INTRODUCTION
Migraine is one of the most common types of primary headaches worldwide (about 20%) besides a tension-type headache. The lack of attention to migraine could lead to uncontrolled or chronic migraine. Uncontrolled migraine impacts the quality of life and has long-term unwanted effects, such as strokes (Stovner et al., 2018) (Øie et al., 2020). Moderate to severe migraine attacks could interfere with daily activities. Thus, productive time is reduced, and this potentially reduces the quality of life. Therefore, migraine needs to be treated adequately to reduce disability due to migraine attacks (Manack et al., 2011).
Several migraine medications have been introduced and used, starting from simple analgesics such as acetaminophen, Nonsteroidal anti-inflammatory drug , and opioids to the ergotamine and triptans groups. Triptans are often used, especially in moderate to severe migraine attacks. However, triptans have some contraindications. Triptans are 5-Hydroxytriptamine (5-HT) 5-HT1b and 5-HT1d receptor agonists (Ong and De Felice 2018). The analgesic effect of triptans on the 5-HT1b receptors makes the intracranial blood vessels vasoconstrictive, whereas the effect on the 5-HT1d receptors blocks several vasoactive peptides and proinflammatory cytokines that can stimulate nociceptors. Several studies have shown that triptans have a vasoconstriction effect in other blood vessels, including the coronary arteries, so their safety profile for patients with vascular comorbidities such as coronary artery disease is still questionable (Dodick et al., 2020). A new class of drugs that acts on 5-HT1f has been developed, called ditans. Ditans specifically act on the intracranial blood vessels, which is safe for patients with comorbid vascular disease (Mecklenburg et al., 2020).
The efficacy and safety of currently available migraine preventive drugs do not meet the expected effect. They are also not specific for migraine, i.e., antiepileptic, antidepressant, and beta-blocker. Various side effects also decrease the patient’s adherence rate. Therefore, some new preventive drugs have been developed (Silberstein et al., 2012).
Drugs acting on calcitonin gene-related protein (CGRP) have been developed recently and have shown positive results. Monoclonal antibodies against the CGRP and CGRP receptors effectively prevent episodic and chronic migraine with minimal side effects. CGRP is known to play a role in migraine pathophysiology. In 2014, anti-CGRP or monoclonal antibodies were developed (Edvinsson et al., 2018). Galcanezumab, eptinezumab, fremanezumab, and erenumab are the monoclonal antibodies that have been developed to date. Among them, eptinezumab is the only monoclonal antibody given intravenously, at a dose of 100 mg or 300 mg every 3 months, while others are given subcutaneously (Datta et al., 2021).
The Headache Impact Test-6 (HIT-6) is an instrument that was developed to measure various factors that contribute to the headache burden. HIT-6 is often used for migraine. There are six items in HIT-6, which are pain, social functioning, vitality, role functioning, cognitive functioning, and psychological distress. The patients are asked to answer the questions with “never,” “rarely,” “sometimes,” “very often,” and “always.” HIT-6 scores ranged from 36 to 78. The higher the score, the more significant the impact of migraine on the quality of life. HIT-6 is easy to use, highly reliable, and consistent. Another instrument used for migraine is monthly migraine days (MMD). A high frequency of MMD is associated with a low quality of life, increased medication use, and loss of productivity. The frequency of MMD is a parameter that could be used as a reference in determining the effectiveness of therapy or prophylaxis. HIT-6 and MMD were used in several studies to determine the success rate of migraine therapy (Di Tanna et al., 2019; Shin et al., 2008).
Many clinical trials about the effectiveness of eptinezumab as a preventive migraine therapy have been conducted. So far, some of these studies have shown positive results. Several researchers have also conducted meta-analyses regarding the efficacy of eptinezumab. However, most of the outcomes they assessed were based only on MMD. Therefore, we conducted an updated meta-analysis to determine the efficacy of eptinezumab (100 mg and 300 mg) as a migraine preventive therapy based on mean MMD and mean HIT-6 scores. In addition, we assessed the safety profile of eptinezumab based on the frequency of the most frequent side effects in each of the available Randomized controlled trial (RCTs).
METHODS
Data sources and search strategy
We systematically searched PubMed and Cochrane Library using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses method to prepare this systematic review. We used the keywords “migraine” OR “migraineurs” OR “migrain” OR “migraineous” OR “migrainous” AND “eptinezumab” AND “placebo” OR “placebos” OR “placeboes” on search engines. The Population, Intervention, Comparison, Outcome, and Study design (PICOS) format was used to establish a search strategy. The PICOS and eligibility criteria are shown in Table 1.
Study selection and screening
Titles and abstracts were screened based on the inclusion and exclusion criteria by two authors (first screening). After screening the titles and abstracts, two authors reviewed the selected full text (second screening). If two authors disagreed, the third author was consulted, and a decision was made by consensus. Articles were included in the meta-analysis when data pooling and outcome measurement were identical.
Inclusion and exclusion criteria
For a study to be included, it had to be an English language study, be a randomized clinical trial, have an available full-text article and a complete manuscript, and be published in the last 5 years. Articles in the forms of case reports, descriptive studies, clinical trials, cross-sectional studies, cohort studies, and case-control studies were excluded. The titles and abstracts were manually screened in accordance with the eligibility requirements.
Data items and collection
Three authors collected and extracted data from the selected articles. The data were extracted for the following variables: inclusion criteria, sample size, mean age, intervention, comparison, outcome measurement, duration of intervention, and statistical data. The statistical analysis between the intervention and control group data was calculated using the RevMan software (version 5.4). Confidence intervals and p-values were used to calculate the missing standard deviation from each study.
Risk of bias (ROB) and quality assessment
We use the JADAD scale to assess the quality assessment for each study. The JADAD scale is used to assess the methodological quality of clinical trials. The Cochrane ROB tool for RCTs was used to assess the ROB. Two reviewers evaluated the included articles independently. The third reviewer resolved disagreements between the two reviewers.
RESULTS
Literature search
There were 112 article results in PubMed and 150 in Cochrane Library. After removing duplicates, 125 articles were screened based on the titles and abstracts. We used Boolean operators to search the papers. We excluded articles that were not randomized controlled trials and were not published in the last 5 years. The full texts of 18 studies were screened by 2 authors. After screening the full text, eight studies were found eligible and included in this review. The flow of the article search system is shown in Figure 1.
ROB and quality assessment
A moderate ROB was present in almost all included studies. Some studies did not report complete statistical results and reported ambiguous details on the dropout rate. The ROB summary is shown in Figure 2. The quality assessment of each study was done using the JADAD scale. The JADAD scale is used to evaluate the methodological quality of clinical trials. There were five questions with one score for each question. The study with a total score range of 3–5 is high quality, and that with 1–2 is low quality. All studies included in this review were of high quality. The results of the quality assessment are shown in Table 2.
Synthesis results
The sample size ranged from 431 to 1,072, with a mean age ranging from 39.9 to 41.4 years. Each study performed an intervention using eptinezumab at doses of 100 mg and 300 mg and had cut-off periods of 12 weeks and 24 weeks. MMD, HIT-6, and adverse events during the intervention were assessed. A summary of the study characteristics of the sample size, mean age, type, and duration of intervention from each study is shown in Table 3.
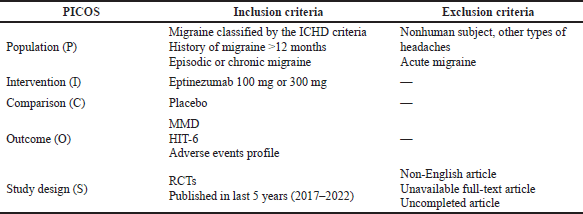 | Table 1. PICOS and eligibility criteria. [Click here to view] |
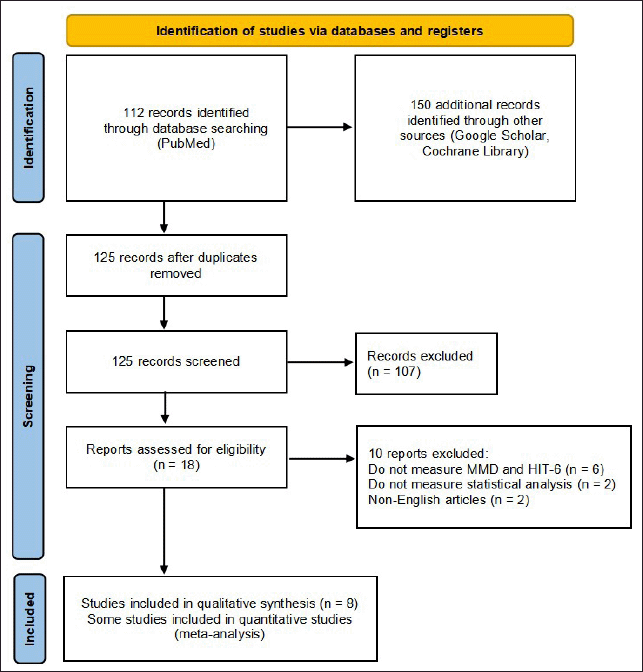 | Figure 1. The flow diagram of article search. [Click here to view] |
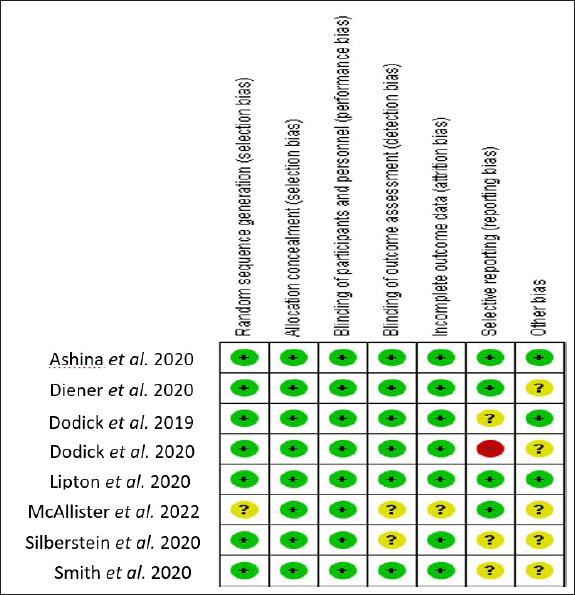 | Figure 2. Risk of bias summary. [Click here to view] |
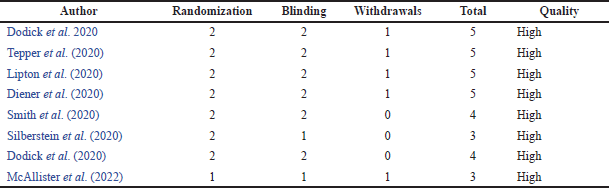 | Table 2. JADAD scale. [Click here to view] |
Main pooled results of meta-analysis
Studies with identical data and the same outcome measure were calculated for meta-analysis. A meta-analysis study was conducted to compare the results of the mean MMD difference between the intervention (eptinezumab 100 mg and 300 mg) and placebo at the cut-off durations of 12 weeks and 24 weeks and the mean difference between before and after intervention (eptinezumab 100 mg and 300 mg). The mean difference in the mean HIT-6 score was also calculated between the intervention (eptinezumab 100 mg) and placebo groups and the pre-and postintervention groups.
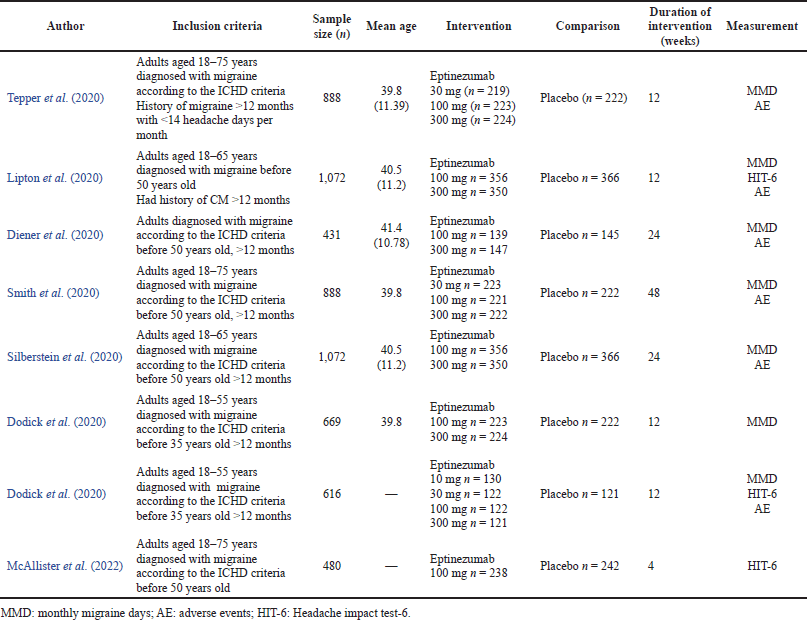 | Table 3. Summary of the study characteristics. [Click here to view] |
Monthly migraine days
The mean differences in MMD were compared between eptinezumab 100 mg and placebo at 12 weeks and 24 weeks (after intervention), eptinezumab 300 mg and placebo at 12 weeks and 24 weeks (after intervention), and also between baseline mean MMD (before intervention) and mean MMD after 12 weeks and 24 weeks (after intervention) of eptinezumab 100 mg and eptinezumab 300 mg.
Eptinezumab 100 mg versus placebo
Following a 12-week intervention, the mean MMD difference between eptinezumab 100 mg and placebo is shown in Figure 3A. The pooled mean difference in the mean MMD is −1.52 (95% CI −2.27 to −0.076, p 0.0001). Figure 3B shows the mean MMD difference between eptinezumab 100 mg and placebo after 24 weeks of intervention using a pooled random-effect data model. When the data from three studies with the same data are combined, the pooled mean difference in the mean MMD is −1.89 (95% CI −3.26 to −0.52, p = 0.007). The mean MMD decreased more significantly when eptinezumab 100 mg was used for 24 weeks than 12 weeks.
Eptinezumab 300 mg versus placebo
Figure 3C shows the mean MMD difference between eptinezumab 300 mg and placebo after 12 weeks of intervention using a pooled random-effect data model. The pooled mean difference in mean MMD for five studies with identical data is 1.90 (95% CI −2.8 to −0.99, p 0.0001). Figure 3D displays the mean MMD difference between eptinezumab 300 mg and placebo after 24 weeks of intervention using a pooled random-effect data model. The pooled mean difference in mean MMD for three studies with identical data is −2.24 (95% CI −3.68 to −0.79, p = 0.002). The MMD rate was decreased more by using eptinezumab 300 mg for 24 weeks than 12 weeks.
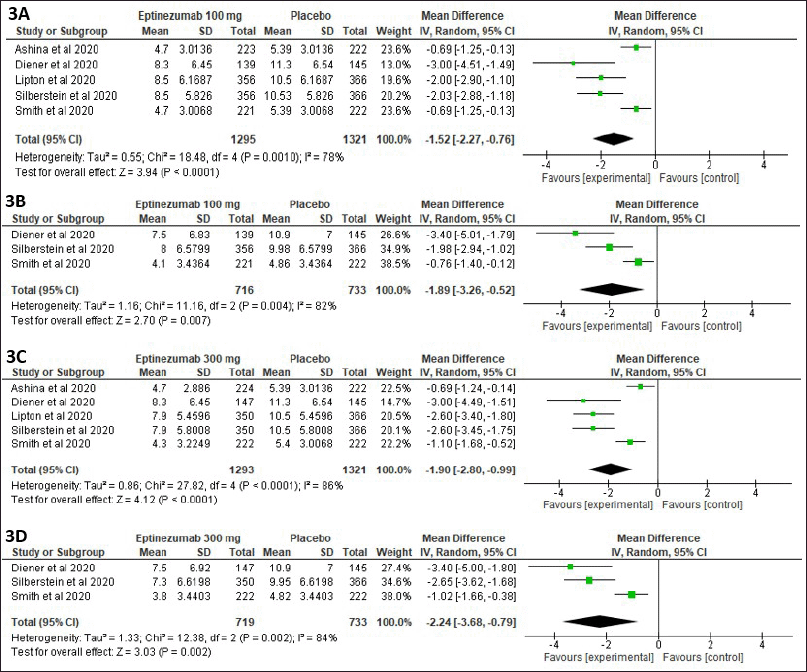 | Figure 3. Pooled data comparing mean differences in mean MMD between eptinezumab 100 mg and placebo group at 12 weeks (A) and at 24 weeks (B) and eptinezumab 300 mg and placebo group at 12 weeks (C) and at 24 weeks (D). [Click here to view] |
Eptinezumab before and after intervention
The mean MMD difference between eptinezumab 100 mg before intervention and eptinezumab 100 mg after 12 weeks of intervention is shown in Figure 4A using a pooled random-effect data model. The pooled mean difference in the mean MMD for two studies with identical data for meta-analysis is −6.16 (95% CI −10.47 to −1.85, p = 0.005). The mean MMD difference between eptinezumab 300 mg before intervention and eptinezumab 300 mg after 12 weeks of intervention is shown in Figure 4B using a pooled random-effect data model. The pooled mean difference in mean MMD for two studies with identical data for meta-analysis is −6.4 (95% CI −10.61 to −2.19, p 0.00001). The use of eptinezumab 300 mg was superior to eptinezumab 100 mg in reducing the mean MMD for 12 weeks.
Headache impact test-6 score
Figure 5A shows the mean difference in HIT-6 score between eptinezumab 100 mg and placebo after 12 weeks of intervention using a pooled random-effect data model. The pooled mean difference in the mean HIT-6 score for two trials with similar data for meta-analysis is −2.87 (95% CI −3.78 to −1.96, p 0.00001). Figure 5B shows the mean difference in mean HIT-6 score between eptinezumab 100 mg before intervention (baseline) and eptinezumab 100 mg after 12 weeks of intervention using a pooled random-effect data model. The pooled mean difference in the mean HIT-6 score for two trials with similar data for meta-analysis is −7.36 (95% CI −8.25 to −6.48, p 0.00001). Using eptinezumab 100 mg for 12 weeks decreased the mean HIT-6 score significantly.
Adverse events
Adverse events were reported in some populations during the intervention. The summarized results of the incidence of any events at each dose (Fig. 6) showed that there was no significant difference between eptinezumab 100 mg and 300 mg (p = 0.07). The most frequently reported adverse events were nasopharyngitis (n = 245), followed by upper respiratory tract infection (n = 237), sinusitis (n = 42), dizziness (n = 44), and urinary tract infection (n = 37). No deaths were reported in these studies. The incidence of side effects at each dose of eptinezumab and placebo can be seen in Table 4.
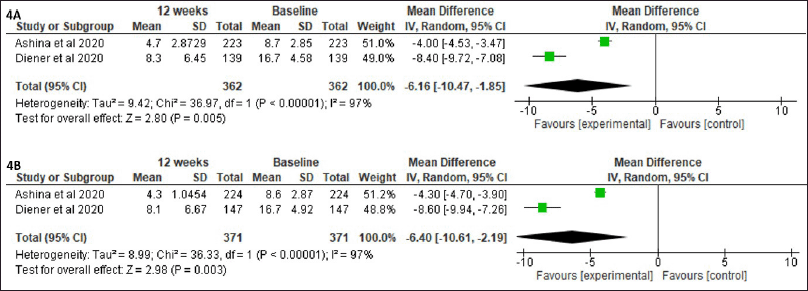 | Figure 4. Pooled data comparing mean differences in mean MMD between eptinezumab 100 mg before intervention (baseline) and eptinezumab 100 mg after 12 weeks of intervention (A) and eptinezumab 300 mg before intervention (baseline) and eptinezumab 300 mg after 12 weeks of intervention (B). [Click here to view] |
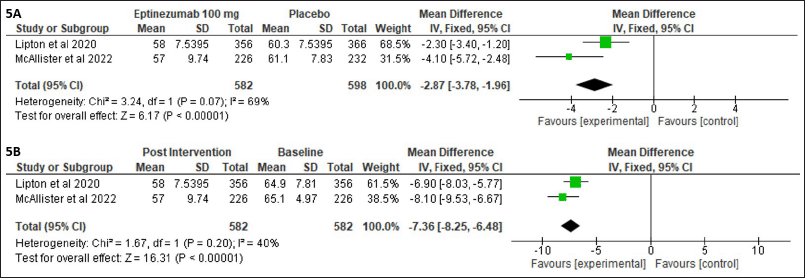 | Figure 5. Pooled data comparing mean differences in mean HIT-6 score between eptinezumab 100 mg and placebo after 12 weeks of intervention (A) and eptinezumab 100 mg before intervention (baseline) and eptinezumab 100 mg after 12 weeks of intervention (B). [Click here to view] |
DISCUSSION
Eptinezumab is the only CGRP monoclonal antibody available intravenously and has a bioavailability of 100%. Steady-state plasma concentrations can be achieved after the first dose. Eptinezumab is not metabolized by cytochrome P450 and is not affected by other drug interactions. Several studies have shown that the administration of sumatriptan does not decrease the concentration and effectiveness of eptinezumab (Datta et al., 2021). The recommended dose of eptinezumab is 100 mg or 300 mg every 3 months. Some patients require a dose of 300 mg. Eptinezumab is given intravenously in 100 ml of a 0.9% NaCl solution for 30 minutes, so its administration requires professional healthcare (Morgan and Joyner 2021).
A study shows that those with more frequent MMD have a lower quality of life, lower productivity, and a high burden of maintenance costs (Johnston et al., 2022). Our meta-analysis showed that the administration of eptinezumab decreased the mean MMD. Administration of eptinezumab 100 mg and 300 mg reduced the mean MMD more than placebo in 12 and 24 weeks. Between the periods before and after intervention, eptinezumab 300 mg reduced the mean MMD more than eptinezumab 100 mg (−6.16 vs. −6.40). Smith et al. (2020) reported that the proportion of patients who experienced >50% and >75% symptom improvement was more significant at 24–48 weeks than at 1–24 weeks. Continuous administration after the first dose (12 weeks) has a better effect and is relatively safe with minimal side effects (Smith et al., 2020).
The HIT-6 score was created to screen and monitor patients with headaches for clinical and research purposes. The HIT-6 score assesses the effects of headaches on pain, social functioning, vitality, role functioning, cognitive function, and psychological distress. The HIT-6 score has been validated and used globally to monitor treatment efficacy for headaches, including migraine (Yang et al., 2011). Our meta-analysis showed that the mean HIT-6 score between eptinezumab 100 mg and placebo was −2.87 (95% CI −3.78 to −1.96, p < 0.00001). Meanwhile, the mean HIT-6 score before and after intervention of eptinezumab 100 mg after the first dose (12 weeks) was −7.36 (95% CI −8.25 to −6.48, p < 0.00001). This shows that eptinezumab 100 mg improves the quality of life and daily functioning. In the Prevention of Migraine via Intravenous ALD403 Safety and Efficacy (PROMISE) PROMISE-2 study, at 4 weeks, eptinezumab 100 mg and eptinezumab 300 mg significantly reduced the mean HIT-6 scores (Lipton et al., 2021).
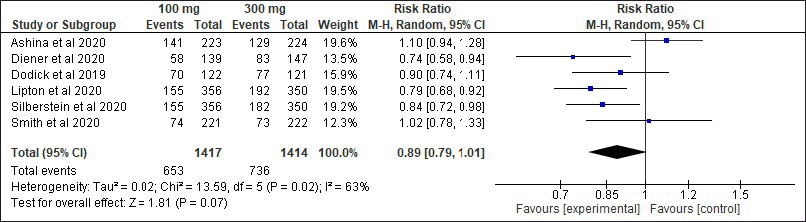 | Figure 6. Pooled risk ratio of any events between eptinezumab 100 mg and 300 mg. [Click here to view] |
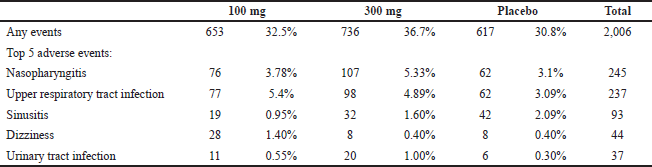 | Table 4. Adverse events that occurred during the intervention period. [Click here to view] |
Side effects after taking eptinezumab have been reported in several studies. Based on the PROMISE-1 and PROMISE-2 trials, nasopharyngitis and upper respiratory tract infection were the most common side effects after taking eptinezumab. In the PREVAIL trial, adverse events occurred in about 71% of the participants. The most common side effects are nasopharyngitis, upper respiratory tract infection, sinusitis, and influenza. In this trial, three participants were pregnant during the intervention period, two of whom miscarried (Kudrow et al., 2021). This report is in line with the results of our meta-analysis. In our meta-analysis, the incidence of side effects was 653 (46.1%) in eptinezumab 100 mg and 736 (52.1%) in eptinezumab 300 mg. The most frequently reported side effects were nasopharyngitis, upper respiratory tract infection, sinusitis, dizziness, and urinary tract infection (Table 4). The side effects reported in this review were consistent with those in previous studies of eptinezumab for migraine. There was no significant difference in the incidence of side effects between eptinezumab 100 mg and 300 mg (p = 0.07). The PREVAIL trial also revealed that eptinezumab 300 mg was the maximum safe and tolerable dose (Tassorelli et al., 2018). The incidence of placebo side effects is mostly related to patient and doctor expectations and disease-specific symptoms (Weihrauch and Gauler, 1999).
Based on the above results, eptinezumab is effective and safe as a migraine preventive therapy. Eptinezumab 300 mg was more effective in reducing the mean MMD and HIT-6 scores, with no significant difference in the incidence of side effects from eptinezumab 100 mg. The use of eptinezumab 300 mg can be considered a preventive therapy for moderate to severe migraine without fear of side effects.
More studies with variations in population types are needed to strengthen the results of the eptinezumab study. In addition, the cost of a single infusion of eptinezumab is known to reach $1,600 (every 3 months). However, these costs are insignificant compared to the improvement of the daily functioning impact caused by migraine (Datta et al., 2021). Previous studies have shown that eptinezumab can be effective for up to 48 weeks. There needs to be a more comprehensive study on whether eptinezumab can be effective and safe for at least the next 3–5 years, considering that migraine is a chronic disease. Other CGRP monoclonal antibodies, such as erenumab and fremanezumab, have been tested to this point (Tepper et al., 2020).
CONCLUSION
Eptinezumab is safe and effective as a migraine preventive therapy. Eptinezumab reduces the mean MMD and HIT-6 scores, and there was no significant difference in the incidence of side effects between eptinezumab 100 mg and 300 mg. Eptinezumab can be considered a preventive therapy for episodic and chronic migraine.
LIMITATIONS
There were not enough studies included in this systematic review. Future research on the efficacy and safety of eptinezumab for migraine needs to be carried out.
AUTHORS’ CONTRIBUTIONS
IMO conceived the idea to carry out this systematic review. IMO is a neurologist and a headache consultant. EHT and IPE performed the study search and data extraction and were approved by IMO. EHT calculated the data for the meta-analysis using RevMan. The results of the meta-analysis calculations were then checked by IPE and confirmed by IMO. EHT prepared this manuscript. IMO and IPE then read and revised the manuscript. The three authors reread the final results of the manuscript and reached a consensus.
ACKNOWLEDGMENTS
The authors thank the Department of Neurology, Faculty of Medicine, Universitas Udayana/Sanglah General Hospital, Bali, Indonesia, for supporting them in preparing this systematic review.
CONFLICTS OF INTEREST
The authors report no financial or other conflicts of interest in this work.
FUNDING
There is no funding to report.
ETHICAL APPROVAL
No ethical approval was required for this study.
DATA AVAILABILITY
All data generated and analyzed are included in this article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
ABBREVIATIONS
5-HT, 5-Hydroxytriptamine; CGRP, Calcitonin gene-related protein; HIT-6, Headache Impact Test-6; MMD, Monthly migraine days; NSAID, Nonsteroidal anti-inflammatory drug; RCT, Randomized controlled trial; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROMISE, Prevention of Migraine via Intravenous ALD403 Safety and Efficacy; PICOS, Population, Intervention, Comparison, Outcome, and Study design.
REFERENCES
Datta A, Maryala S, John R. A review of eptinezumab use in migraine. Cureus, 2021; 13(9):e10832. CrossRef
Dodick DW, Shewale AS, Lipton RB, Baum SJ, Marcus SC, Silberstein SD, Pavlovic JM, Bennett NL, Young WB, Viswanathan HN, Doshi JA, Weintraub H. Migraine patients with cardiovascular disease and contraindications: an analysis of real-world claims data. J Prim Care Community Health, 2020; 11:1–10. CrossRef
Diener HC, Marmura MJ, Tepper SJ, Cowan R, Starling AJ, Diamond ML, Hirman J, Mehta L, Brevig T, Sperling B, Cady R. Efficacy, tolerability, and safety of eptinezumab in patients with a dual diagnosis of chronic migraine and medication-overuse headache: subgroup analysis of PROMISE-2. Headache, 2021; 61(1):125–36. CrossRef
Di Tanna GL, Porter JK, Lipton RB, Brennan A, Palmer S, Hatswell AJ, Sapra S, Villa G. Migraine day frequency in migraine prevention: longitudinal modelling approaches. BMC Med Res Methodol, 2019; 19(1) :20. CrossRef
Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies-successful translation from bench to clinic. Nat Rev Neurol, 2018; 14(6):338–50. CrossRef
Johnston K, Harris L, Powell L, Popoff E, Coric V, L’Italien G, Schreiber CP. Monthly migraine days, tablet utilization, and quality of life associated with rimegepant-post hoc results from an open label safety study (BHV3000-201). J Headache Pain, 2022; 23(1):10. CrossRef
Kudrow D, Cady RK, Allan B, Pederson SM, Hirman J, Mehta LR, Schaeffler BA. Long-term safety and tolerability of eptinezumab in patients with chronic migraine: a 2-year, open-label, phase 3 trial. BMC Neurol, 2021; 21(1):126. CrossRef
Lipton RB, Dodick DW, Ailani J, McGill L, Hirman J, Cady R. Patient-identified most bothersome symptom in preventive migraine treatment with eptinezumab: a novel patient-centered outcome. Headache, 2021; 61(5):766–76. CrossRef
Manack AN, Buse DC, Lipton RB. Chronic migraine: epidemiology and disease burden. Curr Pain Headache Rep, 2011; 15(1):70–8. CrossRef
McAllister P, Winner PK, Ailani J, Buse DC, Lipton RB, Chakhava G, Josiassen MK, Lindsten A, Mehta L, Ettrup A, Cady R. Eptinezumab treatment initiated during a migraine attack is associated with meaningful improvement in patient-reported outcome measures: secondary results from the randomized controlled RELIEF study. J Headache Pain, 2022; 23(1):22. CrossRef
Mecklenburg J, Raffaelli B, Neeb L, Sanchez Del Rio M, Reuter U. The potential of lasmiditan in migraine. Ther Adv Neurol Disord, 2020; 13:1–11. CrossRef
Morgan KW, Joyner KR. Eptinezumab: a calcitonin gene-related peptide monoclonal antibody infusion for migraine prevention. SAGE Open Med, 2021; 9:1–8. CrossRef
Øie LR, Kurth T, Gulati S, Dodick DW. Migraine and risk of stroke. J Neurol Neurosurg Psychiatry, 2020; 91(6):593–604. CrossRef
Ong JJY, De Felice M. Migraine treatment: current acute medications and their potential mechanisms of action. Neurotherapeutics, 2018; 15(2):274–90. CrossRef
Shin HE, Park JW, Kim YI, Lee KS. Headache impact test-6 (HIT-6) scores for migraine patients: their relation to disability as measured from a headache diary. J Clin Neurol (Korea), 2008; 4(4):158–63. CrossRef
Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults. Neurology, 2012; 78(17):1337–45. CrossRef
Smith TR, Janelidze M, Chakhava G, Cady R, Hirman J, Allan B, Pederson S, Smith J, Schaeffler B. Eptinezumab for the prevention of episodic migraine: sustained effect through 1 year of treatment in the PROMISE-1 study. Clin Ther, 2020; 42(12):2254–65. CrossRef
Stovner, L. J. et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol, 2018; 17(11):954–76.
Tassorelli C, Diener HC, Dodick DW, Silberstein SD, Lipton RB, Ashina M, Becker WJ, Ferrari MD, Goadsby PJ, Pozo-Rosich P, Wang SJ, International Headache Society Clinical Trials Standing Committee. Guidelines of the international headache society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia, 2018; 38(5):815–32. CrossRef
Tepper SJ, Ashina M, Reuter U, Brandes JL, Doležil D, Silberstein SD, Winner P, Zhang F, Cheng S, Mikol DD. Long-term safety and efficacy of erenumab in patients with chronic migraine: results from a 52-week, open-label extension study. Cephalalgia, 2020; 40(6):543–53. CrossRef
Weihrauch TR, Gauler TC. Placebo-efficacy and adverse effects in controlled clinical trials. Arzneimittel-Forschung/Drug Research, 1999; 49(5):385–93. CrossRef
Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the headache impact test (HIT-6™) across episodic and chronic migraine. Cephalalgia, 2011; 31(3):357–67. CrossRef