INTRODUCTION
Ovarian cancer is the seventh most common cancer among women globally, with the highest mortality rate among gynecological malignancies. In 2018, GLOBOCAN estimated that new cases of ovarian cancer reached 18 million, with total deaths of nine million worldwide (Reid et al., 2017; Sumanasekera, 2018). Over the past decades, paclitaxel plus platinum-based chemotherapy, including cisplatin and its derivatives, has been used as the first-line treatment for ovarian cancer (Dasari and Tchounwou, 2014). Cisplatin acts as an adduct to both nuclear and mitochondrial DNA, resulting in aberrant mitosis of the cancer cells, followed by apoptosis. Its interaction with mitochondrial DNA also induces the formation of reactive oxygen species, which further triggers cell death. Unfortunately, the ability of cisplatin to induce oxidative stress becomes one of the mechanisms of cisplatin-induced toxicity (Aldossary, 2019; Dasari and Tchounwou, 2014). Dose-dependent side effects have been reported, including hepatotoxicity, ototoxicity, cardiotoxicity, neurotoxicity, and hematotoxicity. These side effects commonly occur in high-dose cisplatin therapy, raising concerns regarding using cisplatin as a first-line agent in ovarian cancer treatment, especially with the increasing resistance to cisplatin, where high-dose therapy might be needed. This undesirable side effect limits cisplatin efficacy as a chemotherapy agent and increases patient morbidity (Florea and Büsselberg, 2011; Tapia and Diaz-Padilla, 2013).
Although the precise mechanism of cisplatin-induced organ damage has not yet been established, earlier investigations have revealed that increased oxidative stress and inflammation have caused a detrimental effect (Dasari and Tchounwou, 2014; Liao et al., 2008; Limaiem and Bouraoui, 2018; Omar et al., 2016; Wang et al., 2014). Palipoch et al. (2014) showed that cisplatin significantly increases the lipid peroxidation product [malondialdehyde (MDA)], which is the marker of liver oxidative stress. Inflammation is also one of the critical factors that might explain the pathophysiology of acute liver damage induced by cisplatin (El-Shitany and Eid, 2017). Other mechanisms, such as stress oxidative and inflammatory response, might also be involved in hepatocyte apoptosis (Guicciardi and Gores, 2005; Papaliagkas et al., 2007).
Previous studies have shown that the use of hepatoprotective agents, such as curcumin from turmeric, could benefit the anticancer activity of cisplatin and ameliorate cisplatin-induced toxicities (Palipoch et al., 2014; Terlikowska et al., 2014; Wang et al., 2014; Waseem et al., 2014). Turmeric’s main ingredient, curcumin, is derived from the Curcuma longa L. plant and is extensively used in Asia as a culinary coloring agent, flavor enhancer, and herbal medicine. Curcumin has been shown to have powerful effects as an antioxidant, anti-inflammatory, antibacterial, and anticancer agent. These properties allow curcumin to be a potential prophylactic agent against cisplatin toxicity. Additionally, the Food and Drug Administration has deemed curcumin a generally safe ingredient (Liu et al., 2018; Waseem et al., 2014).
Although it is promising, the therapeutic efficacy of curcumin is impaired by its physicochemical properties. In physiologic pH, curcumin is poorly soluble in water. It is also rapidly excreted from the body, contributing to its poor bioavailability. However, the administration of curcumin using nanocarriers has been found to solve these issues, improving the solubility and bioavailability of curcumin (Gera et al., 2017; Khadrawy et al., 2019; Yallapu et al., 2012). Chitosan is one of the widely used materials for nanoparticles. It has been used to lower the toxicity and improve the biodistribution of several therapeutic agents, including curcumin (Li et al., 2015).
In our previous study, we developed a curcumin nanoparticle formulation that resulted in about 20 times increase in curcumin area under the curve (AUC0-inf) and increased efficacy of cisplatin in reducing tumor volume in the 7,12-dimethylbenz(a)anthracene (DMBA)-induced ovarian cancer model (Arozal et al., 2021; Sandhiutami et al., 2020). However, the ability of nanocurcumin (NC) to ameliorate cisplatin-induced hepatotoxicity is yet to be known. Therefore, this study aimed to analyze the hepatoprotective effects of curcumin nanoformulations in DMBA-induced ovarian cancer rats treated with cisplatin. In addition, we also aimed to reveal the possible underlying mechanisms of these hepatoprotective effects.
MATERIALS AND METHODS
Nanocurcumin
NC was formulated from curcumin (Plamed, China) by encapsulating the drug into chitosan–sodium tripolyphosphate using the previously described methods (Arozal et al., 2021).
Animals and treatments
The experiment was carried out using 25 female Wistar rats. Five out of the 25 rats were kept healthy and received oral vehicle only throughout the experiment. At the same time, the rest were given DMBA-coated silk to the ovarium to induce ovarian cancer. The experimental method to induce ovarian cancer was described in the Supplementary Material S1. The ethics committee of the Faculty of Medicine, Universitas Indonesia, approved this study prior to the experiment with the number 0531/UN2.F1/ETIK/2018.
The DMBA-induced ovarian cancer rats were further divided into four groups of five rats as follows: (1) DMBA-induced ovarian cancer rats given no treatment and receiving oral vehicles only, (2) DMBA-induced ovarian cancer rats treated with cisplatin 4 mg/kg BW weekly, (3) DMBA-induced ovarian cancer rats treated with cisplatin 4 mg/kg BW weekly plus curcumin 100 mg/kg BW daily, and (4) DMBA-induced ovarian cancer rats treated with cisplatin 4 mg/kg BW weekly plus NC 100 mg/kg BW daily. All drugs were given for 4 weeks. After treatments, the rats were sacrificed under anesthesia at the end of the fourth week. Liver tissues and plasma samples were quickly taken and stored at −80°C until used. A part of the liver samples was used for histopathological examination, while the other part was used for biochemical analysis and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis.
Biochemical analysis
The aspartate transaminase (AST) and alanine transaminase (ALT) levels were measured using commercial colorimetric assay kits from DiaSys Diagnostic Systems GmbH (Germany) according to the manufacturer’s protocol.
Oxidative stress, inflammatory and fibrotic biomarkers
An enzyme-linked immunoassay was used to quantify superoxide dismutase (SOD) activities (Randox, UK), glutathione (GSH) concentrations (Invitrogen, USA), transforming growth factor-β (TGF-β) concentrations (Elabscience, USA), and tumor necrosis factor-α (TNF-) (Invitrogen, USA). MDA concentrations in liver homogenates were quantified using the previous method and measured colorimetrically at 534 nm relative to the MDA content.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis
The qRT-PCR analysis was used to quantify the expressions of nuclear factor-erythroid factor 2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), heme oxygenase-1 (HO-1), nicotinamide adenine dinucleotide phosphate (NADPH) quinone oxidoreductase, caspase-3, and caspase-9. Beta-actin was used as the housekeeping gene. In brief, 100 mg of liver tissues was used to isolate RNA using the Quick-RNA Miniprep Plus Kit (Zymo Research, CA). The ReverTra Ace® qPCR RT Master Mix was used to convert RNA into cDNA (TOYOBO Biotech, Osaka, Japan). Listed in Table 1 are the primer sequences that were used in this research.
Histopathological analysis
The liver tissues were fixed in 10% buffer formalin, dehydrated in ascending grades of ethyl alcohol, cleared in xylol, and embedded in paraffin. The tissue blocks were then sectioned at 5 μm in thickness, and hematoxylin and eosin staining was used for routine histological analysis. As previously described, liver fibrosis was evaluated using Masson’s trichrome staining for collagen deposition in fibrotic tissues (Eryani et al., 2018; Hassan et al., 2020; Lo and Kim, 2017). Afterward, the cumulative percentage of collagen proportionate area (CPA) was quantified using ImageJ. CPA was calculated based on the percentage of total stained fibrosis area per total sample area (Lo and Kim, 2017).
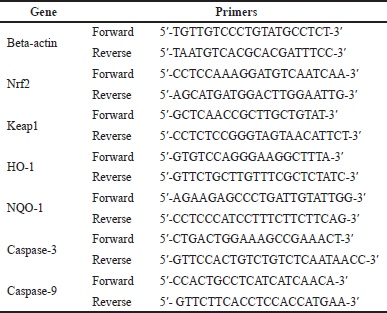 | Table 1. List of primers used in the study. [Click here to view] |
Statistical analyses
The data were displayed in mean ± standard error of the mean (SEM). Statistical analysis was carried out using one-way analysis of variance, followed by Tukey’s post-hoc multiple comparisons carried out in Statistical Package for the Social Sciences v.26. Differences were considered significant at p < 0.05.
RESULTS AND DISCUSSION
Plasma AST and ALT levels
We found an increase in plasma AST and ALT levels after treatment with cisplatin (Fig. 1). Adding curcumin or NC to cisplatin resulted in reducing AST and ALT levels. Figure 2 shows the liver injury caused by cisplatin. The administration of curcumin and/or NC caused restoration of liver architecture, which can be seen through the reduced density of fibrotic tissue and less dilatation of the sinusoid spaces.
Despite cisplatin’s antitumor efficacy, hepatotoxicity has been one of its dose-limiting toxicities, which can be seen even with single low-dose administration (Dkhil et al., 2013). The hepatotoxicity effect of cisplatin was observed by increasing liver enzymes AST and ALT levels in the plasma. The finding of a lower AST level in the group with the combination treatment of cisplatin–curcumin indicates that curcumin was able to ameliorate the liver toxicity caused by cisplatin. Furthermore, an earlier study by Palipoch et al. (2014) showed a similar result, which found that the cisplatin and curcumin combination significantly reduced AST levels compared to cisplatin only. Similarly, the results demonstrated that the administration of NC combined with cisplatin also reduced the AST and ALT levels compared to cisplatin only. However, in contrast to Marslin et al. (2018), we detected somewhat higher AST and ALT levels in the rats treated with cisplatin plus NC versus those treated with cisplatin plus curcumin. The effect might be due to the higher dose of NC used in this study (100 mg/kg BW) than the NC dose used by Marslin et al. (2018) (25–50 mg/kg BW). Nonetheless, the group treated with NC still had lower AST and ALT levels than those treated with cisplatin only.
Hepatic oxidative stress markers
A significant increase in MDA level was observed in the liver tissues of the rats given cisplatin monotherapy compared to the normal group (p = 0.038). While the coadministration of both conventional (p < 0.001) and NC (p = 0.005) with cisplatin significantly reduced hepatic MDA levels, no statistically significant differences were observed between both groups (Fig. 3A). Interestingly, while we found that NC administration restored the hepatic MDA level close to the normal level, the conventional curcumin group had the lowest mean hepatic MDA levels, even lower than the normal group (Fig. 3A). The SOD level decreased in the rats treated with cisplatin, albeit insignificantly compared to the normal group. While coadministration of curcumin raised the SOD level significantly, administration of NC did not (Fig. 3B). Coadministration of both curcumin and NC tended to increase GSH levels; however, they were insignificant (Fig. 3C).
The increased hepatic MDA level in the rats treated with cisplatin was expected since the administration of cisplatin is known to deviate the activity of the mitochondrial electron transport chain by interfering with the expression of the mitochondrial protein complexes, thereby inducing oxidative stress (Marullo et al., 2013). Previous studies also found a significant difference in hepatic MDA levels in animal models treated with cisplatin (Palipoch et al., 2014) or its analog oxaliplatin (Lu et al., 2020), despite the different dosage and duration of treatment between studies. Curcumin has been shown to ameliorate the hepatic oxidative status in animal models induced by platinum-based agents (Lu et al., 2020; Palipoch et al., 2014) or other agents (Peng et al., 2018). A previous study showed that the hepatoprotective properties of NC were shown to be superior to conventional curcumin in rats treated with carbon tetrachloride (Singh et al., 2014). Another study proved that the administration of NC at a dose of 50 mg/kg BW restored hepatic MDA levels close to the average level after administering a single dose of cisplatin (12 mg/kg BW) (El-Gizawy et al., 2020).
Interestingly, we found that the hepatic MDA level in the rats treated with conventional curcumin was lower than that in the rats receiving NC, in line with the AST and ALT levels. The decrease of hepatic GSH levels in the cisplatin-treated rats was expected as GSH has been shown to defend hepatic cells from oxidative stress induced by cisplatin actively and have a role in cisplatin clearance (Martins et al., 2008). Different modes of administration and doses might have been used despite the differences in administration and doses. A previous study has also found a similar decrease in hepatic GSH levels (Martins et al., 2008). Coadministration of curcumin has been shown to increase GSH back to normal levels, owing to its ability to increase GSH biosynthesis and directly interact with free radicals (Biswas et al., 2005). However, curcumin nanoformulation is not better than conventional curcumin in this matter. SOD activities can also show oxidative stress in the tissue status (Katerji et al., 2019), and its activities are lower in cisplatin-induced damaged liver tissues (Karadeniz et al., 2011). Curcumin has been found to help normalize hepatic SOD levels (El-Bahr, 2015), and while this is true for this research, NC coadministration did not produce significant results.
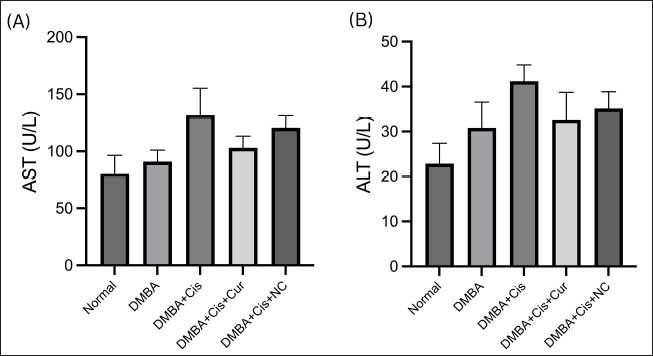 | Figure 1. NC decreases of AST (A) and ALT (B) plasma levels. Histograms show the mean levels (±SEM) of plasma. DMBA = dimethylbenz(a)anthracene; Cis = cisplatin; Cur = curcumin; NC = nanocurcumin. [Click here to view] |
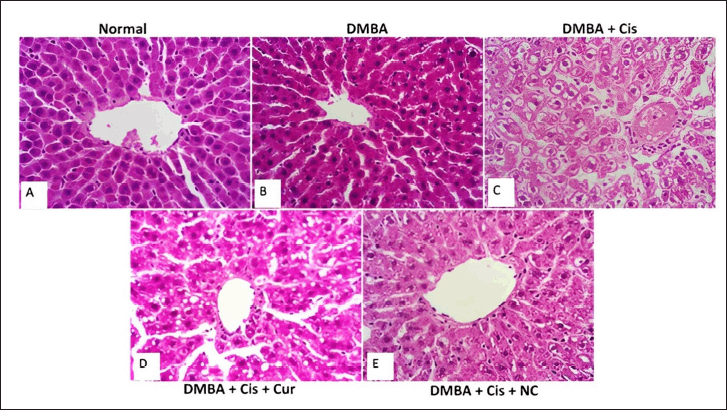 | Figure 2. Hematoxylin and eosin staining in cisplatin-induced liver damage and the effect of curcumin and NC. Microscopy images of liver slices from the normal (A) and DMBA (B) groups indicate normal central vein and well-organized, radially oriented hepatic cords. Cisplatin groups showed disrupted hepatic cords organization, vacuolar degeneration, dense fibrotic septal (arrow), and dilations of portal tracts and sinusoids (C). On the contrary, curcumin administration showed partial improvement (D) and was markedly improved in NC administration (E). The magnification used was 400 times. [Click here to view] |
Nrf2/Keap1 expressions
Significant differences were observed in the Nrf2 expression level between the normal and other treatment groups, with the highest expression observed in the rats receiving coadministration of cisplatin and NC (Fig. 4A). Both cisplatin and curcumin increased the expression of Nrf2 mRNA. Although no statistically significant differences in Keap1 mRNA expression were found between the treatment groups, the rats receiving cisplatin monotherapy tended to express a lower level of Keap1 mRNA. At the same time, the administration of curcumin in both forms increased Keap1 mRNA expression (Fig. 4B). The mRNA expressions of downstream components of the Nrf2/Keap1/ARE signaling pathway were represented by HO-1 and NQO-1 (Fig. 4C and D). Although no significant differences were observed between the rats receiving cisplatin monotherapy and the normal group, cisplatin administration tended to decrease the transcription of the HO-1 and NQO-1 genes. A similar pattern of gene expression was observed between the two genes. The expression of both genes did not significantly differ between the rats receiving conventional and NC. Interestingly, we found that the DMBA group expressed the highest level of both genes compared to the other groups.
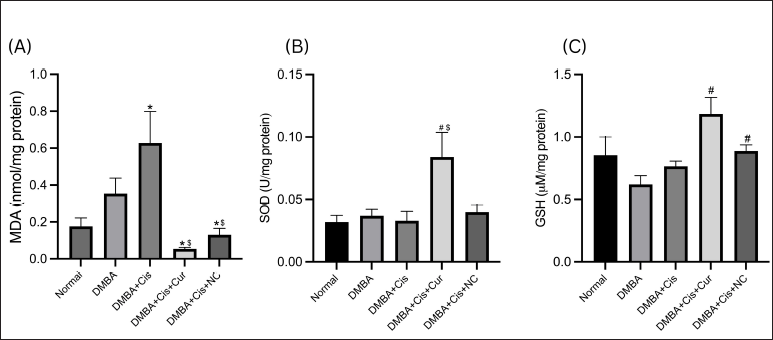 | Figure 3. Hepatic levels and activities of hepatic oxidative markers in rats with and without treatment. (A) NC decreased the MDA level significantly as compared to the DMBA–cisplatin group. (B) Curcumin significantly increased the SOD level. (C) Both curcumin and NC significantly increased the GSH levels (C). *p < 0.05 versus normal; #p < 0.05 versus DMBA; $p < 0.05 versus cisplatin. [Click here to view] |
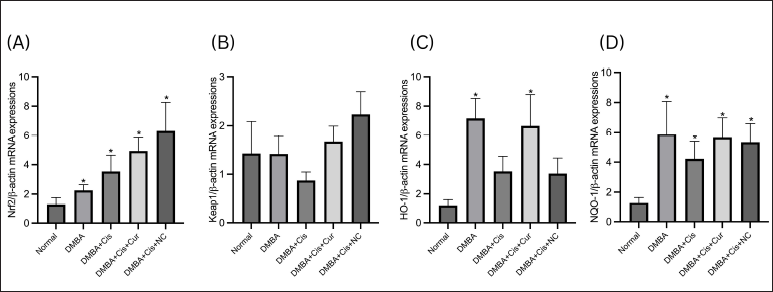 | Figure 4. Relative expressions of Nrf2, Keap1, HO-1, and NQO-1 mRNA. Both curcumin and NC increased the mRNA Nrf2 (A) and Keap1 (B) as compared to the normal group. Curcumin increased the mRNA HO-1 and both curcumin and NC (C) and significantly increased the mRNA NQO-1 (D). *p < 0.05 versus normal. [Click here to view] |
The rats treated with cisplatin significantly expressed a higher level of Nrf2 mRNA than the normal group. The administration of curcumin and NC also increased Nrf2 mRNA expression, even higher than the group receiving cisplatin monotherapy. Although no statistically significant differences were observed in Keap1 mRNA expression between the groups, the rats receiving cisplatin monotherapy exhibited lower Keap1 expression levels than the normal group. On the contrary, the addition of curcumin increased Keap1 mRNA expression. Previous studies showed varying results regarding the expression of Nrf2 and Keap1 at the transcriptional level. Several studies showed that oxidative stress increased the transcription of Nrf2 in an animal model (Peng et al., 2018) or in vitro (Shen et al., 2017) using different inducers of oxidative stress, supporting our findings. Since the protein Nrf2 is known as a transcription factor that regulates the expression of various cytoprotective genes, including NADPH dehydrogenase (quinone-) 1 (NQO-1), heme oxygenase-1 (HO-1), p-glycoprotein, thioredoxin, and many other enzymes in response to oxidative and electrophilic stresses, this increase was expected (Robledinos-Antón et al., 2019; Taguchi and Yamamoto, 2017). Cisplatin, in particular, has also been shown to repress the expression of Keap1 mRNA (Shen et al., 2017). In physiologic conditions, Keap1, a part of the Cullin3-based-e3 ubiquitin ligase, acts as the negative regulator of Nrf2 by forming a complex which represses Nrf2 activity through ubiquitination and proteasomal degeneration. Oxidative or electrophilic stress could modify the cysteine residue of Keap1, allowing Nrf2 to dissociate from Keap1 and translocate into the nucleus, where it will form a complex with the small muscle aponeurosis fibromatosis transcription factor and bind to the antioxidant response element sequence to upregulate the expression of more than 250 cytoprotective and detoxification enzymes (Robledinos-Antón et al., 2019; Taguchi and Yamamoto, 2017).
The administration of curcumin has been shown to increase the transcription of Nrf2 in vivo and in vitro (Li et al., 2021; Peng et al., 2018; Shen et al., 2017). Although an in vitro study showed that coadministration of curcumin with cisplatin lowered Nrf2 expression more than in the group receiving cisplatin only or curcumin only, it was still higher than the normal group, and an increased level of Keap1 was also observed. Shin et al. (2020) demonstrated that while curcumin increased the expression of Nrf2 protein, it did not affect the transcription of Nrf2 mRNA. However, it should be noted that the research was carried out using topically administered curcumin in epithelial cells (Shin et al., 2020). Serafini et al. (2020) also found that, in the SH-SY5Y cell line, curcumin did not affect the transcription of Nrf2 and Keap1 and Keap1 protein levels. However, it did significantly increase the expression of Nrf2 protein (Serafini et al., 2020). The mRNA level might not correlate directly with protein expression in some conditions, especially in dynamic phases such as cellular response to stress. Posttranscriptional modifications might cause a substantial deviation from the ideal correlation between mRNA and protein (Liu et al., 2016).
The transcription factor Nrf2 controls the expression of various cytoprotective genes, including NQO-1 and HO-1. NQO-1 has been popularly used to evaluate the Nrf2-Keap1 pathway (Taguchi and Yamamoto, 2017). It plays an enormous role in cellular adaptation to oxidative stress by acting as a direct superoxide reductase or a cell membrane redox system (Ross and Siegel, 2017). In comparison, HO-1 is a cytoprotective enzyme that catalyzes heme degradation reaction, resulting in the formation of bilirubin, a potent antioxidant (Loboda et al., 2016). A previous study has identified the protective effect of HO-1 in attenuating cisplatin-induced ototoxicity through the activation of the Nrf2/HO-1 pathway by curcumin (Fetoni et al., 2014). Our study found an elevated expression of both HO-1 and NQO-1 mRNA compared to the normal group, albeit insignificantly. The increase was expected since it was known that oxidative stress induces Nrf2 translocation into the nucleus, resulting in an increased expression of HO-1 and NQO-1 (Fetoni et al., 2014; Li et al., 2021; Taguchi and Yamamoto, 2017).
Interestingly, the DMBA group expressed the highest level of both genes. In a previous study by Arivazhagan and Subramanian (2015) using a DMBA-induced breast cancer model, the liver suffered oxidative damage, as exhibited by the increased level of oxidative markers (hydroperoxides and carbonyls) and reduced level of antioxidants, suggesting that DMBA might induce hepatic injury via oxidative stress. Intriguingly, the study showed a decreased level of HO-1 and NQO-1 mRNA, a decreased level of Nrf2 mRNA, and an increased level of Keap1 mRNA. These findings contrast other previous studies (Fetoni et al., 2014; Li et al., 2021; Loboda et al., 2016; Taguchi and Yamamoto, 2017). Thus, other studies evaluating the expression dynamics of the downstream genes of Nrf2/Keap1 genes at the transcriptional level are needed.
Inflammatory markers
The mean concentration of the TNF-α protein level in the liver tissue in the group treated with cisplatin only tended to increase compared to the group of the ovarian cancer model mice without therapy. The TNF-α protein levels were slightly lower in the group treated with cisplatin plus curcumin and cisplatin plus NC (Fig. 5A).
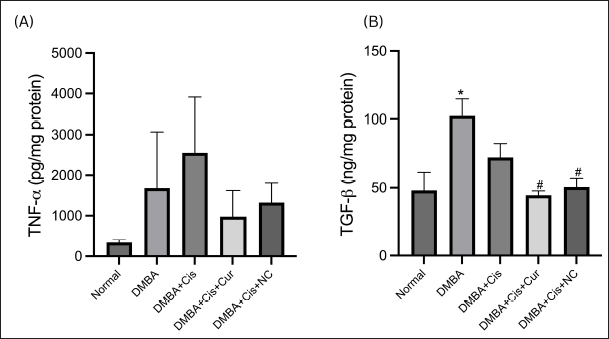 | Figure 5. NC decreased hepatic inflammatory and fibrosis markers. Hepatic TNF-α (A) and TGF-β1 (B) concentrations were reduced by curcumin and NC. [Click here to view] |
Fibrotic markers
The mean concentration of the TGF-β1 protein levels in the liver tissue in the group treated with cisplatin only tended to be lower than the ovarian cancer model group with no treatment. A significant decrease was seen in the cisplatin plus curcumin treatment group (p = 0.004) and cisplatin plus NC treatment group (p = 0.010) when compared to the ovarian cancer model group with no therapy (Fig. 5B).
These results were consistent with Masson’s trichrome staining, which showed marked fibrosis in the group treated with cisplatin. The administration of curcumin and NC appeared to reduce the fibrotic area, as shown by the reduction of the CPA (Fig. 6).
To further analyze apoptotic cell death in the liver, we examined caspase-3 and caspase-9 gene expressions. As shown in Figure 7, there were no significant differences between the five groups in both mRNA expressions of caspase-3 and caspase-9 (p = 0.111; p = 0.395). The mean expressions of caspase-3 and caspase-9 mRNA in the group of the ovarian cancer model mice without therapy tended to increase compared to the healthy rats group. The mRNA expression of caspase-3 showed a tendency to decrease in the cisplatin treatment group, while it increased in the cisplatin plus curcumin and cisplatin plus NC treatment groups. Meanwhile, the mRNA expression of caspase-9 tended to decrease in the cisplatin plus curcumin treatment group, while it increased in the NC-combined therapy group.
In the present study, we also demonstrated a tendency for TNF-α levels to increase in the cisplatin therapy group compared to the DMBA-induced group. This result corresponds with the previous study showing that cisplatin has a toxic effect on the liver, thereby increasing the levels of the inflammatory cytokines in the liver through the extrinsic pathway, where the ligand binds to TNF-α and then recruits procaspase-8 and forms a death-inducing signaling complex (Dasari and Tchounwou, 2014). The study shows that cisplatin administration significantly increases inflammatory cytokines, such as IL-6 and TNF-α. In addition, there was a decrease in TNF-α levels in the liver tissue in the cisplatin–curcumin and cisplatin–NC treatment groups compared to the cisplatin monotherapy group, indicating the anti-inflammatory effect of curcumin and its nanoparticles on the liver tissue (Cüre et al., 2016). Our findings are supported by a previous study that demonstrated curcumin’s anti-inflammatory effects by reducing various inflammatory cytokines, such as TNF-α, IL-6, and IL-1β (Zhou et al., 2011). In a CCI4-induced mouse model, curcumin has been shown to reduce inflammatory effects by reducing inflammatory cytokines, including TNF-α, IL-6, and IFN-γ, in both tissue and serum (Fu et al., 2008).
Moreover, a study investigating the cisplatin–nephrotoxic and hepatotoxic amelioration effects of NC in rats found a significant increase in serum TNF-α levels in the group treated with cisplatin. Meanwhile, the administration of NC has been shown to reduce the TNF-α levels to control values (El-Gizawy et al., 2020). A study by Singh et al. (2014) also demonstrated that curcumin resulted in the downregulation of the TNF-α-induced activation of NF-kB and AP-1.
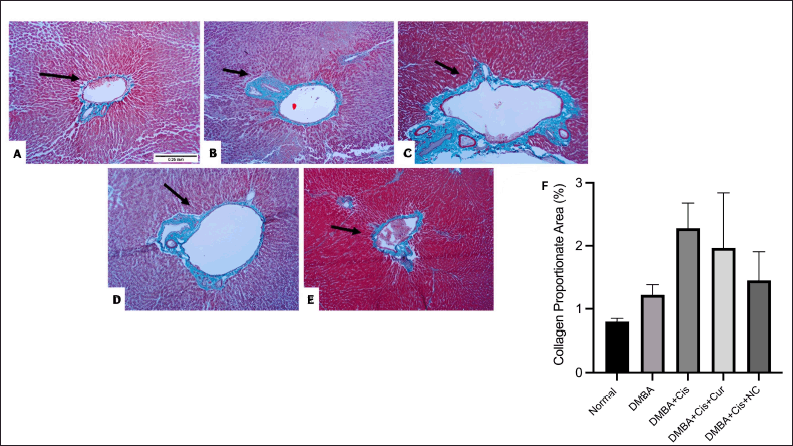 | Figure 6. Masson’s trichrome staining of histological liver sections: (A) Healthy control liver showed normal architecture; (B) DMBA group showed relatively normal architecture of the of the liver with few fine collagen fibers (stained blue) around the central vein and portal tract; (C) cisplatin group showed disruption in hepatic architecture, marked dense bridging fibrous septa and collagen fibers’ accumulation and dilatations of sinusoid spaces; (D) curcumin administration showed moderate fibrous septa and collagen fibers accumulation, (E) NC administration markedly reduced the fibrous septa and collagen administration; (F) percentage of CPA reduction by curcumin and NC upon ImageJ analysis. The magnification used was 100 times. [Click here to view] |
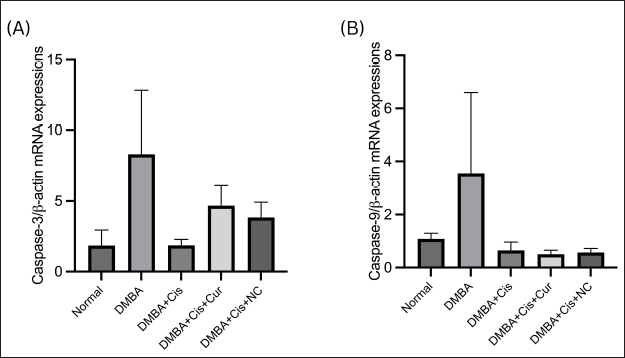 | Figure 7. Relative mRNA expression of (A) caspase-3 and (B) caspase-9 in rats with and without treatment. Histograms show the mean mRNA expressions (±SEM). [Click here to view] |
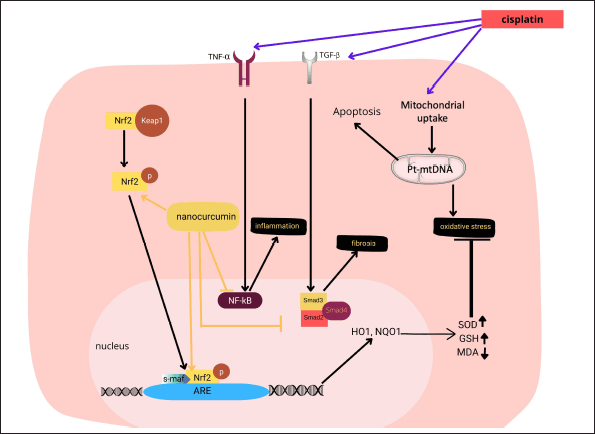 | Figure 8. Proposed mechanism of nanocurcumin action in cisplatin-induced hepatotoxicity in rats with ovarian cancer. Nanocurcumin ameliorates liver damage caused by curcumin by targeting the Nrf2 signaling pathway, which affects the modulation in the downstream expression of the Nrf2 target genes, including Keap1, HO-1, NQO1, and finally increases antioxidant gene expressions. In addition, nanocurcumin also improves inflammatory, fibrosis, and apoptosis marker expressions. [Click here to view] |
We also demonstrated that the TGF-β1 levels in the liver tissue showed statistically significant differences in the dual therapy group of cisplatin–curcumin and cisplatin–NC against the DMBA induction group without any treatment. This is in line with past studies that found the hepatoprotective effect of curcumin and its effects on suppressing TGF-β1 (Rahmani et al., 2018; Xu et al., 2018). In CCl4-induced rats, the groups treated with curcumin had higher levels of TGF-β1 than the normal controls. Curcumin acts by inhibiting the liver’s hepatic stellate cells, which results in the reduction of TGF-β1 levels (Fu et al., 2008). The analysis of caspase-3 and caspase-9 mRNA expression levels in all groups did not show significant differences. The increasing expressions of both caspases’ mRNA in the cisplatin-treated ovarian cancer group can be explained by the hepatotoxicity due to cisplatin administration, as shown by the increase of AST and ALT levels, which increases the apoptosis of hepatocyte cells (Omar et al., 2016). The decreased expressions of caspase-9 in the cisplatin + curcumin and cisplatin + NC groups are expected due to its liver protection properties (Alhusaini et al., 2018).
CONCLUSION
In conclusion, the present study displayed the beneficial effects of NC in alleviating hepatotoxicity due to cisplatin administration through its antioxidant and anti-inflammatory activities.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING
This work was funded by Universitas Indonesia grants (Contract No. NKB-1293/UN2.RST/PPM.00.03.01/2020).
AUTHORS’ CONTRIBUTIONS
ML, NMDS, VS, and DS contributed to the conception, study design, and data interpretation. AMP, AAS, DKM, ERF, MFA, NT, NNBA, and NMDS worked on data collection, data analysis, and interpretation. ML acquired the funding. All the authors participated in drafting the article, critical revision, and final approval of the manuscript.
ETHICAL APPROVALS
The ethics committee of the Faculty of Medicine, Universitas Indonesia, approved this study prior to the experiment with the number 0531/UN2.F1/ ETIK/2018.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aldossary SA. Review on pharmacology of cisplatin: clinical use, toxicity and mechanism of resistance of cisplatin. Biomed Pharmacol J, 2019; 12(1):7–15. CrossRef
Alhusaini A, Hasan IH, Aldowsari N, Alsaadan N. Prophylactic administration of nanocurcumin abates the incidence of liver toxicity induced by an overdose of copper sulfate: role of CYP4502E1, NF-κB and bax expressions. Dose-response, 2018; 16(4):1559325818816284; doi:10.1177/1559325818816284 CrossRef
Arivazhagan L, Subramanian SP. Tangeretin, a citrus flavonoid attenuates oxidative stress and protects hepatocellular architecture in rats with 7, 12-dimethylbenz(a)anthracene induced experimental mammary carcinoma. J Functional Foods, 2015; 15:339–53; doi:10.1016/j.jff.2015.03.041 CrossRef
Arozal W, Louisa M, Rahmat D, Chendrana P, Sandhiutami NMD. Development, characterization and pharmacokinetic profile of chitosan-sodium tripolyphosphate nanoparticles based drug delivery systems for curcumin. Adv Pharm Bull, 2021; 11(1):77–85; doi:10.34172/apb.2021.008 CrossRef
Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-κB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal, 2005; 7(1-2):32–41. CrossRef
Cüre MC, Cüre E, Kalkan Y, K?rba? A, Tümkaya L, Y?lmaz A, Türky?lmaz AK, ?ehito?lu ?, Yüce S. Infliximab Modulates Cisplatin-Induced Hepatotoxicity in Rats. Balkan Med J, 2016; 33(5):504–11; doi:10.5152/balkanmedj.2016.150576 CrossRef
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol, 2014; 740:364–78; doi:10.1016/j.ejphar.2014.07.025 CrossRef
Dkhil MA, Al-Quraishy S, Aref AM, Othman MS, El-Deib KM, Abdel Moneim AE. The potential role of Azadirachta indica treatment on cisplatin-induced hepatotoxicity and oxidative stress in female rats. Oxid Med Cell Longev, 2013; 2013:741817; doi:10.1155/2013/741817. CrossRef
El-Gizawy MM, Hosny EN, Mourad HH, Razik AE, Amira N. Curcumin nanoparticles ameliorate hepatotoxicity and nephrotoxicity induced by cisplatin in rats. Naunyn-Schmiedeberg’s Arch Pharmacol, 2020; 393(10):1941–53. CrossRef
El-Bahr S. Effect of curcumin on hepatic antioxidant enzymes activities and gene expressions in rats intoxicated with aflatoxin B1. Phytother Res, 2015; 29(1):134–40. CrossRef
El-Shitany NA, Eid B. Proanthocyanidin protects against cisplatin-induced oxidative liver damage through inhibition of inflammation and NF-κβ/TLR-s4 pathway. Environ Toxicol, 2017; 32(7):1952–63. CrossRef
Eryani A, Sukmawati D, Damayanti L, Angmalisang EC, Pawitan JA. The healing effect of adipose-derived stem cell conditioned medium on burn wound model. Trends Biomater Artif Org, 2018; 32(1):18–25.
Fetoni AR, Eramo SL, Paciello F, Rolesi R, Podda MV, Troiani D, Paludetti G. Curcuma longa (curcumin) decreases in vivo cisplatin-induced ototoxicity through heme oxygenase-1 induction. Otol Neurotol, 2014; 35(5):e169–77; doi:10.1097/mao.0000000000000302. CrossRef
Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers, 2011; 3(1):1351–71. CrossRef
Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol, 2008; 73(2):399–409; doi:10.1124/mol.107.039818 CrossRef
Gera M, Sharma N, Ghosh M, Huynh DL, Lee SJ, Min T, Kwon T, Jeong DK. Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget, 2017; 8(39):66680. CrossRef
Guicciardi M, Gores G. Apoptosis: a mechanism of acute and chronic liver injury. Gut, 2005; 54(7):1024–33. CrossRef
Hassan HM, Al-Wahaibi LH, Elmorsy MA, Mahran YF. Suppression of cisplatin-induced hepatic injury in rats through alarmin high-mobility group box-1 pathway by Ganoderma lucidum: theoretical and experimental study. Drug Des Dev Ther, 2020; 14:2335. CrossRef
Karadeniz A, Simsek N, Karakus E, Yildirim S, Kara A, Can I, Kisa F, Emre H, Turkeli M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid Med Cell Longev, 2011; 2011:981793; doi:10.1155/2011/981793 CrossRef
Katerji M, Filippova M, Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxid Med Cell Longev, 2019; 2019:1279250; doi:10.1155/2019/1279250 CrossRef
Khadrawy YA, El-Gizawy MM, Sorour SM, Sawie HG, Hosny EN. Effect of curcumin nanoparticles on the cisplatin-induced neurotoxicity in rat. Drug Chem Toxicol, 2019; 42(2):194–202. CrossRef
Li S, Wang X, Xiao Y, Wang Y, Wan Y, Li X, Li Q, Tang X, Cai D, Ran B, Wu C. Curcumin ameliorates mercuric chloride-induced liver injury via modulating cytochrome P450 signaling and Nrf2/HO-1 pathway. Ecotoxicol Environment Saf, 2021; 208:111426; doi:10.1016/j.ecoenv.2020.111426 CrossRef
Li Z, Jiang H, Xu C, Gu L. A review: using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocolloids, 2015; 43:153–64. CrossRef
Liao Y, Lu X, Lu C, Li G, Jin Y, Tang H. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol Res, 2008; 57(2):125–31. CrossRef
Limaiem F, Bouraoui S. Chemotherapy-induced liver injury in metastatic colorectal cancer: about 48 cases. Pan Afr Med J, 2018; 30:198; doi:10.11604/pamj.2018.30.198.15548 CrossRef
Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell, 2016; 165(3):535–50; doi:10.1016/j.cell.2016.03.014 CrossRef
Liu Z, Huang P, Law S, Tian H, Leung W, Xu C. Preventive effect of curcumin against chemotherapy-induced side-effects. Front Pharmacol, 2018; 9:1374. CrossRef
Lo RC, Kim H. Histopathological evaluation of liver fibrosis and cirrhosis regression. Clin Mol Hepatol, 2017; 23(4):302. CrossRef
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci, 2016; 73(17):3221–47; doi:10.1007/s00018-016-2223-0 CrossRef
Lu Y, Wu S, Xiang B, Li L, Lin Y. Curcumin attenuates oxaliplatin-induced liver injury and oxidative stress by activating the Nrf2 pathway. Drug Des Dev Ther, 2020; 14:73–85. CrossRef
Marslin G, Prakash J, Qi S, Franklin G. Oral delivery of curcumin polymeric nanoparticles ameliorates CCl4-induced subacute hepatotoxicity in wistar rats. Polymers, 2018; 10(5):541. CrossRef
Martins N, Santos N, Curti C, Bianchi M, Santos A. Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J Appl Toxicol, 2008; 28(3):337–44. CrossRef
Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, Doetsch PW. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PloS One, 2013; 8(11):e81162. CrossRef
Omar HA, Mohamed WR, Arab HH, Arafa ESA. Tangeretin alleviates cisplatin-induced acute hepatic injury in rats: targeting MAPKs and apoptosis. PLoS One, 2016; 11(3):e0151649. CrossRef
Palipoch S, Punsawad C, Koomhin P, Suwannalert P. Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatin-induced oxidative stress. BMC Complement Alterm Med, 2014; 14(1):1–8. CrossRef
Papaliagkas V, Anogianaki A, Anogianakis G, Ilonidis G. The proteins and the mechanisms of apoptosis: a mini-review of the fundamentals. Hippokratia, 2007; 11(3):108.
Peng X, Dai C, Liu Q, Li J, Qiu J. Curcumin attenuates on carbon tetrachloride-induced acute liver injury in mice via modulation of the Nrf2/HO-1 and TGF-β1/Smad3 pathway. Molecules, 2018; 23(1):215. CrossRef
Rahmani AH, Alsahli MA, Aly SM, Khan MA, Aldebasi YH. Role of curcumin in disease prevention and treatment. Adv Biomed Res, 2018; 7:38; doi:10.4103/abr.abr_147_16 CrossRef
Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med, 2017; 14(1):9–32; doi:10.20892/j.issn.2095-3941.2016.0084
Robledinos-Antón N, Fernández-Ginés R, Manda G, Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid Med Cell Longev, 2019; 2019:9372182; doi:10.1155/2019/9372182 CrossRef
Ross D, Siegel D. Functions of NQO1 in cellular protection and CoQ10 metabolism and its potential role as a redox sensitive molecular switch. Front Physiol, 2017;8; doi:10.3389/fphys.2017.00595 CrossRef
Sandhiutami NMD, Arozal W, Louisa M, Rahmat D, Wuyung PE. Curcumin nanoparticle enhances the anticancer effect of cisplatin by inhibiting PI3K/AKT and JAK/STAT3 pathway in rat ovarian carcinoma induced by DMBA. Front Pharmacol, 2020; 11:603235; doi:10.3389/fphar.2020.603235 CrossRef
Serafini MM, Catanzaro M, Fagiani F, Simoni E, Caporaso R, Dacrema M, Romanoni I, Govoni S, Racchi M, Daglia M, Rosini M, Lanni C. Modulation of Keap1/Nrf2/ARE signaling pathway by curcuma- and garlic-derived hybrids. Front Pharmacol, 2020; 10:1597; doi:10.3389/fphar.2019.01597 CrossRef
Shen J, Chen YJ, Jia YW, Zhao WY, Chen GH, Liu DF, Chen YY, Zhang C, Liu XP. Reverse effect of curcumin on CDDP-induced drug-resistance via Keap1/p62-Nrf2 signaling in A549/CDDP cell. Asian Pac J Trop Med, 2017; 10(12):1190–6; doi:10.1016/j.apjtm.2017.10.028 CrossRef
Shin JW, Chun KS, Kim DH, Kim SJ, Kim SH, Cho NC, Na HK, Surh YJ. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem Pharmacol, 2020; 173:113820; doi:10.1016/j.bcp.2020.113820 CrossRef
Singh N, Khullar N, Kakkar V, Kaur IP. Attenuation of carbon tetrachloride-induced hepatic injury with curcumin-loaded solid lipid nanoparticles. BioDrugs, 2014; 28(3):297–312. CrossRef
Sumanasekera W. Epidemiology of ovarian cancer: risk factors and prevention. Biomed J Sci Technical Res, 2018; 11:2:8405–27; doi:10.26717/BJSTR.2018.11.002076 CrossRef
Taguchi K, Yamamoto M. The KEAP1–NRF2 system in cancer. Front Oncol, 2017; 7:85; doi:10.3389/fonc.2017.00085 CrossRef
Tapia G, Diaz-Padilla I. Molecular mechanisms of platinum resistance in ovarian cancer. In: Ovarian cancer - a clinical and translational update [Internet], IntechOpen, London,UK, 2013; doi:10.5772/55562 CrossRef
Terlikowska KM, Witkowska AM, Zujko ME, Dobrzycka B, Terlikowski SJ. Potential application of curcumin and its analogues in the treatment strategy of patients with primary epithelial ovarian cancer. Int J Mol Sci, 2014; 15(12):21703–22. CrossRef
Wang Y, Hu PC, Gao FF, Lv JW, Xu S, Kuang CC, Wei L, Zhang JW. The protective effect of curcumin on hepatotoxicity and ultrastructural damage induced by cisplatin. Ultrastruct Pathol, 2014; 38(5):358–62. CrossRef
Waseem M, Pandey P, Tomar B, Raisuddin S, Parvez S. Ameliorative action of curcumin in cisplatin-mediated hepatotoxicity: an in vivo study in Wistar rats. Arch Med Res, 2014; 45(6):462–8. CrossRef
Xu XY, Meng X, Li S, Gan RY, Li Y, Li HB. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients, 2018; 10(10):1553; doi:10.3390/nu10101553 CrossRef
Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today, 2012; 17(1-2):71–80. CrossRef
Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets, 2011; 12(3):332–47; doi:10.2174/138945011794815356 CrossRef
SUPPLEMENTARY MATERIAL
SUPPLEMENTARY MATERIAL S1.
7,12-dimethylbenz[a]anthracene (DMBA)-induced ovarian cancer in the rats
Our study used a rat model of ovarian cancer by implanting 7,12-dimethylbenz[a]anthracene (DMBA) directly in the right ovary of female Wistar rats. The DMBA was preheated at 124°C before use. Approximately 2 cm of sterile 3-0 silk sutures were coated with DMBA (2 mg) for 10 seconds and dried. DMBA-coated silk sutures can be stored for 1 week at -20°C before being implanted in the ovaries of female rats. Wistar female rats aged 5 weeks were quarantined and acclimatized for one week, kept in a controlled animal room at room temperature maintained at 25 C, relative humidity of 65%, and air ventilation 11-13 times per hour, and 12 hours of dark and light cycle daily. The animals were fed with standard pellets and adequate drinking water.
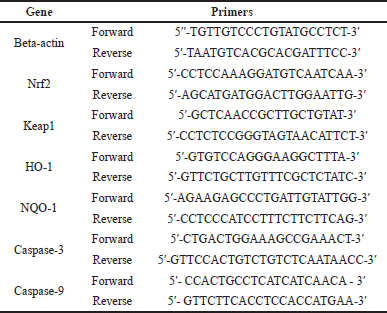 | Supplementary Table 1. List of primers used in the study [Click here to view] |
Before surgery, rats were put to sleep using intraperitoneal anesthesia ketamine and xylazine. Surgery was performed on the retroperitoneal section, and fatty tissues were cleaned. A 3-0 silk suture coated with DMBA was implanted in the ovarian tissue, and the wound was closed again. After the surgical process, the rats were reared and fed according to the standard conditions mentioned above. It took at least 28 weeks from the implantation process to the tumor mass formation
The development and growth of ovarian tissue at week 20 were observed using an ultrasound machine (Ultrasonography Chison Q8TM). The rats were anesthetized intraperitoneally using ketamine (75 mg/kg BW) and xylazine (8.8 mg/kgBW). The peritoneal section of the rats was shaved and cleaned, then examined using an ultrasound. Enlargement of the rat ovarium was observed and noted. Then, at week 24, 5 rats as experimental animal models of ovarian cancer were euthanized with cervical dislocation. The rats were dissected, the left and right ovaries were taken, the morphology was observed, and the left and right ovaries were measured. The ovaries were fixed with 10% formalin buffer for 24-48 hours. Then the tissue was implanted into a paraffin medium. The processed tissue preparations were then stained with Haematoxylin-Eosin (HE) staining. An anatomical pathologist performed histopathological analysis. The complete result of the analysis of ovarian cancer has been provided in the previous article (Dwi Sandhiutami et al., 2019; Sandhiutami et al., 2020)
REFERENCES
Dwi Sandhiutami, N.M., Arozal, W., Louisa, M., Rahmat, D., Wuyung, P.E., and Ulum, M.F. (2019). Induction of Epithelial Ovarian Cancer by Implantation of 7, 12-dimethylbenz (a) athracene (DMBA) Coated Silk in Rats. Journal of Young Pharmacists 11(1).
Sandhiutami, N.M.D., Arozal, W., Louisa, M., Rahmat, D., and Wuyung, P.E. (2020). Curcumin Nanoparticle Enhances the Anticancer Effect of Cisplatin by Inhibiting PI3K/AKT and JAK/STAT3 Pathway in Rat Ovarian Carcinoma Induced by DMBA. Front Pharmacol 11, 603235. doi: 10.3389/fphar.2020.603235.