 spike protein: implications on immune evasion and vaccine-induced immunity. Semin Immunol, 2021; 55:101533; https://doi.org/10.1016/j.smim.2021.101533 CrossRef
Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol, 2020; 20(6):355–62; https://doi.org/10.1038/s41577-020-0331-4 CrossRef
Moriyama M, Moriyama H, Uda J, Kubo H, Nakajima Y, Goto A, Akaki J, Yoshida I, Matsuoka N, Hayakawa T. Beneficial effects of the genus aloe on wound healing, cell proliferation, and differentiation of epidermal keratinocytes. PLoS One, 2016; 11(10):1–15; https://doi.org/10.1371/journal.pone.0164799 CrossRef
Mousavi SS, Karami A, Haghighi TM, Tumilaar SG, Fatimawali, Idroes R, Mahmud S, Celik I, A?agündüz D, Tallei TE, Emran TB, Capasso R. In silico evaluation of Iranian medicinal plant phytoconstituents as inhibitors against main protease and the receptor-binding domain of SARS-CoV-2. Molecules, 2021; 26(18):5724; https://doi.org/10.3390/molecules26185724 CrossRef
Muthukumaran P, Divya R, Indhumathi E, Keerthika C. Total phenolic and flavonoid content of membrane processed Aloe vera extract: a comparative study. Int Food Res J, 2018; 25(4):1450–6; http://ifrj.upm.edu.my/25%20(04)%202018/(17).pdf
Nalimu F, Oloro J, Kahwa I, Ogwang PE. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe ferox. Futur J Pharm Sci, 2021; 7(1):145; https://doi.org/10.1186/s43094-021-00296-2 CrossRef
Nur S, Aisyah AN, Lukitaningsih E, Rumiyati, Juhardi RI, Andirah R, Hajar AS. Evaluation of antioxidant and cytotoxic effect against cancer cells line of Angiopteris ferox Copel tuber and its compounds by LC-MS analysis. J Appl Pharm Sci, 2021; 11(8):54–61; https://doi.org/10.7324/JAPS.2021.110808 CrossRef
O’Flaherty JT, Hu Y, Wooten RE, Horita DA, Samuel MP, Thomas MJ, Sun H, Edwards IJ. 15-Lipoxygenase metabolites of docosahexaenoic acid inhibit prostate cancer cell proliferation and survival. PLoS One, 2012; 7(9):e45480; https://doi.org/10.1371/journal.pone.0045480 CrossRef
Omotuyi O, Olubiyi O, Nash O, Afolabi E, Oyinloye B, Fatumo S, Femi-Oyewo M, Bogoro S. SARS-CoV-2 Omicron spike glycoprotein receptor binding domain exhibits super-binder ability with ACE2 but not convalescent monoclonal antibody. Comput Biol Med, 2022; 142:105226; https://doi.org/10.1016/j.compbiomed.2022.105226 CrossRef
Ortega JT, Jastrzebska B, Rangel HR. Omicron SARS-CoV-2 variant spike protein shows an increased affinity to the human ACE2 receptor: an in silico analysis. Pathog, 2022; 11(1):45; https://doi.org/10.3390/pathogens11010045 CrossRef
Ortega JT, Pujol FH, Jastrzebska B, Rangel HR. Mutations in the SARS-CoV-2 spike protein modulate the virus affinity to the human ACE2 receptor, an in silico analysis. EXCLI J, 2021; 20:585–600; https://doi.org/10.17179/excli2021-3471 CrossRef
Ottolenghi S, Zulueta A, Caretti A. Iron and sphingolipids as common players of (Mal) adaptation to hypoxia in pulmonary diseases. Int J Mol Sci, 2020;21(1):307; https://doi.org/10.3390/ijms21010307 CrossRef
Pieretti JC, Rubilar O, Weller RB, Tortella GR, Seabra AB. Nitric oxide (NO) and nanoparticles – potential small tools for the war against COVID-19 and other human coronavirus infections. Virus Res, 2021; 291:198202; https://doi.org/10.1016/j.virusres.2020.198202 CrossRef
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev, 2017; 2017:8416763; https://doi.org/10.1155/2017/8416763 CrossRef
Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res, 2010; 86(2):243–53; https://doi.org/10.1093/cvr/cvq016 CrossRef
Raad B, Ali S, Rehman K, Akhtar N, Ullah B, Wali S. Phytochemical screening and biological activities of Aloe vera (L.) Burm. F. Pure Appl Biol, 2020; 10(2):360–7; http://dx.doi.org/10.19045/bspab.2021.100039
Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta Mol Cell Biol Lipids, 2015; 1851(4):331–9; https://doi.org/10.1016/j.bbalip.2014.08.012 CrossRef
Ravichandran S, Coyle EM, Klenow L, Tang J, Grubbs G, Lie S, Wang T, Golding H, Khurana S. Antibody signature induced by SARS-CoV-2 spike protein immunogens in rabbits. Sci Transl Med, 2020; 12(550):eabc3539; https://doi.org/10.1126/scitranslmed.abc3539 CrossRef
Sammar M, Abu?Farich B, Rayan I, Falah M, Rayan A. Correlation between cytotoxicity in cancer cells and free radical?scavenging activity: in vitro evaluation of 57 medicinal and edible plant extracts. Oncol Lett, 2019; 18(6):6563–71; https://doi.org/10.3892/ol.2019.11054 CrossRef
Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP. Pharmacological update properties of Aloe Vera and its major active constituents. Molecules, 2020; 25(6):1324; https://doi.org/10.3390/molecules25061324 CrossRef
Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol, 2020; 21(7):363–83; https://doi.org/10.1038/s41580-020-0230-3 CrossRef
Stuehr DJ, Vasquez-Vivar J. Nitric oxide synthases-from genes to function. Nitric oxide Biol Chem, 2017; 63:29; https://doi.org/10.1016/j.niox.2017.01.005 CrossRef
Tallei TE, Tumilaar SG, Niode NJ, Fatimawali, Kepel BJ, Idroes R, Effendi Y, Sakib SA, Emran TB. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: a molecular docking study. Scientifica (Cairo), 2020; 2020:6307457; https://doi.org/10.1155/2020/6307457 CrossRef
Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, Psaltopoulou T, Gerotziafas G, Dimopoulos MA. Hematological findings and complications of COVID-19. Am J Hematol, 2020; 95(7):834–47; https://doi.org/10.1002/ajh.25829 CrossRef
Uddin MJ, Xu S, Crews BC, Aleem AM, Ghebreselasie K, Banerjee S, Marnett LJ. Harmaline analogs as substrate-selective cyclooxygenase-2 inhibitors. ACS Med Chem Lett, 2020; 11(10):1881–5; https://doi.org/10.1021/acsmedchemlett.9b00555 CrossRef
Zhang H, Lei Y, Yuan P, Li L, Luo C, Gao R, Tian J, Feng Z, Nice EC, Sun J. ROS-mediated autophagy induced by dysregulation of lipid metabolism plays a protective role in colorectal cancer cells treated with gambogic acid. PLoS One, 2014; 9(5):1–14; https://doi.org/10.1371/journal.pone.0096418 CrossRef
Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, Zhang S, Cao T, Yang C, Li M, Guo G, Chen X, Chen Y, Lei M, Liu H, Zhao J, Peng P, Wang CY, Du R. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med, 2020; 172(9):629–32; https://doi.org/10.7326/M20-0533 CrossRef
SUPPLEMENTARY MATERIAL
Supplementary data can be downloaded from the journal’s website