INTRODUCTION
Elaeis guineensis Jacq. or E. guineensis (EG) or oil palm tree is a native plant of Africa that is distributed in West Africa, Angola, and Namibia (Purugganan and Fuller, 2009) and is popular as an industrial plant (Andoko and Widodoro, 2013). This plant has different names in many countries, such as oelpalme in Germany, palma da olio in Italy, pam namman in Thailand, palmeira-caiaue in Brazil, palma de aceite in Spain, and palmier a huile in France (CABI, 2021). Indonesia is well known as the first and biggest crude palm oil producer and exporter in the world. EG or “kelapa sawit” is widely cultivated for industrial palm oil production, especially in East Kalimantan, an Indonesian province with the largest industrial palm oil cultivation. However, the high level of palm oil production linearly increases the amount of biomass production, reaching 16 tons dry weight per hectare per year, with the oil palm leaves as the largest amount of biomass. Thus far, the utilization of EG is still limited to the fruit for palm oil production. As a consequence, overloading of biomass occurred and led to hazardous environmental pollution in the industrial area. Innovation is urgently required as the alternative solution to convert the useless biomass, in this case, its leaves, to renewable and economic-valuable products. Owing to this problem, many studies have been conducted to explore the therapeutic potential of EG leaves (EGL).
In this mini review, we summarize and highlight the phytoconstituents and some pharmacological actions of EGL from several reported preclinical studies to improve the reader’s insight and to provide the scientific basis of EGL as a potential biomass for herbal medicine resources. Our perspective on the challenge and prospect of this plant in herbal medicine aspects is also briefly discussed in this article.
BOTANICAL ASPECTS OF E. GUINEENSIS JACQ.
Morphology and taxonomy
EG is a monocotyle plant with a height of 20 m or more at maturity with the taxonomy classification in Table 1.
The trunk of this tree is solid and is spirally arranged by 20−40 leaves and 100−160 pairs per leaf blade. Its root system consists of primary and secondary roots. Leaves are numerous, erect, spreading to drooping with the length of 3−5 m in a mature tree, and they are characterized by a short stalk, broad base, and fibrous along the margin and jagged spines, especially in the adult leaves. Additionally, the leaves consist of leaflets, lamina, rachis, and petiole; the leaflet that fails in the growth process to be lamina will develop into a spine (Pahan, 2008).
The flower is single and/or pairs in the between of branches with three sepals, three petals with edges touching in the bud, six stamens and a small sterile pistil and two to three small bracts with three sepals, three petals overlapping in the bud in a ring of small, sterile stamens, and a three-celled ovary with three spreading stigmas (Pahan, 2008).
The fruit is arranged in a bunch with an average weight of 23 kg and may be up to 82 kg per bunch. A bunch contains 200−2,000 oval drupes, 4 cm long and 2 cm broad with a pointed apex. The fruit color varies from yellow to orange or nearly black (Orwa et al., 2009). The morphology of the tree and leaves of EG is shown in Figure 1.
Geographical distribution
EG is native to Africa and widely distributed in many parts of Africa, such as in Angola, Benin, Cameroon, the Central African Republic, Chad, Congo, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Gulf of Guinea Island, Ivory Coast, Kenya, Liberia, Malawi, Mozambique, Nigeria, Senegal, Sierra Leone, Sudan, Tanzania, Togo, Uganda, and Zaire (Kew, 2002). Moreover, this plant is also well cultivated in many tropical countries, such as Malaysia and Indonesia. The Indonesian Central Agency on Statistics (BPS) reported that the area of oil palm plantation in Indonesia reached 14,858.300 hectares in 2020 (BPS, 2022). The geographical distribution of EG in the world is shown in Figure 2.
Phytochemical aspects of E. guineensis Jacq. leaves
Several lines mention that the main bioactive compounds of EGL are reported as phenolic compounds, flavonoids, tannins, coumarins, alkaloids, saponins, terpenoids, and steroids (Yin et al., 2013). The phenolic compounds, especially flavonoids, such as flavan-3-ols, flavones, flavone-C-glycoside, and flavone-O-glycoside, are the most abundant in EGL (Tow et al., 2021). The phenolic compound is well known as the most potent compound group that possesses many pharmacological activities, especially antioxidant-related activity. The reported chemical constituents of EGL and the chemical structure are presented in Table 2.
 | Table 1. Taxonomy classification of EG (Andoko and Widodoro, 2013 ; Pahan, 2008 ). [Click here to view] |
Pharmacological aspect of E. guineensis Jacq. leaves
Some investigations are conducted to evaluate the pharmacological activity of EGL. According to the previous in vitro and in vivo studies, it was concluded that the extract of EGL showed 11 potential pharmacological actions, as shown in Figure 3.
Antioxidant activity
Based on the study conducted by Che Zain et al. (2020), the EGL extract exhibited a potent antioxidant activity. The antioxidant activity was investigated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and nitric oxide (NO) methods. The results revealed that the methanolic extract had a reducing power value of as much as 71.62−101.48 mg AAE/g extract on the FRAP assay with the half inhibitory concentration (IC50) being 3.53−6.54 µg/mL and 7.64 µg/mL on the DPPH and NO assay, respectively (Che Zain et al., 2020). Correspondingly, the investigations by Yusof et al. (2016) and Zumaro et al. (2021) reported that the ethanolic extract of EGL also inhibited the DPPH radical with IC50 values of 247 µg/ml and 133.58 µg/ml, respectively (Yusof et al., 2016; Zumaro et al., 2021). The antioxidant properties of EGL were related to (+)-catechin, (−)-epicatechin, orientin, isoorientin, vitexin, and isovitexin contents in the extract (Che Zain et al., 2020).
Antimicrobial activity
Chong et al. (2008) reported that EGL showed a good potency for bacterial growth inhibition, especially on Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, and Proteus mirabilis, with the zone of inhibition and minimum inhibitory concentration of as much as 14 mm, 12.5 mg/ml; 12 mm, 6.25 mg/ml; 13 mm, 12.5 mg/ml; 11 mm, >50 mg/ml; 14 mm, 12.5 mg/ml; 12 mm, 6.25 mg/ml; and 13 mm, >50 mg/ml, respectively (Chong et al., 2008; Vijayarathna et al., 2012). Aritonang et al. (2021) also reported that its ethanolic extract effectively inhibited the growth of Propionibacterium acnes with a zone of inhibition of as much as 5.38 mm. The high contents of saponin, tannin, and flavonoid in the extract were considered active compounds responsible for this activity to coagulate bacterial protein and then impacted growth nutrition blockage in microorganisms (Ajayi et al., 2016; Yin et al., 2013).
Antihypertensive Activity
Jaffri et al. (2011) observed that the EGL extract showed antihypertensive and cardioprotective properties. Catechin with its antioxidant activity plays a role in normalizing the nitric oxide serum level and inhibiting arterial wall thickening. These mechanisms were effective in reducing the risk of hypertension, atherosclerosis, and coronary heart disease (Jaffri et al., 2011).
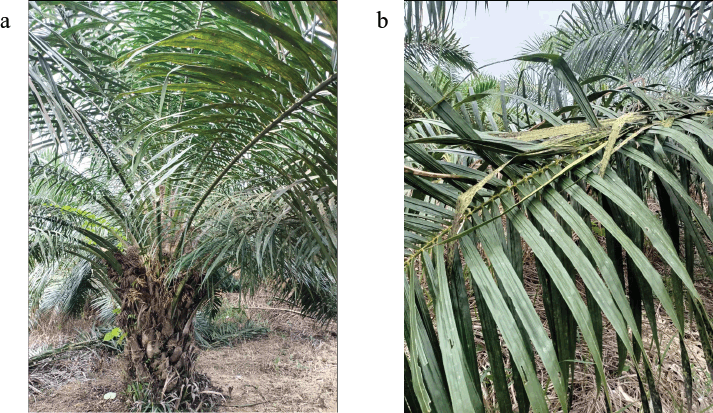 | Figure 1. The morphology of (a) the tree and (b) the leaves of EG (authors’ collection). [Click here to view] |
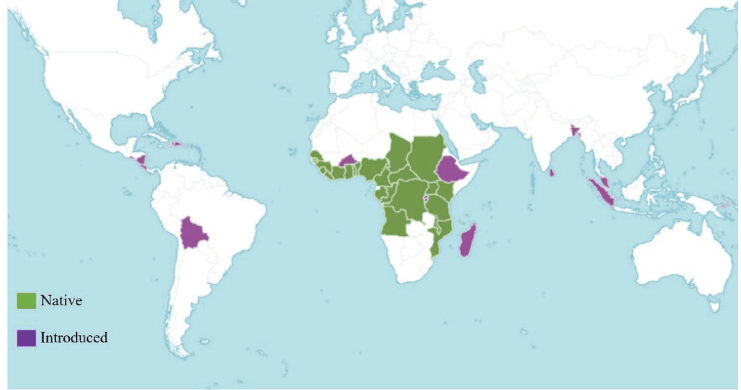 | Figure 2. Geographical distribution of EG (Kew, 2022 ). [Click here to view] |
Antidiabetic activity
Banu (2019) reported that polar and nonpolar extracts of EGL had an antidiabetic effect in alloxan-induced diabetic rats with an effective dose of as much as between 100 and 200 mg/kg BW. Based on this previous study, the administration of 200 mg/kg BW nonpolar and polar extract for 28 days effectively reduced the blood glucose level from 338 to 111 mg/dl and 297 to 90 mg/dl, respectively (Banu, 2019).
Antityrosinase activity
Tyrosinase was a key enzyme in the melanogenesis activation that induced skin hyperpigmentation in humans. Yusof (2016) investigated the antityrosinase activity of the EGL extract, and the result showed a potent tyrosinase inhibition activity on the concentration 7.8−500 µg/ml with the percentage of inhibition and IC50 of as much as 29.69%−64.62% and 254 µg/ml, respectively. The catechin content in the extract was related to this activity. Additionally, a previous study reported that catechin showed the melanin synthesis inhibition activity in the melanoma B16 cell. Thus, this extract can be potentially developed to be a topical brightening skin formula (Yusof, 2016).
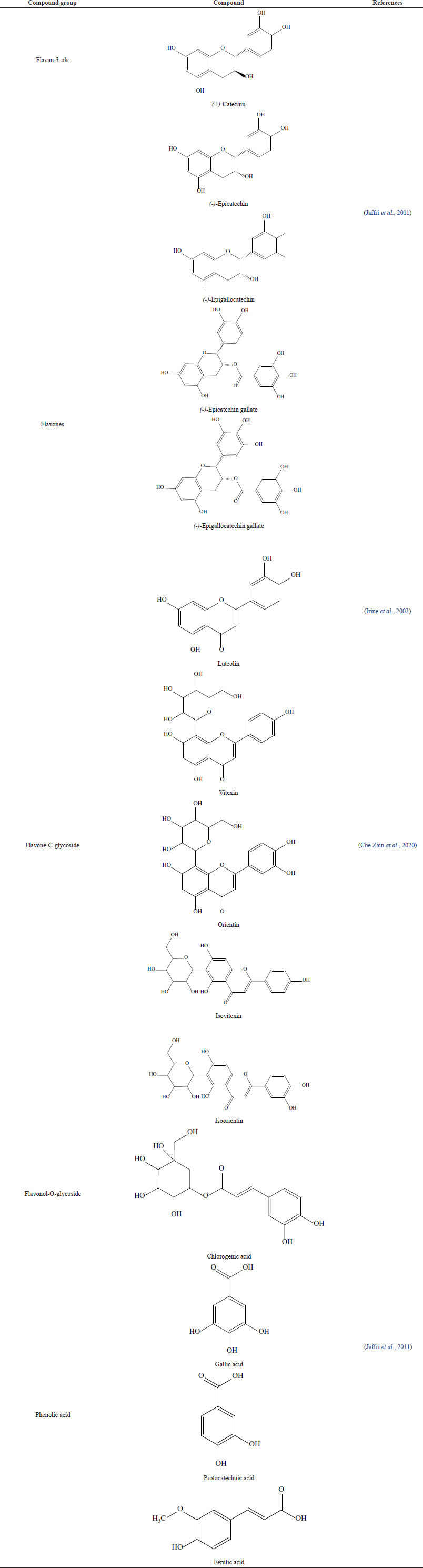 | Table 2. The reported chemical constituents of EGL. [Click here to view] |
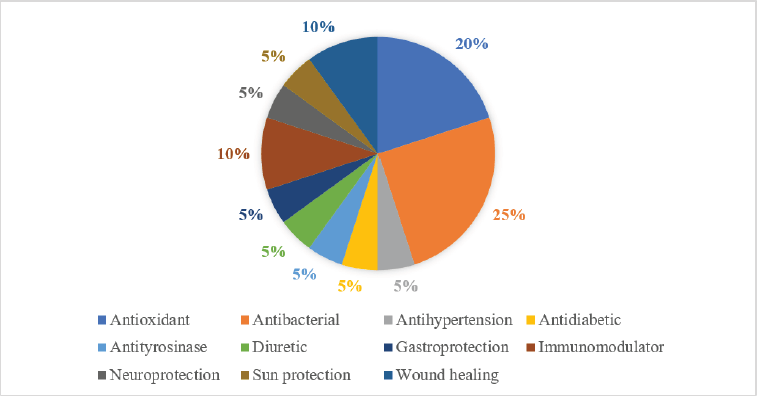 | Figure 3. The pharmacological actions of EGL. [Click here to view] |
Diuretic activity
Simorangkir (2020) examined the diuretic effect of the extract combination of EGL (50 mg/kg BW) and Gymnanthemum amygdalinum leaves (67.5 mg/kg BW). This combination was reported as a potent diuretic agent with a urine volume of as much as 21.6 mL for 6 h. Moreover, the other combination dose of these extracts, 135:100 mg/kg BW and 270:200 mg/kg BW, also showed a more potent diuretic activity with the urine volume being 22.09 and 23.01 ml for 6 h. Previously, the diuretic activity of this combination was reported as not significantly different in comparison with that of furosemide, the available diuretic agent, with the urine volume that was produced by furosemide being as much as 24.48 mL for 6 h (Simorangkir, 2020).
Gastroprotection activity
The gastroprotection activity of the ethanolic and ethyl acetate extracts of EGL was examined by Wahyuni (2021). This study presented a potent gastroprotective activity of these extracts, based on the ability to decrease ulcer quality and quantity, total acidity, and gastric fluid volume in ethanolic-induced peptic ulcer male rats. The gastroprotection activities of the ethanolic and ethyl acetate extracts on the concentrations of 100 and 200 mg/kg BW were similar, with the percentage of inhibition as much as 23.35, 39.63, 22.16, and 32.61%, respectively, and these results were also not significantly different in comparison to those of sucralfate (43.24%), the available gastroprotective agent (Wahyuni, 2021).
Immunomodulator activity
The immunomodulatory effect of the EGL extract was examined by Nabila (2021) and Situngkir (2021). Its ethanolic, n-hexane, ethyl acetate, and water extracts indicated the immunomodulatory effect based on the leucocyte profile and antibody titer in male rats. The administration of these extracts significantly (p < 0.05) increased the total of leucocytes, especially in the lymphocyte, neutrophil, monocyte, and eosinophil parameters and also on the antibody titer in comparison to the CMC-Na 0.5% control group. The best activity was represented by 200 mg/kg BW of the n-hexane extract, and its activity was not significantly different from Levamisol®, the approved immunomodulator for anthelmintic and cancer therapy (Nabila, 2021; Situngkir, 2021).
Neuroprotection activity
A previous in vivo study was conducted by Mohamed et al. (2013) to assess the neuroprotective and neurogenesis effect of the ethanolic extract of EGL based on the nitric oxide (NO) deficiency animal model. The viability of neurons was observed in the hippocampus area, especially in CA1, CA3, and DG. The NO deficiency condition led to the chronic neurodegeneration in CA1, CA3, and DG with percentages of degeneration of as much as 53%, 80%, and 32%, respectively. The oral administration of the ethanolic extract of EGL significantly (p < 0.001) inhibited the pyramidal cell degeneration condition and effectively retained the neuron in CA1, CA3, and DG with percentages of protection activity of as much as 74%, 72%, and 76%. A previous study mentioned that the ethanolic extract of EGL was more potent than captopril in the neuroprotection activity. In this case, the percentage of protection activity of captopril was only in the range of 47−56%. Interestingly, the extract-treated control group showed higher neuron viability than the extract-untreated control group. This result also indicated that the ethanolic extract of EGL had a potent neurogenesis effect in the hippocampus area (Mohamed et al., 2013).
Skin protection activity
In a separate study conducted by Yusof (2016), skin protection activity was also shown by the EGL extract at concentrations of 1, 5, and 10% with percentages of UV transmission of as much as 104, 66.9, and 52.62%, respectively. This decrease in UV transmission profile represented that the extract effectively absorbed UVB and increased the sun protection factor. Moreover, the increase in its concentration strongly associated with increase in the UVA/UVB ratio. Thus, the concentrations of 5% and 10% were considered for superior protection against UVA. Based on these results, the EGL extract revealed a good potency to be developed as a herbal-based topical sunscreen for skin protection from UVA and UVB radiation (Yusof, 2016).
Wound healing activity
In a previous investigation conducted by Che Zain et al. (2020), the EGL extract represented the wound healing activity based on the ability to increase the proliferation and migration in the 3T3 cell. Based on a previous study, the methanolic extract of EGL showed the high cell migration activity with a percentage of cell migration of as much as 85.83−93.34% on the scratch-wound assay (Che Zain et al., 2020). Additionally, the study conducted by Ginting (2020) showed that the administration of EGL and G. amygdalinum leaves in a gel formula presented a similar potency in the burn-wound healing process and similar duration of wound healing of as much as 23 . Interestingly, this extracts combination was more effective than the single formula corresponding with a shorter healing duration, just 18 d. These results indicated that the extract of EGL with the phenolic content had a promising activity in the wound healing process (Ginting, 2020).
Challenge and future prospects of E. guineensis Jacq. leaves as herbal medicine
Several studies showed that E. guineensis Jacq. leaves (EGL) are a promising prospect material with various pharmacological effects, and these are appropriated to overcome many health problems. The previous studies only provided preliminary experimental data. Therefore, comprehensive studies in the future are still required to complete and clarify all the pharmaceutical-related issues, which include toxicity, pharmacodynamic, pharmacokinetic, and pharmaceutics technology aspects. These are scientific challenges to the reutilization of EGL as herbal medicine resources. A good collaboration between researcher and herbal industry to develop it as pharmaceutical raw material in herbal medicine.
CONCLUSION
Based on the current literature, we conclude that E. guineensis Jacq. leaves (EGL) present broad pharmacological actions. We identified 11 different activities that are represented by the EGL extract, including antioxidant, antibacterial, antihypertensive, antidiabetic, immunomodulatory, diuretic, sun protection, wound healing, neuroprotective, gastroprotective, and antityrosinase activities. Flavonoid and phenolic acids are considered the major bioactive compound groups in the EGL extract, and they are promising for the development of various therapeutic agents after further pharmaceutical studies on the toxicity, pharmacodynamic, pharmacokinetic, and pharmaceutic technology aspects.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the Faculty of Pharmacy, Mulawarman University, for the financial support during the preparation of this manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve the use of animals or human subjects.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ajayi O, Awala S, Ogunleye A, Okogbue, F. Antimicrobial screening and phytochemical analysis of Elaeis guineensis (Ewe Igi Ope) against Salmonella strains. British J Pharm Res, 2016; 10(3):1–9. CrossRef
Andoko A, Widodoro. Berkebun Kelapa Sawit Si Emas Cair. Agro Media, Jakarta, Indonesia, 2013.
Aritonang SC, Wisnu CP, Angga CN, Riski S, Helmi. Optimization of VCO concentration on physical stability of facewash and antibacterial test of oil palm (Elaeis guineensis Jacq.) leaf extract on acne-causing bacteria. Proceeding of 14th Mulawarman Pharmaceuticals Conferences; 2021 Dec 10–12; Faculty of Pharmacy University of Mulawarman, Samarinda, Indonesia, 2021. CrossRef
Banu K. Perbandingan efektivitas antidiabetik ekstrak non polar dan polar daun kelapa sawit (Elaeis guineensis Jacq) terhadap tikus yang diinduksi aloksan. BSc. Thesis. University of North Sumatera, Medan, Indonesia, 2019.
BPS, 2022. Luas Tanaman Perkebunan Menurut Provinsi (Ribu Hektar) Tahun 2018-2020. Available via https://www.bps.go.id/indicator/54/131/1/luas-tanaman-perkebunan-menurut-provinsi.html (Accessed 26 January 2022).
CABI, Elaeis guineensis (African oil palm), 2022. Available via https://www.cabi.org/isc/datasheet/20295 (Accessed 26 January 2022).
Che Zain MS, Lee SY, Nasir NM, Fakurazi S, Shaari K. Metabolite characterization and correlations with antioxidant and wound healing properties of oil palm (Elaeis guineensis Jacq.) leaflets via 1H-NMR-based metabolomics approach. Mol, 2020; 25(23):5636. CrossRef
Chong KH, Zuraini Z, Sashidaran S, Devi PVK, Latha LY, Ramanathan S. Antimicrobial activity of Elaeis guineensis leaf. Pharmacologyonline, 2008; 3:379–86.
Ginting SS. Perbandingan efek penyembuhan luka bakar antara gel ekstrak daun afrika (Gymnanthemum amygdalinum Del.) dengan gel ekstrak etanol daun kelapa sawit (Elaeis guineensis Jacq.) serta kombinasinya pada kelinci tahun 2019, J Penelitian Farm Herb, 2020; 3(1):82–90. CrossRef
Irine R, Noordin MM, Radzali M, Azizah H, Hapizah N, Mahinda YA, Suhaila M. Antioxidant and hypocholesterolemic effects of Elaeis guineensis frond extract on hypercholesterolemic rabbits. ASEAN Food J, 2003; 12:137–47.
Jaffri JM, Mohamed S, Rohimi N, Ahmad IN, Noordin MM, Manap YA. Antihypertensive and cardiovascular effects of catechin-rich oil palm (Elaeis guineensis) leaf extract in nitric oxide–deficient rats. J Med Food, 2011; 14(7–8):775–83. CrossRef
Kew RBG. Elaeis guineensis Jacq Encyclopedia, 2022 [ONLINE] Available via https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:666802-1 (Accessed 30 January 2022).
Mohamed S, Lee Ming T, Jaffri JM. Cognitive enhancement and neuroprotection by catechin-rich oil palm leaf extract supplement: cognitive-enhancing effects of oil palm leaf extract. J Sci Food Agric, 2013; 93(4):819–27. CrossRef
Nabila AK. Efek Imunomodulator Ekstrak n-Heksana dan Etil Asetat Daun Kelapa Sawit (Elaeis guineensis Jacq) terhadap jumlah total Leukosit dan Titer Antibodi Sel Imun Tikus Jantan, BSc. Thesis, University of North Sumatera, Medan, Indonesia, 2021.
Orwa C, Mutua A, Jamnadass R. Agroforestree database: a tree reference and selection guide version 4.0, 2009. [ONLINE] Available via http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp (Accessed 25 January 2022).
Pahan I. Paduan Lengkap Kelapa Sawit. Penebar Swadaya, Jakarta, Indonesia, 2008.
Purugganan MD, Fuller DQ. The nature of selection during plant domestication. Nature, 2009; 457(7231):843–8. CrossRef
Simorangkir D. Pengujian efektivitas diuretik kombinasi ekstrak etanol daun afrika (Gymnanthemum amygdalinum Del.) dan daun kelapa sawit (Elaeis guineensis Jacq.) pada tikus jantan. J Penelitian Farm Herb, 2020; 3(1):106–11. CrossRef
Situngkir OR. Efek Imunomodulator Ekstrak Etanol dan Air Daun Kelapa Sawit (Elaeis gueneensis Jacq) terhadap total Leukosit dan Titer Antibodi Sel Imun Tikus Jantan. BSc. Thesis, Univeristy of North Sumatera, Medan, Indonesia, 2021.
Tow WK, Asly PTG, Usha S, Uma DP, Yasodha S. Flavonoid composition and pharmacological properties of Elaeis guineensis Jacq. leaves extracts: a systematic review. Pharmaceuticals, 2021; 14(10):961. CrossRef
Vijayarathna S, Zakaria Z, Chen Y, Latha LY, Kanwar JR, Sasidharan S. The antimicrobial efficacy of Elaeis guineensis: Characterization, in vitro and in vivo studies. Mol, 2012; 17(5):4860–77. CrossRef
Wahyuni, S. Uji ekstrak etanol dan etil asetat daun kelapa sawit (Elaeis guineensis Jacq.) sebagai gastroprotektor pada lambung tikus putih jantan yang diinduksi dengan etanol. BSc.Thesis, University of North Sumatera, Medan, Indonesia, 2021.
Yin NS, Abdullah S, Phin CK. Phytochemical constituents from leaves of Elaeis guineensis and their antioxidant and antimicrobial activities. Inter J Pharm Pharm Sci, 2013; 5, 137-140.
Yusof NZ. Potetial uses of oil palm (Elaeis guineensis) leaf extract in topical application. J Oil Palm Res, 2016; 28(4):520–30. CrossRef
Zumaro M, Hifdzur RR, Angga CN, Riski S, Helmi. Antioxidant activity of oil palm (Elaeis guineensis Jacq.) leaf ethanol extract. Proceeding of 14th Mulawarman Pharmaceuticals Conferences; 2021 Dec 10–12; Samarinda, Indonesia: Faculty of Pharmacy University of Mulawarman, 2021. CrossRef