INTRODUCTION
Skin, hair, and nail fungal infections are a widespread public health concern worldwide but are seldom addressed according to a population-based survey (dos Santos et al., 2010). Cutaneous fungal infections are projected to be prevalent in 20%–25% of the world’s population (Male, 1990). Dermatophytes, including Trichophyton, Microsporum, and Epidermophyton, could infect the stratum corneum and keratinized tissues and are the major culprits of skin fungal infections (Kwon-Chung and Bennett, 1992). Even though fungal infection epidemiology has become a remarkable global concern, issues regarding access to antifungal drugs and their variable cost are continuously faced by the world (Kneale et al., 2016). Several antifungal compounds have been recently discovered and developed. In particular, those from plants exhibit low toxicities and contain abundant bioactive secondary metabolites, such as tannins, terpenoids, saponins, alkaloids, and flavonoids (Arif et al., 2009).
Numerous plants, including Inga dulcis, Schinus terebinthifolius Raddi, Alternanthera brasiliana Kuntze, Piper regnellii, Herissantia crispa, Rubus ulmifolius, Rumex acetosa, and Baccharis dracunculifolia, have antifungal properties (Johann et al., 2007). However, the antifungal activity of Zingiber zerumbet (L.) rhizome extract in humans has rarely been documented. Zingiber zerumbet is a ginger species that has leafy stems and reaches a height of 1.2 m (Roskov et al., 2014). Although it originated in Asia, this plant can now be found in various tropical nations. Zingiber zerumbet is also known as awapuhi, bitter ginger, shampoo ginger, and pinecone ginger (Akbar, 2020). Its rhizomes have been used as a flavoring agent and appetizer in various cuisines, and its rhizome extracts have been employed in traditional herbal medicine for anti-inflammatory properties, pain relief, and treatment of worm infestation and diarrhea (Yob et al., 2011). Zingiber zerumbet rhizome extract has been recently reported as an antifungal agent in plants. The primary constituent of edible wild ginger rhizome, Z. zerumbet (L.), is zerumbone, which exhibits various pharmacological properties, including anticancer, anti-inflammatory, antioxidant, antibacterial, antinociceptive, hepatoprotective, and immunomodulatory ones (Kitayama et al., 2003). Thus, this investigation examined the novel antifungal activity of Z. zerumbet rhizome extract.
This study determined the feasibility of developing a new antifungal agent from Z. zerumbet rhizome extract. Three solvents (hexane, dichloromethane, and ethanol) were used to extract the rhizome. The obtained extracts were discovered to have antifungal, antibacterial, antioxidant, and anti-inflammatory properties. Finally, the cytotoxicity of the extracts was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test.
MATERIALS AND METHODS
Chemicals and materials
Ethanol (Batch No. 20060068), dichloromethane (Batch No. 21080094), and hexane (Batch No. 21070007) were purchased from RCI Labscan, Thailand. Standard gallic acid (Batch No. A0422721) and zerumbone (Batch No. S00321110) were obtained from Acros Organics and ChemFaces (China), respectively, and 2,2?-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) (Batch No. Z10G016) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Batch No. Q16G021) were purchased from Alfa Aesar, UK. All other chemicals and reagents were of analytical grade.
Extraction of Z. zerumbet (L.) rhizome
Fresh rhizomes of Z. zerumbet (L.) were collected in February 2022 from Chanthaburi Province. The rhizomes were cleaned by washing with tap water, and a portion was dried in a hot air oven at 50°C for 48 hours. The dried rhizomes were ground using a cutting mill (SM100, Retsch GmbH, Haan, Germany) until they can pass through a 0.5 mm sieve, kept in sealed containers, and protected from light until further use. The specimens were identified by Dr. Boonyadist Vongsak, Faculty of Pharmaceutical Sciences, Burapha University, Thailand. The voucher specimens (KM No. 0415001, KM No. 0415002, and KM No. 0415003) were deposited at the Faculty of Pharmaceutical Sciences, Burapha University, Thailand. For the extraction, the Z. zerumbet (L.) rhizomes were soaked in hexane, dichloromethane, and ethanol as presented in Figure 1.
Biomarker monitoring by high-performance liquid chromatography (HPLC)
Zerumbone, the major active compound of Z. zerumbet rhizomes, was selected as the biomarker from the extract (Kitayama et al., 2003). This compound was monitored and quantified using HPLC (DGU-20A5R, Shimadzu, Kyoto, Japan) following the validated method of Rahman (Rahman et al., 2013). Stainless steel with 5 μm particle size (4.6 mm internal diameter × 250 mm length) analytical symmetry column (Merck, Darmstadt, Germany) packed with a dimethyl octylsilane- (C18-) bonded amorphous silica stationary phase was used. The mobile phase was composed of a binary mixture of HPLC-grade acetonitrile and purified water at a gradient ratio of 65%:35%–75%:25% (v/v) that was blended within 26 minutes, freshly prepared for each run, and degassed before use. The injection volume was 10 μl, and the flow rate was 1 ml per minute. The samples were examined by ultraviolet (UV) detection at a wavelength of 250 nm. For the standard curve, the reference standard was injected and determined at five concentration levels.
Antibacterial activity test
Minimal inhibitory concentration (MIC) of Z. zerumbet rhizome extract
The MIC of Z. zerumbet rhizome extract was calculated through macrobroth dilution to determine its appropriate loading dose for topical formulation. Staphylococcus aureus and Staphylococcus epidermidis were transferred to Mueller–Hinton broth (MHB) and incubated at 37°C for 3–4 hours. The extracts of Z. zerumbet rhizomes were diluted to concentrations of 1,000, 500, 250, 125, 62.5, 31.25, 15.6, 7.8, and 3.9 μg/ml in test tubes. Afterward, 5 ml of MHB was added to each test tube containing the extract. The extracts of Z. zerumbet rhizomes and microbe mixes were then incubated at 37°C for 20–24 hours. Microbe growth was monitored by determining the turbidity of the mixture in the test tubes following their incubation. The lowest concentration of Z. zerumbet rhizome extract that can inhibit microorganism growth was defined as the MIC (Wiegand et al., 2008).
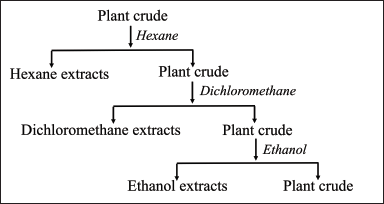 | Figure 1. Extraction of Z. zerumbet Linn crude extract. [Click here to view] |
Minimal bactericidal concentration (MBC) of Z. zerumbet rhizome extract
The MBC of Z. zerumbet rhizome extract was determined by streaking the turbid mixture from the MIC test onto the surface of Mueller–Hinton agar (MHA) and incubating it at 37°C for 20–24 hours. The lowest concentration of Z. zerumbet rhizome extract that can inhibit the formation of microbial colonies on the MHA plate was defined as the MBC.
Antifungal activity test
MIC of Z. zerumbet rhizome extract
The MIC of Z. zerumbet rhizome extract was determined through broth dilution. Candida albicans was incubated in Sabouraud dextrose broth at 37°C for 20–24 hours and then diluted to a turbidity of 0.5 McFarland at 1 × 108 CFU/ml C. albicans. The specimens were then incubated in Z. zerumbet rhizome extracts at different concentrations (1,000, 500, 250, 125, 62.5, 31.25, 15.6, 7.8, 3.9, and 1.9 μg/ml) at 37°C for 20–24 hours. Microbe growth was monitored by determining the turbidity of the mixture in the test tubes following their incubation. The lowest concentration of Z. zerumbet rhizome extract that can inhibit microorganism growth was defined as the MIC (Anyanwu, 2012).
Minimal fungicidal concentration (MFC) of Z. zerumbet rhizome extract
The MFC of Z. zerumbet rhizome extract was determined by streaking the turbid mixture from the MIC test onto the surface of Sabouraud dextrose agar (SDA), which was then incubated at 37°C for 20–24 hours. The lowest concentration of Z. zerumbet rhizome extract that can inhibit the formation of fungal colonies on the SDA plate was defined as the MFC (Anyanwu, 2012).
Antioxidant activity test
The antioxidant activity of Z. zerumbet rhizome extract was determined by a radical-scavenging assay using 2,20-azino-bis-ABTS and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. Zingiber zerumbet rhizome extract was prepared at concentrations of 0.3125–1 mg/ml. The antioxidant activity of Z. zerumbet rhizome extract was then benchmarked with the gallic acid standard in the concentration range of 2.5–0.1953 μg/ml.
ABTS assay
ABTS radical-scavenging assay is a widely used method for determining the antioxidant activity of hydrophilic and lipophilic compounds. This technique was adopted in this study following the modified version by Arumugam et al. (2014). In brief, 7 mM ABTS solution was prepared and kept in the dark at room temperature (25°C) for 18 hours. Different concentrations of Z. zerumbet rhizome extracts (0.3125–1 mg/ml) were mixed with 160 μl of 7 mM ABTS solution in 96-well microplates for testing. The final absorbance (Abs) was determined at 734 nm. ABTS radical-scavenging activity was calculated using the following equation:
where Abs control is the Abs of ABTS radical and methanol and Abs sample is the Abs of ABTS radical and Z. zerumbet rhizome extracts. All experiments were performed in triplicate.
DPPH assay
DPPH free radical-scavenging assay is a simple method for determining the antioxidant activity of extracts in a short period of time. This technique is also used to measure the antiradical activity of an antioxidant by measuring the decrease in DPPH Abs at 517 nm. This decrease appears as a change in color from purple to yellow due to hydrogen being donated to form a stable DPPH-H molecule. This study used the modified version of the DPPH free radical-scavenging assay by Omokhua-Uyi et al. (2020). Six different concentrations (0.5, 0.25, 0.125, 0.625, and 0.3125 mg/ml) of the extract were evaluated. The DPPH solution was prepared at a concentration of 60 μM and then added with 40 μl of the ethanol extract. The mixture was vigorously shaken and allowed to stand in the dark for 45 minutes. A spectrophotometer was used to determine the decrease in Abs at 517 nm relative to a blank sample (ethanol). The DPPH radical-scavenging activity was calculated using a calibration curve that was obtained with various extract concentrations and described in the following equation:
where Abs control is the Abs of DPPH radical and methanol and Abs sample is the Abs of DPPH radical and Z. zerumbet rhizome extracts. All experiments were replicated three times.
Anti-inflammatory activity test
Antiprotein denaturation assays were used to determine the anti-inflammatory activity of Z. zerumbet rhizome extracts. In brief, 0.2% bovine serum albumin (BSA) was dissolved in Tris-HCl buffer (pH 6.8). Zingiber zerumbet rhizome extracts were then added and mixed with the BSA solution, and the mixture was heated to 75°C for 10 minutes and allowed to cool for 20 minutes. The turbidity of the sample was then determined using a UV/Vis spectrometer set to 660 nm. The inhibition of protein denaturation (BSA) was calculated as a percentage using the following equation:
where Abs control is the Abs of the sample before heat and Abs treated is the Abs of the sample after heat treatment. Diclofenac sodium was applied as a positive control. All experiments were performed in triplicate.
Cytotoxicity test
Cell viability was assayed using the colorimetric method with. Medical research council cell strain 5 (MRC-5) MRC-5 cells were seeded at a density of 10,000 cells per well in a 96-well plate and incubated at 37°C in a 5% CO2 atmosphere for 24 hours. The cells were then treated in triplicate with 15.625, 31.25, 62.5, 125, 250, 500, and 1,000 g/ml Z. zerumbet rhizome extract for 24 hours. Afterward, 10 μl of 5 mg/ml MTT in phosphate buffered saline solution was added directly to each well, followed by incubation at 37°C for 3 hours. After the cultured medium was aspirated, 100 μl aliquot of absolute dimethyl sulfoxide was added. Abs [optical density (OD)] values were determined at a wavelength of 570 nm using a microplate reader (Metertech, Taiwan). The cells treated with various concentrations of Z. zerumbet rhizome extract were photographed to monitor their viability. The following equation was used to determine the percentage of viable cells:
Statistical analysis
Data were statistically analyzed using one-way ANOVA, followed by post hoc Tukey’s honestly significant difference test for the data from three independent experiments.
RESULTS AND DISCUSSION
Extraction yield and appearance
The yield percentage and physical appearance of Z. zerumbet rhizome crude extracts obtained using various solvents are shown in Table 1. The yield from hexane extraction was markedly higher than that from dichloromethane and ethanol extractions. This phenomenon occurred because Z. zerumbet rhizome crude extract contains a high proportion of sesquiterpenes (15-carbon skeletons), a low-polar chemical group, which caused the high extraction efficiency from hexane, a low-polar solvent (Koga et al., 2016). The viscosity and thickness of the extracts were recorded with (+) symbols, where (+) signifies low viscosity and (+++++) signifies extremely thick and viscous. According to Table 1, the viscosity and thickness of the ethanol extract were noticeably higher than those of dichloromethane and hexane. ?Compared with the dichloromethane and ethanol extracts, the hexane extract showed a liquid appearance.
Biomarker monitoring by HPLC
Zerumbone was chosen as the biomarker for extraction monitoring because it is the predominant active component of Z. zerumbet rhizome (Kitayama et al., 2003). The retention time of standard zerumbone at concentrations ranging from 10 μg/ml to 500 μg/ml was around 11 minutes, which is consistent with a previous finding (Akhtar et al., 2019). As shown in Table 1, the hexane extract contained a higher zerumbone concentration than the dichloromethane and ethanol extracts. This result is consistent with the findings on extraction yield: zerumbone has a low polarity and hence dissolves more readily in hexane than in the other solvents (Chia et al., 2021).
Antibacterial activity test
The MIC of Z. zerumbet rhizome extracts obtained using different solvents was determined by broth dilution, and their MBC was measured by subculturing the broths used for MIC determination onto fresh agar plates. As presented in Table 2, the ethanol extract could not inhibit the growth of S. epidermidis and S. aureus. Meanwhile, the dichloromethane and hexane extracts had similar MIC and MBC of 0.03125 mg/ml against S. epidermidis. The MIC and MBC of the hexane extract against S. aureus were both 0.01562 mg/ml, which was lower than the MIC and MBC of the dichloromethane extract. Therefore, the hexane extract was more effective than the dichloromethane extract possibly because of its high concentrations of zerumbone and sesquiterpenes (Kader et al., 2011; Rana et al., 2017). Zerumbone and sesquiterpenes have been reported for their antimicrobial activity against the cariogenic bacterium Streptococcus mutans (da Silva et al., 2018). Staphylococcus epidermidis and S. aureus are both Gram-positive bacteria; therefore, zerumbone could work against this group of bacteria. Additionally, the Z. zerumbet extract obtained using hexane and ethyl acetate exhibited antibacterial activity against S. epidermidis (Vishwantha et al., 2012).
Antifungal activity test
The MIC and MFC of Z. zerumbet rhizome extracts against C. albicans are presented in Table 2. The ethanol extract did not exhibit antifungal activity within the test concentration range. Meanwhile, the dichloromethane and hexane extracts had MIC values of 0.0625 and 0.03125 mg/ml, respectively. The MFC of the dichloromethane and hexane extracts was also similar to their MIC. This finding suggested that the hexane extracts, which contain a great quantity of zerumbone, exhibited a higher level of antifungal activity than the dichloromethane extract. This result follows the same pattern as the antibacterial test (Section 3.3). Kader et al. (2021) previously reported the antifungal activity of Z. zerumbet rhizome extracts and found that the crude ethanol extract and its petroleum ether and chloroform fractions exhibited antifungal activity against C. albicans, Aspergillus niger, and Saccharomyces cerevisiae. This finding proved that Z. zerumbet rhizome extracts can be applied as fungicides (Kader et al., 2011).
 | Table 1. Extraction yield percentage, viscosity, thickness, and zerumbone concentration of Z. zerumbet rhizome extracts obtained using hexane, dichloromethane, and ethanol. [Click here to view] |
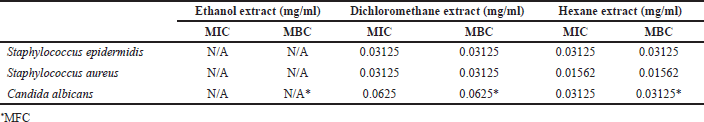 | Table 2. MIC, MBC, and MFC of Z. zerumbet rhizome extracts against S. epidermidis, S. aureus, and C. albicans. [Click here to view] |
Antioxidant activity test
The antioxidant activity of Z. zerumbet rhizome extracts obtained using various solvents was determined using ABTS and DPPH assays as illustrated in Figure 2a and b, respectively. The IC50 (concentration of antioxidant required to reduce the initial ABTS or DPPH concentration by 50%) of each extract was determined using the graph of radical-scavenging activity as shown in Table 3. Among the extracts, the ethanol extract had the strongest antioxidant activity (IC50 values of 28.52 ± 5.03 and 221.03 ± 6.74 g/ml for ABTS and DPPH, respectively) possibly due to its high concentration of flavonoids, which act as antioxidants by scavenging free radicals in an organism (Saija et al., 1995). The antioxidant activity of the dichloromethane and hexane extracts was lower than that of the ethanol extract. Sambou et al. (2020) successfully extracted phenolic compounds, the phytochemicals responsible for the majority of the antioxidant activity in plants, using various solvents in the following order: methanol, ethanol, dichloromethane, and hexane (Sambou et al., 2020).
Anti-inflammatory activity test
The anti-inflammatory efficacy of Z. zerumbet rhizome extracts was determined using antiprotein denaturation assays as presented in Figure 3. The extracts were compared with diclofenac sodium (a positive control), a nonsteroidal anti-inflammatory drug. The ethanol extract showed a greater anti-inflammatory effect than the other extracts. This finding is consistent with the antioxidant activity result, which stated that the ethanol extract had a high concentration of flavonoids. Flavonoids could protect against tissue damage or fibrosis. Multiple investigations in vitro and in animal models also revealed that flavonoids may suppress the initiation and progression of inflammatory conditions (Costamagna et al., 2016; Maleki et al., 2019).
Cytotoxicity test
The cell viability after treatment with Z. zerumbet rhizome extracts is presented in Figure 4. All three extracts (ethanol, dichloromethane, and hexane) showed similar cell viability. The cell viability percentage was higher than 50% when the extract concentration was lower than 15.625 μg/ml. This result was confirmed and corroborated by the microscope images of MRC-5 cells in Figure 5. When the extract (ethanol, dichloromethane, and hexane) concentration was greater than 31.25 μg/ml, the cells appeared to lysate and lose their density. The LC50 (concentration of extract that kills half of the tested cells in culture) of all extracts was determined using the cell viability trendline, and the values for the ethanol, dichloromethane, and hexane extracts were 122.78, 220.76, and 67.96 μg/ml, respectively. These findings suggested that the ethanol and dichloromethane extracts are moderately hazardous (LC50 values of 100–500 μg/ml) according to Clarkson’s toxicity criterion (Clarkson et al., 2004).
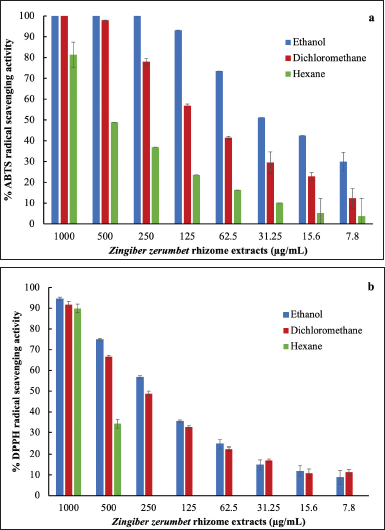 | Figure 2. Radical-scavenging activity of Z. zerumbet rhizome extracts obtained using different solvents evaluated by (a) ABTS and (b) DPPH assays. [Click here to view] |
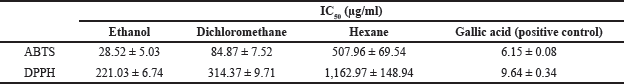 | Table 3. IC50 values (μg/ml) of Z. zerumbet rhizome extracts determined by ABTS and DPPH assays. [Click here to view] |
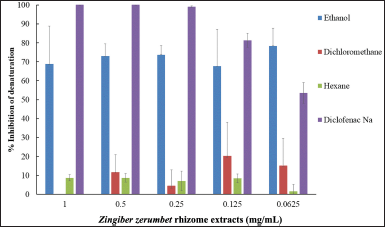 | Figure 3. Anti-inflammatory activity of Z. zerumbet rhizome extracts tested by antiprotein denaturation assays. [Click here to view] |
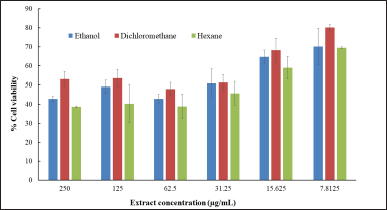 | Figure 4. Cell viability after treatment with Z. zerumbet rhizome extracts obtained using various solvents. [Click here to view] |
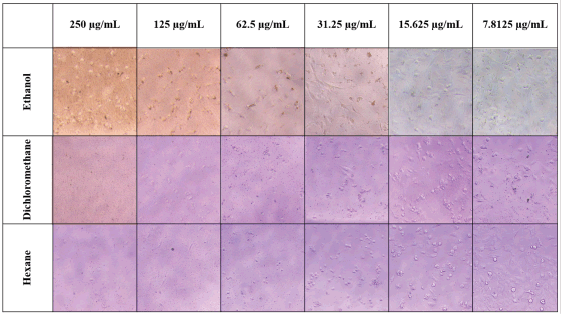 | Figure 5. Microscope images of MRC-5 cells after treatment with different concentrations of Z. zerumbet rhizome extracts obtained using various solvents. [Click here to view] |
CONCLUSION
This study demonstrated that Z. zerumbet rhizome extract possessed antibacterial and antifungal properties. In particular, the hexane and dichloromethane extracts were successful in inhibiting the growth of S. epidermidis, S. aureus, and C. albicans. HPLC was used to monitor and measure zerumbone, which was selected as a biomarker for Z. zerumbet rhizome extracts. Compared with the hexane and dichloromethane extracts, the ethanol extract exhibited greater antioxidant and anti-inflammatory activities. When added to the cell culture, the ethanol and dichloromethane extracts presented a moderate level of toxicity. In summary, the combination extracts of Z. zerumbet rhizome (ethanol, dichloromethane, and hexane) show a potential to be developed as oral or topical, antifungal, and antibacterial agents.
ACKNOWLEDGMENT
This research was funded by Burapha University’s Faculty of Pharmaceutical Sciences with Grant number Rx2/2564. The authors express their gratitude to the Faculty of Pharmaceutical Sciences, Burapha University, for providing laboratory facilities and equipment support. The assistance of Worraphatch Sirisakorn, Monpariya Lamsamoot, Sasinipa Diregrungruang, and Wipada Siritanyong on laboratory work is greatly appreciated.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akbar S. Zingiber zerumbet (L.) Sm. (Zingiberaceae). In: Akbar S (ed.). Handbook of 200 medicinal plants: a comprehensive review of their traditional medical uses and scientific justifications, Cham, Switzerland, Springer, pp 1999–2006, 2020. CrossRef
Akhtar NMY, Jantan I, Arshad L, Haque M. Standardized ethanol extract, essential oil and zerumbone of Zingiber zerumbet rhizome suppress phagocytic activity of human neutrophils. BMC Complement Altern Med, 2019; 19(1):1–12. CrossRef
Anyanwu CU. Investigation of in vitro antifungal activity of honey. J Med Plant Res, 2012; 6(18):3512–6. CrossRef
Arif T, Bhosale J, Kumar N, Mandal T, Bendre R, Lavekar G, Dabur R. Natural products–antifungal agents derived from plants. J Asian Nat Prod Res, 2009; 11(7):621–38. CrossRef
Arumugam B, Manaharan T, Heng CK, Kuppusamy UR, Palanisamy UD. Antioxidant and antiglycemic potentials of a standardized extract of Syzygium malaccense. LWT, 2014; 59(2):707–12. CrossRef
Chia JSM, Farouk AAO, Mohamad TAST, Sulaiman MR, Zakaria H, Hassan NI, Perimal EK. Zerumbone ameliorates neuropathic pain symptoms via cannabinoid and PPAR receptors using in vivo and in silico models. Molecules, 2021; 26(13):3849. CrossRef
Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Pillay P, Matsabisa MG, Bhagwandin N, Smith PJ, Folb PI. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol, 2004; 92(2–3):177–91. CrossRef
Costamagna MS, Zampini IC, Alberto MR, Cuello S, Torres S, Pérez J, Quispe C, Schmeda-Hirschmann G, Isla MI. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem, 2016; 190:392–402. CrossRef
da Silva TM, Pinheiro CD, Orlandi PP, Pinheiro CC, Pontes GS. Zerumbone from Zingiber zerumbet (L.) smith: a potential prophylactic and therapeutic agent against the cariogenic bacterium Streptococcus mutans. BMC Complement Altern Med, 2018; 18(1):1–9. CrossRef
dos Santos MM, Amaral S, Harmen SP, Joseph HM, Fernandes JL, Counahan ML. The prevalence of common skin infections in four districts in Timor-Leste: a cross sectional survey. BMC Infect Dis, 2010; 10(1):1–6. CrossRef
Johann S, Pizzolatti MG, Donnici CL, de Resende MA. Antifungal properties of plants used in Brazilian traditional medicine against clinically relevant fungal pathogens. Braz J Microbiol, 2007; 38:632–7. CrossRef
Kader G, Nikkon F, Rashid MA, Yeasmin T. Antimicrobial activities of the rhizome extract of Zingiber zerumbet Linn. Asian Pac J Trop Biomed, 2011; 1(5):409–12. CrossRef
Kitayama T, Yokoi T, Kawai Y, Hill RK, Morita M, Okamoto T, Yamamoto Y, Fokin VV, Sharpless KB, Sawada S. The chemistry of zerumbone. Part 5: structural transformation of the dimethylamine derivatives. Tetrahedron, 2003; 59(26):4857–66. CrossRef
Kneale M, Bartholomew JS, Davies E, Denning DW. Global access to antifungal therapy and its variable cost. J Antimicrob Chemother, 2016; 71(12):3599–606. CrossRef
Koga AY, Beltrame FL, Pereira AV. Several aspects of Zingiber zerumbet: a review. Rev Bras Farmacogn, 2016; 26(1):385–91. CrossRef
Kwon-Chung KJ, Bennett JE. Medical mycology. Philadelphia, PA, Springer, pp 397–446, 1992.
Male O. The significance of mycology in medicine. In: Hawksworth DL (ed.). Front Mycology, Wallingford, UK, CAB International, pp 131–156, 1990.
Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem, 2019; 299(1):125124. CrossRef
Omokhua-Uyi AG, Abdalla MA, Leonard CM, Aro A, Uyi OO, Staden JV, McGaw LJ. Flavonoids isolated from the South African weed Chromolaena odorata (Asteraceae) have pharmacological activity against uropathogens. BMC Complement Med Ther, 2020; 20(1):1–15. CrossRef
Rahman HS, Rasedee A, How CW, Abdul AB, Zeenathul NA, Othman HH, Saeed MI, Yeap SK. Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int J Nanomedicine, 2013; 8(1):2769. CrossRef
Rana VS, Ahluwalia V, Shakil NA, Prasad L. Essential oil composition, antifungal, and seedling growth inhibitory effects of zerumbone from Zingiber zerumbet Smith. J Essent Oil Res, 2017; 29(4):320–9. CrossRef
Roskov Y, Kunze T, Orrell T, Abucay L, Paglinawan L, Culham A, Bailly N, Kirk P, Bourgoin T, Baillargeon G, Decock W, de Wever A, Didžiulis V. Species 2000 & ITIS catalogue of life, 2014 annual checklist. 2014 [ONLINE]. Available via http://www.catalogueoflife.org/annual-checklist/2014 (Accessed 16 January 2022).
Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med, 1995; 19(4):481–6. CrossRef
Sambou M, Jean-François J, Moutombi FJN, Doiron JA, Hébert MP, Joy AP, Mai-Thi N, Barnett DA, Surette ME, Boudreau LH, Touaibia M. Extraction, antioxidant capacity, 5-lipoxygenase inhibition, and phytochemical composition of propolis from Eastern Canada. Molecules, 2020; 25(10):2397. CrossRef
Vishwantha HN, Niraguna Babu P, Gowrishankar BS, Shridhar SB. Antimicrobial activity of Zerumbone from Zingiber zurumbet against Staphylococcus epidermidis and Aspergillus spp. Int J Appl Biol Pharm, 2012; 3(4):40–3.
Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc, 2008; 3(2):163–75. CrossRef
Yob NJ, Jofrry SM, Affandi MM, Teh LK, Salleh MZ, Zakaria ZA. Zingiber zerumbet (L.) Smith: a review of its ethnomedicinal, chemical, and pharmacological uses. Evid Based Complement Alternat Med, 2011; 2011(1):543216. CrossRef