INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the third leading cause of global deaths (World Health Organization, Geneva, Switzerland, 2020). COPD affects the quality of life of patients (Bogart et al., 2018) and results in significant financial implications for patients and healthcare institutions (WHO, Geneva, Switzerland, 2008). The pharmacotherapy for COPD patients may include selective short and long-acting β2 agonists, antimuscarinics, and glucocorticoids, which are delivered through inhalation.
Inhaled corticosteroids (ICS) have been a major pharmacotherapy option in maintenance therapy for COPD due to their presumed benefit of reducing the underlying inflammatory features linked to chronic bronchitis in COPD (Halpin et al., 2021). For patients with frequent exacerbations, ICS are normally used in combination with a long-acting bronchodilator, such as long-acting β2 agonists (LABA) and long-acting muscarinic antagonists (LAMA) (Halpin et al., 2021). Several early observational findings demonstrated significant death reductions with ICS usage. However, such outcomes were not observed in larger randomized controlled trials (RCTs) (Calverley et al., 2007; Sin and Tu, 2001; Soriano et al., 2002), and several studies have shown that ICS use was associated with multiple long-term adverse effects (Agusti et al., 2018; Crim et al., 2009; Pavord et al., 2016).
Multiple guidelines suggest that ICS could be safely discontinued in patients who have not experienced exacerbations, asthma, or low blood eosinophil count (Chalmers et al., 2020; Miravitlles et al., 2017). Despite such recommendations, it has been reported that ICS has been widely overprescribed in clinical settings. Moreover, ICS prescribing has been reported to be performed without personalized consideration of its benefit-to-risk ratio (Burgel et al., 2014).
This study aimed to (1) identify the prevalence of ICS-containing and non-ICS therapy prescribing among a sample of COPD patients at a Malaysian tertiary care center, (2) investigate the factors associated with the prescribing of ICS-containing and non-ICS therapy among the patients, and (3) examine the patterns of COPD inhaled therapy prescriptions among the patient sample, covering the period from 2017 to 2020. The study also aimed to determine the proportion of patients using ICS-containing therapy who would be considered suitable for ICS withdrawal during their most recent follow-up, based on the 2020 European Respiratory Society (ERS) guidelines (Chalmers et al., 2020). The findings from this study not only provide insights into ICS-containing therapy prescribing in the local setting but also highlight the current level of adherence to recent clinical practice guidelines regarding COPD treatment.
METHODS
Study design
This was a retrospective study involving a review of the electronic medical records (EMR) of COPD patients attending the Respiratory Clinic at Hospital Sultanah Nur Zahirah (HSNZ) in the state of Terengganu, Malaysia. This respiratory clinic provides specialized respiratory medical care and receives referrals from all levels of healthcare throughout the state. The EMR of patients were retrieved from the hospital information system (HIS), which contains patients’ health histories (including records of medical appointments and hospital admissions), the drugs prescribed, and any radiography and laboratory findings (Rani et al., 2021). Data collection was carried out in June 2021.
Study population
In this study, we included all patients who (1) had been clinically diagnosed with COPD in the years 2017–2020, (2) who were prescribed a COPD inhaled therapy (ICS-containing or non-ICS therapy) that was either initiated or escalated/de-escalated from a previous regimen, and (3) who had a most recent follow-up date of at least June 1, 2021. Patients who had been initiated with, escalated to, or de-escalated to any type of inhaled short-acting bronchodilators (e.g., salbutamol metered-dose inhalers) were excluded.
Study procedure
Collection of patients’ data
The EMR of COPD patients attending the HSNZ Respiratory Clinic between January 2017 and December 2020 were identified from the HIS. The patients’ EMR were reviewed to obtain data including sociodemographic details, body mass index (BMI), comorbidities, smoking status, eosinophil count, spirometry results [including forced expiratory volume in one second (FEV1) and forced vital capacity (FVC)], the modified Medical Research Council (mMRC) dyspnea scale score, and any history of COPD exacerbations in the preceding year.
Determination of the prevalence of ICS-containing and non-ICS therapy prescribing
For each patient included in the study, the first prescription of COPD inhaled therapy upon patient inclusion was identified. This was used in the analysis to determine the prevalence of ICS-containing and non-ICS therapy prescribing among the patients. In this study, the prescriptions of COPD inhaled therapy include those that result from either the initiation, escalation, or de-escalation of the therapy (which could be either an ICS-containing or a non-ICS therapy), based on the latest Global Initiative for Chronic Obstructive Lung Disease (GOLD) treatment recommendations, as follows (Gupta et al., 2021):
- Initiation of COPD inhaled therapy: a patient was prescribed maintenance inhaled therapy (LABA only, or LAMA only, or LABA/LAMA combination therapy, or ICS/LABA combination therapy) upon their initial COPD diagnosis.
- Escalation of COPD inhaled therapy: a patient’s therapy was escalated to either LABA/LAMA, or ICS/LABA, or ICS/LABA/LAMA combination therapies. Treatment escalation occurred when patients had experienced inadequate clinical outcomes with the previous therapy.
- De-escalation of COPD inhaled therapy: this refers to the reduction of a patient’s therapy in the event of unsatisfactory clinical outcomes or the occurrence of side effects, or in patients who had partial or near-complete symptoms resolution. For example, a patient using a LABA/LAMA combination therapy could be de-escalated to either LABA or LAMA monotherapy if long-term symptoms resolution had been achieved with the use of the previous regimen.
In the present study, patients prescribed an ICS-containing therapy (e.g., ICS monotherapy, ICS/LABA combination therapy, or ICS/LABA/LAMA triple therapy) are classified as ICS users. The term “non-ICS users” refers to those prescribed long-acting bronchodilators in the absence of ICS (e.g., LAMA monotherapy, LABA monotherapy, or LABA/LAMA dual therapy) (Chalmers et al., 2017; Lee et al., 2019).
Assessment of the factors associated with the prescribing of ICS-containing and non-ICS therapy
Consequently, the factors associated with the first prescription of COPD inhaled therapy (which could be either ICS-containing or non-ICS therapy) among the patients upon their inclusion in the study were identified. In this procedure, variables such as gender, ethnicity, age, BMI, comorbidities, smoking status, serum eosinophil level, mean post-bronchodilator FEV1 % predicted, FEV1 /FVC, COPD staging/group, mMRC score, and COPD exacerbation history were analyzed to determine their association with the prescription of ICS-containing or non-ICS therapy.
Determination of the trend of COPD inhaled therapy prescribing from 2017 to 2020 among the study sample
Next, patterns illustrating COPD inhaled therapy prescribing among the study sample between 2017 and 2020 were observed. This procedure involved compiling and tabulating all the prescriptions for COPD inhaled therapy received by the patients (that comprised ICS-containing or non-ICS therapy that was either initiated, escalated, or de-escalated) according to the year. The specific drug groups in the ICS-containing or non-ICS therapy were also tabulated according to the year.
Identification of patients suggestive of ICS withdrawal
For each patient included in the study, the prescription of COPD inhaled therapy during the patient’s most recent clinic follow-up was examined. The aim was to identify the proportion of patients using ICS-containing therapy among all patients prescribed a COPD inhaled therapy. These ICS users were then assessed for their eligibility for ICS withdrawal based on the 2020 ERS guidelines (Chalmers et al., 2020). In this procedure, the patients were categorized as follows:
- Strongly recommended for ICS continuation (eosinophils ≥ 300 cells/μl regardless of exacerbations history) or
- Conditionally recommended for ICS withdrawal (eosinophils < 300 cells/μl with less than two moderate exacerbations and no hospitalizations in the preceding year) or
- No recommendation is possible due to the lack of evidence (eosinophils <300 cells/μl with more than or equal to two moderate exacerbations or one hospitalization in the preceding year).
Statistical analysis
Continuous data are presented as mean and standard deviation (SD), whereas categorical data are expressed as frequencies and percentages. The chi-squared test or Fisher’s exact test were used to determine the association between the sociodemographic and clinical data, and the prescribing of ICS-containing and non-ICS therapy. Continuous data from the two groups were compared using independent sample t-tests. Descriptive statistics were utilized to display the patterns of COPD inhaled therapy usage and the proportion of patients demonstrating features suggestive of ICS discontinuation.
RESULTS
Patients’ characteristics
Between January 2017 and December 2020, 384 patients diagnosed with COPD were identified. Of all the patients, 228 had completed their clinic follow-up by June 1, 2021. Out of these 228 patients, 75 did not meet the inclusion criteria. Therefore, only 153 patients were included in the study.
Tables 1 and 2 illustrate the sociodemographic and clinical characteristics of the patients upon admission into the study, respectively. Most patients were male (140/153, 91.5%) and Malays (147/153, 96.1%). The mean (± SD) age of the patients was 66.2 (± 8.3) years. The history and presence of concurrent asthma were noted in 13.7% (21/153) and 6.5% (10/153) of the patients, respectively. The mean (± SD) serum eosinophil count was 323.8 (± 372.3) cells/μl. Despite this, only 39.2% (60/153) of the patients had eosinophilia. Most patients (98/153, 64.1%) had experienced no moderate exacerbations in the previous year. Nearly one-fifth of the patients (30/153, 19.6%) had frequent moderate exacerbations.
Prevalence of COPD inhaled therapy prescribing during the study period (2017–2020)
Upon inclusion in the study, 45.1% (69/153) and 54.9% (84/153) of the patients had been prescribed ICS-containing and non-ICS therapy (either as an initiated, escalated, or de-escalated regimen), respectively (Table 1).
Factors associated with the prescribing of ICS-containing and non-ICS therapy
There was no association between the sociodemographic characteristics of the patients and ICS-containing therapy prescribing upon patient inclusion in the study (Table 1). Several clinical characteristics of the patients were associated with the prescribing of ICS-containing therapy (Table 2). Patients who received ICS-containing therapy had significantly higher mean FEV1% predicted (p = 0.013) and mean serum eosinophils (p = 0.001) than those who received non-ICS therapy prescriptions. In addition, ICS-containing therapy prescribing was associated with concurrent asthma (p = 0.044), a history of moderate COPD exacerbations in the preceding year (p = 0.027), and serum eosinophil level of ≥ 300 cells/μl (p = 0.001).
Trends in COPD inhaled therapy prescribing between 2017 and 2020 among the study sample
The COPD inhaled therapy prescriptions among the included patients between 2017 and 2020 are shown in Figures 1 and 2. During the study period (2017–2020), the study sample (n = 153) used 239 prescriptions of COPD inhaled therapy. Overall, the number of prescriptions for ICS-containing therapy decreased from 72% (36/50) in 2017 to 33.3% (22/66) in 2020. However, there was a rise in the number of prescriptions for ICS-containing therapy from 2019 (20/74, 27%) to 2020 (22/66, 33.3%).
LAMA was the most frequently prescribed monotherapy inhaled therapy within the study period. No prescribing of ICS monotherapy was observed after 2019 (Fig. 1). The combination of LABA/LAMA, which comprised less than 10% of all prescriptions in 2017 and 2018, rose to about 30% in 2019 and 2020. The opposite pattern was observed for triple ICS/LABA/ LAMA therapy prescribing from 2017 to 2020, which showed a decline (Fig. 2).
The proportion of patients suggestive of ICS withdrawal
During the most recent clinic follow-up, 50.3% (77/153) and 49.7% (76/153) of the patients were ICS and non-ICS users, respectively. Of the 77 ICS users, 63.6% (49/77) had high eosinophil count and strong recommendations to continue with ICS therapy, whereas 26% (20/77) could benefit from ICS withdrawal due to their low eosinophil count and infrequent exacerbations (Table 3).
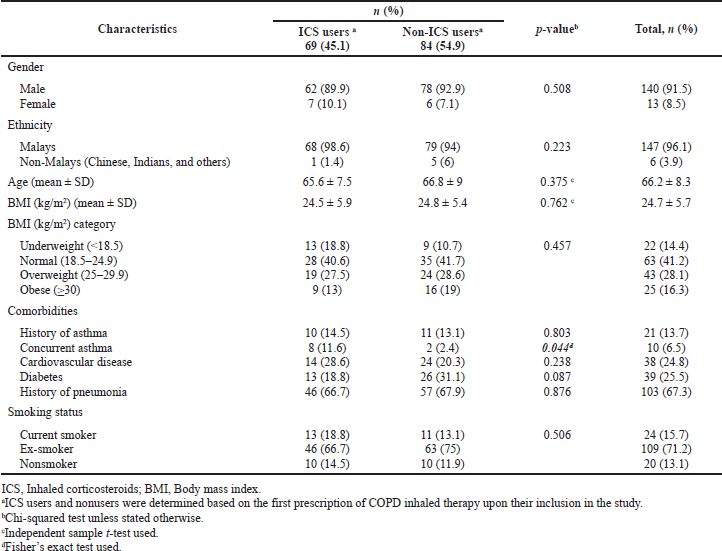 | Table 1. Sociodemographic characteristics of the patients and the association between these characteristics and the prescription of ICS-containing and non-ICS therapy among the study sample, n (%). [Click here to view] |
DISCUSSION
The present study showed that patients with concurrent asthma were significantly associated with the prescribing of ICS-containing therapy, which aligns with the recommendations in the GOLD report (Gupta et al., 2021). This finding was also recorded in reports from previous studies conducted in the United Kingdom (UK), Norway, and Spain (Chalmers et al., 2017; Drivenes et al., 2014; Roman-Rodriguez et al., 2016). The present study also found that blood eosinophilia was strongly associated with the prescribing of ICS-containing therapy. This aligns with the recommendation in the 2019 revision of the GOLD report. In the report, determining the blood eosinophil count to identify patients who potentially could be responsive to ICS is recommended. This recommendation was supported by previous post-hoc analyses of RCTs (Agusti et al., 2018; Bafadhel et al., 2018; Calverley et al., 2007; Lipson et al., 2018).
In addition, the present study showed that the history of moderate COPD exacerbations in the preceding year was significantly associated with the prescribing of ICS-containing therapy. Approximately 45% of the patients with a history of at least one moderate exacerbation in the preceding year, were prescribed an ICS-containing therapy. Moderate exacerbations were also identified as a significant predictor for the prescribing of ICS-containing therapy in previous studies (Chalmers et al., 2017; Drivenes et al., 2014; Roman-Rodriguez et al., 2016).
In this study, we categorized COPD patients using ICS-containing therapy based on the currently available ICS withdrawal algorithms. A retrospective cohort study in Korea attempted to identify ICS users with evidence-based factors for ICS treatment continuation recommendations, such as frequent exacerbations, history of asthma, and eosinophilia. The study reported a relatively high percentage of ICS users (47.5%) who did not present with any of the characteristics (Lee et al., 2019).
In fact, it has been reported that only a fraction of patients with COPD are actually considered appropriate for ICS therapy, based on the prescribing criteria (Vestbo et al., 2014). Additionally, the rates of ICS prescribing, as either monotherapy or combination therapy, ranged between 39% and 86%, as reported in previous studies (Chalmers et al., 2017; Izquierdo et al., 2010; Lee et al., 2019; Lung Health Study Research Group, 2000). Given the recommendation to limit ICS use to those for whom the treatment benefits would outweigh the risks, the current study highlights the need for measures to optimize ICS de-escalation among COPD patients.
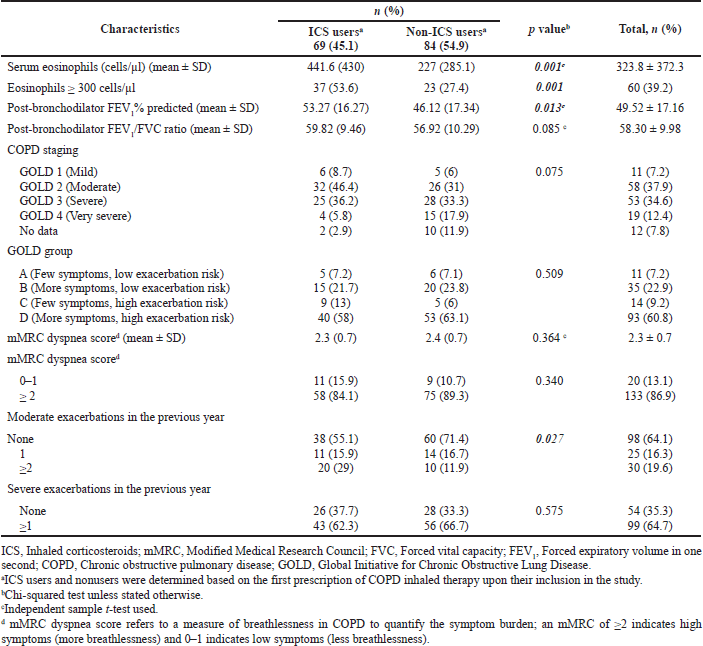 | Table 2. Clinical characteristics of the patients and the association between these characteristics and the prescription of ICS-containing and non-ICS therapy among the study sample, n (%). [Click here to view] |
In Malaysia, the opportunity to intensify ICS de-escalation among COPD patients could be performed through a medication therapy management program known as the Respiratory Medication Therapy Adherence Clinic (RMTAC). The pharmacist-led program that is run in collaboration with clinicians focuses on enhancing the treatment of patients with respiratory diseases, including COPD. It is accessible to patients at most public hospitals nationwide (Pharmaceutical Services Program, 2015). The RMTAC may assist in COPD patient management by monitoring stable patients with COPD and conducting assessments to determine whether ICS discontinuation is required. In the United States (US), a pharmacist-led ICS de-escalation program has been implemented to identify stable COPD patients on triple therapy who may have received excessive ICS-containing therapy. This program was associated with a high rate of de-escalation of ICS-containing therapy and resulted in a low occurrence of disease exacerbations among the patients within 3 months (Melzer et al., 2020).
The current study illustrates trends related to COPD inhaled therapy usage among COPD patients between 2017 and 2020 in HSNZ. The results showed that, of all prescriptions for COPD inhaled therapy in 2017, 72% included ICS-containing therapy. However, this percentage had been reduced to 33.3% by 2020. This result may be explained by the recent substantial revisions to the COPD management guideline in the GOLD reports regarding ICS usage in COPD patients. These updates were made due to knowledge of the increased risk of ICS-related adverse effects, particularly pneumonia, as well as the recommendation to restrict ICS use to only those patients for whom the treatment benefits are judged to outweigh the risks.
These results corroborate the findings from previous similar studies in the UK and South Korea. Chalmers et al. (2017)in the UK reported a decrease in ICS prescriptions as initiation therapy, from 77% in 2005 to 47% 10 years later. Similarly, the ICS prescribing rates in South Korea declined from 46.3% in 2014 to 38.8% 3 years later (Lee et al., 2019). Both studies attributed the decreasing trend of ICS prescriptions to the introduction of the dual long-acting bronchodilator regimen into the market, as well as the updates in COPD management guidelines.
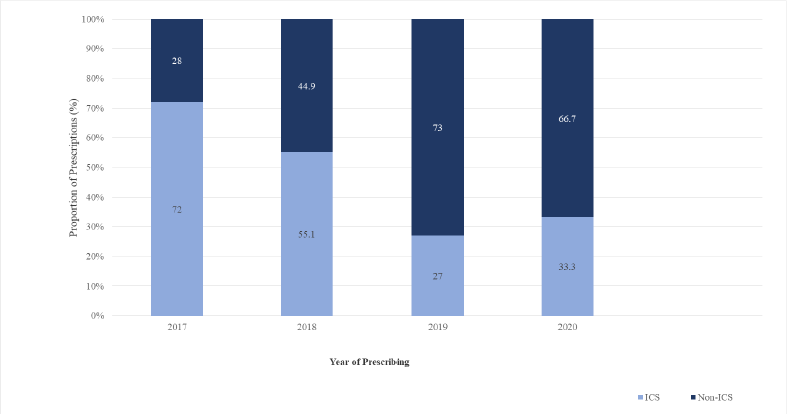 | Figure 1. Prescriptions of ICS-containing and non-ICS therapy from 2017 to 2020 among the study sample. [Click here to view] |
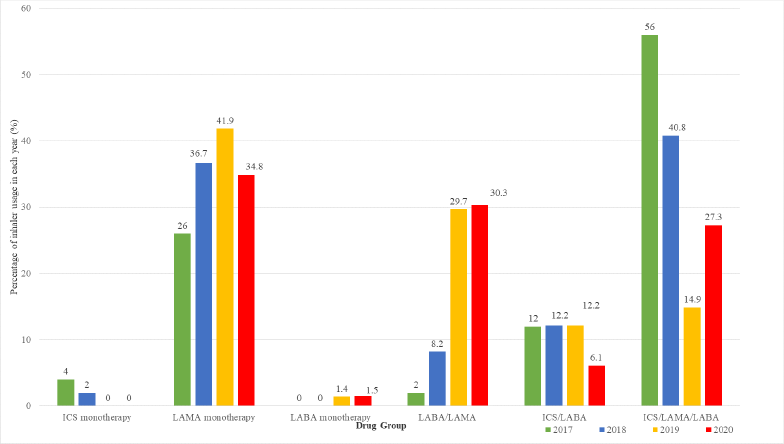 | Figure 2. Prescriptions of ICS-containing and non-ICS therapy according to the drug class from 2017 to 2020 among the study sample (ICS, LAMA, and LABA). [Click here to view] |
 | Table 3. Patients using ICS-containing therapy during their most recent follow-up and their category based on the 2020 European Respiratory Society (n = 77). [Click here to view] |
The increase in ICS prescribing from 2019 to 2020, as observed in the present study, should warrant the attention of healthcare professionals regarding possible nonadherence to clinical practice guidelines. Adherence to the evidence-based guidelines for COPD management, particularly the GOLD report, must be continually investigated (WHO, Geneva, Switzerland, 2003). Several barriers to nonadherence to clinical practice guidelines among prescribers have been reported. These include the lack of updated guidelines in the local settings, feeling insecure about ICS withdrawal, too frequent updates of guidelines, and the local prescribing policy (Tsiligianni et al., 2019). At the time of writing, the Malaysian Clinical Practice COPD Guidelines were last updated in 2012, as opposed to the yearly GOLD report revisions since 2011 (Ministry of Health Malaysia, 2017).
The overall changes in the prescribing of COPD inhaled therapy trends between 2017 and 2020 could be explained by the underlying shift in the treatment options for the initiation, escalation, and de-escalation of therapy. This study noted an increase in LABA/LAMA combination therapy prescribing with a corresponding decrease in the prescribing of ICS/LABA/LAMA combination therapy. LABA/LAMA combination therapy replaced ICS/LABA/LAMA combination therapy in treatment escalation, and this was the preferred regimen in ICS/LABA/LAMA combination therapy de-escalation. This result corroborates the findings from other studies that explored trends in ICS-containing and non-ICS prescriptions over time (Chalmers et al., 2017; Lee et al., 2019). Studies have shown that LABA/LAMA combination therapy is superior to the single long-acting bronchodilator therapy in improving lung functions, patient-reported outcomes, and symptoms. ICS withdrawal from ICS/LABA/LAMA combination therapy to LABA/LAMA combination therapy could be safely performed in patients with infrequent exacerbations and low eosinophil count (Magnussen et al., 2014; Maltais et al., 2019; Rossi et al., 2014; Suissa et al., 2015; Vogelmeier et al., 2017).
The high prescribing of LAMA monotherapy in the present study was consistent with the finding by Lee et al. (2019). According to the GOLD guidelines, LAMA monotherapy could be prescribed for the initial pharmacological treatment of Group B (high symptoms and low exacerbation risk), Group C (low symptoms and high exacerbation risk), and Group D (high symptoms and high exacerbation risk) patients (Gupta et al., 2021). Another important finding from the present study was the changing pattern of ICS monotherapy prescribing. No prescribing of ICS monotherapy was observed after 2019. This aligns with the treatment recommendation in the GOLD guidelines, which outline that ICS therapy only had minor beneficial effects on symptoms and exacerbations, and the benefits are outweighed by the long-term side effects, which include pneumonia (Vogelmeier et al., 2017; Yang et al., 2012).
Study limitations
The main limitation of this study was the inclusion of patients from only one tertiary care center. Therefore, the application and generalization of the study findings in other settings could be limited. Additionally, the small patient sample size hindered the application of multivariate statistical analysis. Future studies may address these issues by including more patients and involving multiple centers. In this study, the first prescription of COPD inhaled therapy upon patient inclusion was used in the analysis to determine the prevalence, factors, and trends of ICS-containing and non-ICS therapy prescribing. The findings of this study might be different if the prevalence, factors, and trends were determined from the prescription of COPD inhaled therapy at different time points. Also, the investigations conducted in this study only cover the period from 2017 to 2020. Additional studies should be conducted to investigate the trends of COPD inhaled therapy prescribing in recent years. Finally, in this study, the prescription of COPD inhaled therapy consisted of a therapy that was initiated, escalated, or de-escalated, resulting in a heterogeneous study sample in terms of their COPD inhaled therapy use.
CONCLUSION
A history of moderate exacerbations, presence of concurrent asthma, and eosinophilia were significantly associated with ICS-containing therapy prescribing, indicating that the current prescribing practice aligns with the GOLD treatment recommendations. The decreasing trend in ICS-containing therapy prescribing from 2017 to 2020 could be attributed to the substantial revisions in the COPD management guidelines. However, we noted a quarter of ICS users displayed features suggestive of ICS withdrawal, which necessitates a de-escalation program to identify and support patients who have been unnecessarily prescribed ICS-containing therapy. A pharmacist-led de-escalation program could be enhanced to optimize the safety and benefits of medication therapy in patients with COPD.
ACKNOWLEDGMENTS
The authors would like to thank the Director-General of Health, Malaysia, for permission to publish this paper.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
FUNDING
None.
AUTHORS’ CONTRIBUTIONS
All authors made significant contributions to the study conceptualization, methodology, formal analysis, investigation, data curation, writing the original draft, and reviewing and editing the final draft.
ETHICAL APPROVAL
The study received ethical approval from the Medical Review and Ethics Committee of the Ministry of Health Malaysia (NMRR-21-735-59206) and the Research and Ethics Committee of Universiti Teknologi MARA (REC/04/2021 [MR/262]).
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agusti A, Fabbri LM, Singh D, Vestbo J, Celli B, Franssen FME, Rabe KF, Papi A. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J, 2018; 52(6). CrossRef
Bafadhel M, Peterson S, De Blas MA, Calverley PM, Rennard SI, Richter K, Fageras M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med, 2018; 6(2):117–26. CrossRef
Bogart M, Stanford RH, Reinsch T, Hull M, Buikema A, Hulbert E. Clinical characteristics and medication patterns in patients with COPD prior to initiation of triple therapy with ICS/LAMA/LABA: a retrospective study. Respir Med, 2018; 142:73–80. CrossRef
Burgel PR, Deslée G, Jebrak G, Brinchault G, Caillaud D, Chanez P, Escamilla R, Nesme-Meyer P, Paillasseur JL, Perez T, Roche N. Real-life use of inhaled corticosteroids in COPD patients versus the GOLD proposals: a paradigm shift in GOLD 2011? Eur Respir J, 2014; 43(4):1201–3. CrossRef
Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J, investigators T. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med, 2007; 356(8):775–89. CrossRef
Chalmers JD, Laska IF, Franssen FME, Janssens W, Pavord I, Rigau D, McDonnell MJ, Roche N, Sin DD, Stolz D, Suissa S, Wedzicha J, Miravitlles M. Withdrawal of inhaled corticosteroids in COPD: a European respiratory society guideline. Eur Respir J, 2020; 55(6). CrossRef
Chalmers JD, Tebboth A, Gayle A, Ternouth A, Ramscar N. Determinants of initial inhaled corticosteroid use in patients with GOLD A/B COPD: a retrospective study of UK general practice. NPJ Prim Care Respir Med, 2017; 27(1):1–8. CrossRef
Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Willits LR, Yates JC, Vestbo J. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J, 2009; 34(3):641–7. CrossRef
Drivenes E, Ostrem A, Melbye H. Predictors of ICS/LABA prescribing in COPD patients: a study from general practice. BMC Fam Pract, 2014; 15(1):42. CrossRef
Gupta N, Malhotra N, Ish P. GOLD 2021 guidelines for COPD—what’s new and why. Adv Respir Med, 2021; 89(3):344–6. CrossRef
Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, Agusti AA, Vogelmeier CF. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med, 2021; 203(1):24–36. CrossRef
Izquierdo JL, Martin A, de Lucas P, Rodriguez-Gonzalez-Moro JM, Almonacid C, Paravisini A. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis, 2010; 5:241–9. CrossRef
Lee SH, Lee JH, Yoon HI, Park HY, Kim TH, Yoo KH, Oh YM, Jung KS, Lee SD, Lee SW. Change in inhaled corticosteroid treatment and COPD exacerbations: an analysis of real-world data from the KOLD/KOCOSS cohorts. Respir Res, 2019; 20(1):62. CrossRef
Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, Kilbride S, Lange P, Lomas DA, Martinez FJ, Singh D, Tabberer M, Wise RA, Pascoe SJ, Investigators I. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med, 2018; 378(18):1671–80. CrossRef
Lung Health Study Research Group, Wise R, Connett J, Weinmann G, Scanlon P, Skeans M. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med, 2000; 343(26):1902–9. CrossRef
Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, Towse L, Finnigan H, Dahl R, Decramer M, Chanez P, Wouters EF, Calverley PM, Investigators W. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med, 2014; 371(14):1285–94. CrossRef
Maltais F, Bjermer L, Kerwin EM, Jones PW, Watkins ML, Tombs L, Naya IP, Boucot IH, Lipson DA, Compton C. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res, 2019; 20(1):1–15. CrossRef
Melzer A, Bolduc J, Chezik L, Kunisaki K, Hegland A. Pharmacist driven de-escalation of inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease [ONLINE], 2020. Available via https://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2748 (Accessed 08 May 2022).
Ministry of Health Malaysia. Clinical practice guidelines: management of chronic obstructive pulmonary disease [ONLINE], 2017. Available via https://www.moh.gov.sg/docs/librariesprovider4/guidelines/copd.pdf (Accessed 08 May 2022).
Miravitlles M, Cosio BG, Arnedillo A, Calle M, Alcazar-Navarrete B, Gonzalez C, Esteban C, Trigueros JA, Rodriguez Gonzalez-Moro JM, Quintano Jimenez JA, Baloira A. A proposal for the withdrawal of inhaled corticosteroids in the clinical practice of chronic obstructive pulmonary disease. Respir Res, 2017; 18(1):198. CrossRef
Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, Barnes NC. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax, 2016; 71(2):118–25. CrossRef
Pharmaceutical Services Program. Respiratory medication therapy adherence clinic protocol: asthma/COPD (adult & paediatric) [ONLINE]. 2nd edition, 2015. Available via https://www.pharmacy.gov.my/v2/en/documents/respiratory-medication-therapy-adherence-clinic-protocol-asthma-copd-adult-paediatric-2nd-edition.html (Accessed 08 May 2022).
Rani NS, Wahab MSA, Zulkifly HH, Mohamad SH. Factors associated with disease progression among hormone receptor-positive breast cancer patients treated with endocrine therapy: A 5-year cross-sectional, retrospective follow-up study. J Appl Pharm Sci, 2021; 11(01):72–7.
Roman-Rodriguez M, van Boven JF, Vargas F, Contreras CC, Lamelas G, Gestoso S, Gongora M, Corredor M, Esteva M. Factors associated with inhaled corticosteroids prescription in primary care patients with COPD: a cross-sectional study in the Balearic Islands (Spain). Eur J Gen Pract, 2016; 22(4):232–9. CrossRef
Rossi A, van der Molen T, del Olmo R, Papi A, Wehbe L, Quinn M, Lu C, Young D, Cameron R, Bucchioni E, Altman P. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J, 2014; 44(6):1548–56. CrossRef
Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med, 2001; 164(4):580–4. CrossRef
Soriano JB, Vestbo J, Pride NB, Kiri V, Maden C, Maier WC. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J, 2002; 20(4):819–25. CrossRef
Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the Risk Reduction of Pneumonia. Chest, 2015; 148(5):1177–83. CrossRef
Tsiligianni I, Kampouraki M, Ierodiakonou D, Poulonirakis I, Papadokostakis P. COPD patients’ characteristics, usual care, and adherence to guidelines: the Greek UNLOCK study. Int J Chron Obstruct Pulmon Dis, 2019; 14:547–56. CrossRef
Vestbo J, Vogelmeier C, Small M, Higgins V. Understanding the GOLD 2011 strategy as applied to a real-world COPD population. Respir Med, 2014; 108(5):72936. CrossRef
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med, 2017; 195(5):557–82. CrossRef
World Health Organization. Introduction to drug utilization research [ONLINE], 2003. Available via https://apps.who.int/iris/handle/10665/42627 (Accessed 08 May 2022).
World Health Organization. The global burden of disease: 2004 update [ONLINE], 2008. Available via https://apps.who.int/iris/handle/10665/43942 (Accessed 08 May 2022).
World Health Organization. The top 10 causes of death [ONLINE], 2020. Available via https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (Accessed 08 May 2022).
Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev, 2012; 7:CD002991. CrossRef