INTRODUCTION
Background and definition
Innovation within the pharmaceutical industry continues to draw considerable interest from industry and academia. This is not surprising given the important impact of innovation on various aspects of life (Baregheh et al., 2009). From an economic perspective, innovation has contributed to improved quality of life in many countries, which is reflected as improvement in their economies (Atkinson & Wial, 2008). Despite this interest, many pharmacists still find it challenging to differentiate among terms such as pharmaceutical innovation, invention, discovery, and entrepreneurship. Innovation may be defined as a new idea, creative thoughts, or new imaginations in the form of a device or a method (Arruda, 2016).
Pharmacy is an area that has had a significant share of innovation over the years with many pharmacists, working in various sectors, contributing to the development of new and innovative ideas, products, and services. Pharmaceutical innovations range from the design and discovery of new medicines to novel methods of service delivery, all to enhance patient care (Morgan et al., 2008). It can be said that the discovery, development, production, and delivery processes that increase the availability of medical products and people’s access to them summarize the concept of pharmaceutical innovation (Berger et al., 2016).
Opportunities for innovation within clinical pharmacy and pharmacy practice sectors are many. Examples of previously reported innovations in these areas include innovation focused on clinical performance and regularly monitoring performance data, implementing and prioritizing an appointment-based medication synchronization program, offering patient-centered care by directly delivering services to customers, providing electronic services, and actively participating in the local and greater pharmacy community.
Difference between innovation, invention, discovery, and entrepreneurship
Terms of entrepreneurship and innovation complement each other. However, entrepreneurship is simply the design and launch of new business; innovation is the process of creating new ideas that contribute to business development. In other words, innovation is considered a tool for entrepreneurship (Soriano & Huarng, 2013). There is also a great convergence between the terms “invention” and “innovation”; yet, invention involves the process of creating a new thing that did not exist before, whether it was a product or service, while innovation is to find an idea to introduce this invention into practice (Toner & Tompkins, 2008). Discovery is defined as the process by which new things are known that were not previously known, while innovation comes to transform these discoveries into useful products (Sams-Dodd, 2005).
Innovative design can also be used to describe websites. Thus, in the present digital environment, pharmaceutical institutions should be aware of their websites. In that respect, the following aspects are used to assess the level of innovative design: minimalism, diversity, immersive 3D elements, responsive design, dark mode, micro-interactions, video content, mixing photography with graphics, attractiveness, and fast loading (Shneiderman & Leavitt, 2006).
Pharmaceutical innovation worldwide
Pharmaceutical research and development have traditionally been dominated by multinational companies in the United States and Europe. More recently, other countries such as China and Brazil have developed their pharmaceutical industries to compete in the global pharmaceutical market (Akkari et al., 2016). The rate of technological progress, based on innovation, is a key factor in determining the rate of economic growth. For example, in the United States of America, the increased longevity resulting from pharmaceutical innovation has elevated national wealth by an estimated $3.2 trillion annually (Lichtenberg, 2010). The economic benefits of innovation are not the only stimulus for increased innovative interest, but the positive consequences on population health (Lichtenberg et al., 2017) and reduced mortality and improved quality of medical services (Lichtenberg, 2014a) continue to drive change.
Factors promoting pharmaceutical innovation and obstacles
Many factors influence pharmaceutical innovation both globally and within a country. One of the key promoting factors for innovation is the legislative change, for example, amending legislation to facilitate the innovation process. This may involve modification of some of the existing systems to increase access to larger marks, such as exclusive rights and intellectual property. Providing governmental and societal support for pharmaceutical innovations also stimulates the development of innovative practices. This requires an initial direct investment from governments and societies, reduced tax measures or rescheduling of finances, and so forth. It is hoped that the initial investment will generate future health and economic benefits. Access to expert knowledge and other resources also helps to promote innovation and reduces the risk of failure (Rovira, 2009). Many other factors might also promote innovation, such as proper education, continuous development and training, and the availability of infrastructure. On the other hand, obstacles that hinder innovation include lack of financial investment and support, lack of experience in the field of innovation, and legislative processes that convolute the process (Strobel & Kratzer, 2017).
Innovation in Jordan
Although Jordan is a small country suffering from a scarcity of natural resources with a small population (Dandan, 2011), a large part of its economy depends on industry, where it contributed around 11% of the GDP in 2009 (Kreishan, 2010). The pharmaceutical industry is considered one of the most important pillars of the Jordanian economy (Al-Wazaify & Albsoul-Younes, 2005). Jordanian pharmaceutical production is one of the largest in the Middle East, where Jordanian pharmaceutical companies export more than 70% of their products to 65 countries around the world (Alawi & Alabbadi, 2015). Recently, many business incubators were established in Jordanian universities and public–private institutions. Examples include Umniah and Zinc Business Incubators and iPark, which may play an important role in supporting emerging innovative ideas. At least three annual national competitions have been created to encourage healthcare providers and students to create and lead innovative projects: Hakeem Academy Annual Competition (Hakeem Academy, 2020), Crown Prince Competition for the Best Government Application (The Crown Prince Foundation, 2018), and Be the Solution Competition. However, only the last one solely targets the pharmacy sector (The Arab Innovation Network, 2020). The private sector also funds many competitive financial investments, often advertised via social media platforms (Al Bawab et al., 2018).
Even though the pharmaceutical industry and pharmaceutical care are some of the national Jordanian priorities, almost all innovations in Jordan are related to business administration and computer sciences (Obeidat et al., 2016). Pharmaceutical innovation in Jordan faces many problems; for example, the rules and regulations are not consistent, the financial support is less than needed for such innovation, and sometimes experts who can turn theoretical context into practicalities are not present. Accordingly, the new product development process in the Jordanian pharmaceutical sector is considered highly risky and difficult (Ghannajeh et al., 2015).
The role of pharmaceutical innovations in pharmaceutical practice can be strengthened by developing cooperation between pharmacy practice and academia. This should help to implement innovations that more closely align with the needs of the local community and therefore creates an environment that supports the innovations. (Alsharif, 2019) Ultimately it is hoped that this will result in improved patient outcomes. However, upon review of the curriculum of 17 pharmacy schools in Jordan, we could not find a special module within pharmacy schools that targets innovation and entrepreneurship in pharmacy. To the best of our knowledge, this is the first research paper that aims to investigate the knowledge, attitude, and perceptions (KAP) of pharmaceutical innovation among Jordanian pharmacists and final-year bachelor’s pharmacy students.
METHOD
Study design and settings
This study was carried out through an online validated and reliable questionnaire by using a Google Forms template to explore the KAP toward pharmaceutical innovation among pharmacists and final-year pharmacy students in Jordan. Data collection was carried out during the period from February to August 2020 in Amman, Jordan. The questions were categorized in three sections: Demographic characteristics shared questions, then tailored tracks for Bachelor of Pharmacy Students, but not Doctor of Pharmacy (PharmD) finally tailored track for pharmacists.
The shared questions were designed to investigate five domains: first, the definitions of pharmaceutical innovation, pharmaceutical discovery, pharmaceutical invention, and pharmaceutical entrepreneurship; second, assessment of knowledge and perception of the innovative design of the websites for the responder’s institutions and other Jordanian public institutions (Jordanian Food and Drug Administration ‘”JFDA,” Ministry of Health and the Jordanian Pharmacist Association); third, perception of government and private sector support for pharmaceutical innovation and knowledge about the available fund in Jordan; fourth, perception of the future of pharmaceutical innovation in the next 10 years in addition to the expected impact on both the health and economic sectors; fifth, perception of comparing innovation among other countries in the Middle East in terms of presence and acceptance of innovations within the pharmacy fields. Tailored tracks were designed to take into consideration different attitudes among students and pharmacists, in addition to identifying gaps between attitudes and knowledge.
Questionnaire development and face validity
The questionnaire was designed in the English language. The preliminary version was circulated to a pilot group for review and face validation (Bolarinwa, 2015). The pilot group consisted of 20 members: 6 pharmacy professional innovators (entrepreneurs) who manage innovative business related to pharmacy, 3 academic associate professors in pharmacy practice, 5 undergraduate pharmacy students, and 6 postgraduate pharmacy students.
Feedback from the pilot group to enhance face validity was used to modify the final version of the questionnaire. It was then translated into Arabic as all the respondents were native Arabic speakers. The questionnaire was translated by two independent bilingual translators who were native Arabic speakers and proficient in English. One of these translators (Translator 1: T1) was aware of the underpinning concepts and objectives of the questionnaire, while the other translator (Translator 2: T2) was not. This was useful to elicit unexpected meanings from the original version and helped to detect errors and divergent interpretations of ambiguous items in the original tool and to ensure the translation was bias-free (Degroot et al., 1994).
Construct validity and reliability measures
The assessment tool used in this study was a questionnaire designed by the research investigators based on previous studies with some modifications. To confirm the construct validity (Bolarinwa, 2015; Heale & Twycross, 2015; Taherdoost, 2016) of the questionnaire, the association between the scale items (correlation matrices) was evaluated. The correlation coefficient (r2) was 0.74, which permitted the construct validity.
Reliability was confirmed by Cronbach’s alpha and Pearson’s r analysis. The questionnaire was tested on a pilot sample of 40 responders. Responders were contacted first and 15 days later to complete the questionnaire again (pretest/posttest reliability was carried out) to justify moving forward with a large-scale pilot test.
The questionnaire was overall reliable regarding overall internal consistency and stability, which was estimated using the coefficient alpha (Cronbach’s alpha = 0.62) and test-retest reliability (Pearson’s r = 0.88). The coefficient alpha result reflects acceptable internal consistency. The results of the stability coefficient indicated stronger test-retest reliability, reflecting that the measurement error of the questionnaire is less likely to be attributable to changes in the individuals’ responses over time (Berchtold, 2016).
Ethical consideration
The protocol of this study was approved by the IRB of the Hashemite University, Jordan (reference number: 4/2019/2020, 22nd Jan 2020 – No.4). Eligible responders were those participants who wanted to voluntarily respond to the questionnaire. The questionnaire was distributed using social media platforms (Facebook) and WhatsApp application through the study panel members.
It was clarified that responses would be treated confidentially and anonymously. It was illustrated that no personal identifier where possible in the questionnaire. Moreover, it was stated that responders at any time during the questionnaire have the free choice to withdraw from answering, as their participation is voluntary. Accordingly, participants who submitted the response with their answers were considered to have given informed consent for their participation. At the end of the pilot group trial and modifications, the final form of the questionnaire was published as an online Google Form.
Sample size calculation
The target population of the current study was divided into two substrata based on specialty and education, i.e., working pharmacists versus students. As recommended by Taherdoost (2017) and carried out by the sample size calculator (Select Statistical Consultants United Kingdom, 2019), the representative sample size for the pharmacy discipline (actual pharmacists and final-year pharmacy students) was calculated to be 381 participants providing a 95% confidence interval and a 5% margin of error for a population of no more than 40,000 pharmacists according to the Jordanian Pharmacists Association.
Statistical analytical interpretation
A chi-squared test of association was conducted between answers to the questions within the survey and the specialty of responders, according to the following hypothesis: The null hypothesis is H0: there is no association relationship between answers and specialty (final-year pharmacy students vs. pharmacists). The alternative hypothesis is HA: an association relationship exists between answers and specialty. The measure of effect size and magnitude and strength of association of a nominal-by-nominal relationship was assessed by the phi (φ) coefficient when having two dichotomous variables or Cramer’s V coefficient when having more than two dichotomous variables. The following “crude estimate” of absolute value of the coefficient was considered for interpreting the strengths of relationships: negligible relationship (<0.19), weak relationship (0.2–0.29), and moderate relationship (0.30–0.39) (McHugh, 2013).
RESULTS
Descriptive results
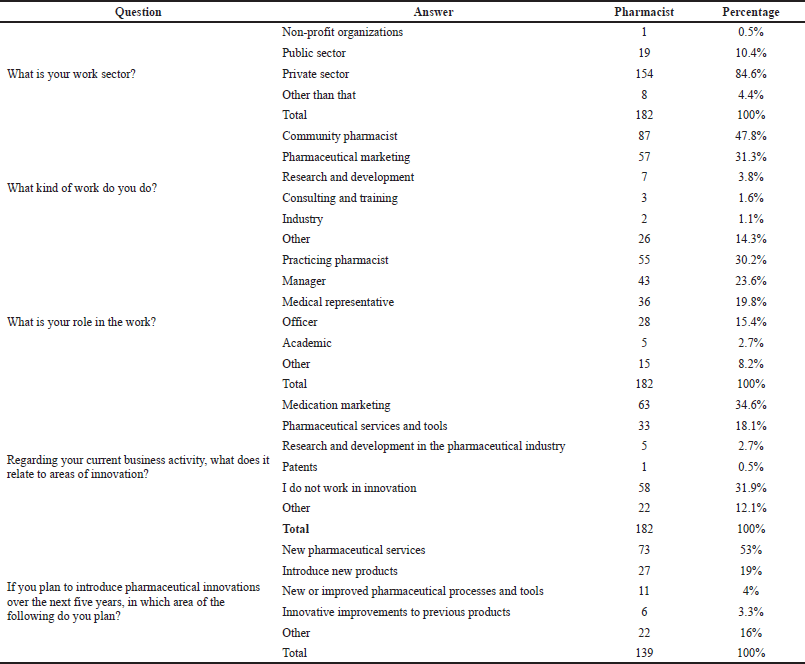
A total of 397 participants answered the questionnaire. Almost 54.2% were final-year bachelor of pharmacy students, and 45.8% were pharmacists. Unlike students, pharmacists had an approximate balance gender distribution, i.e., 44% female vs. 56% male. In comparison, 80% of the students who responded were female. Most of the participating pharmacists worked in the private sector (84.6%). The participants worked in a variety of positions as follows: officers in drug stores (15.4%), medical representatives (19.8%), management (23.6%), practicing community pharmacists (30.2%), and academics (2.7%). There were other categories also (8.2% included freelancers, pharmacists working in health media, manufactories, quality assurance, sports trainers, consultation services, and insurance companies).
Knowledge about terminology
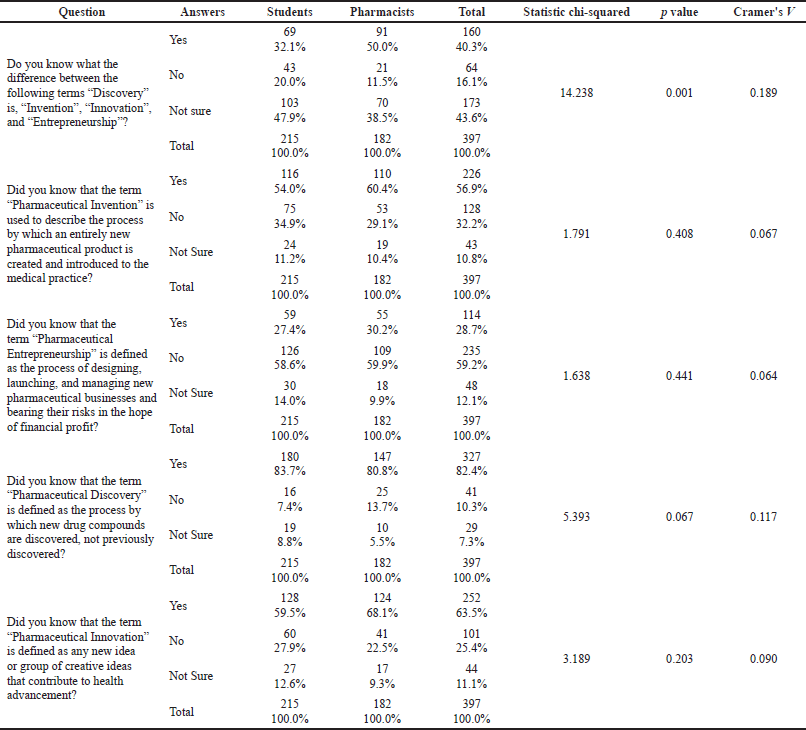
Only 32.1% of the students and 50% of the pharmacists claimed that they knew the differences between the terms (discovery, invention, innovation, and entrepreneurship). Such a gap in knowledge between pharmacists and students was statistically significant [X2(2) =14.238, p = 0.001; Cramer’s V = 0.189]. The most recognized term seemed to be “pharmaceutical discovery,” where it was known to 83.7% of the students and 80.8% of the pharmacists. The most problematic term was “pharmaceutical entrepreneurship,” where it was known to only 27.4% of the students and 30.2% of the pharmacists. More details are presented in Table 1.
Perceptions
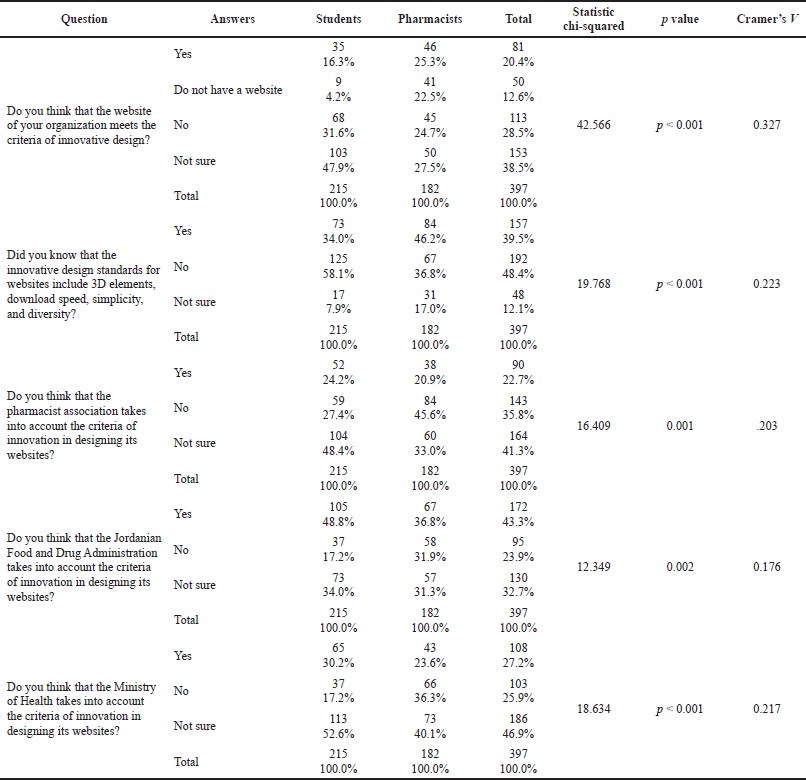
Pharmacists who had websites demonstrated a higher perceived significant level of trust in the innovative design of their institution’s website, with a moderately strong association, 25.3% who claimed to have such innovative design versus 16.3% for the students (p < 0.001, Cramer’s V = 0.327). However, almost one-fifth of the pharmacists’ institutions did not have a website, and almost half of the students were not sure about their answers. Surprisingly, approximately 60% of the students did not know the innovative design standards for websites compared with 37% of the pharmacists. The gap in such knowledge was statistically significant with a moderately strong association (p < 0.001; Cramer’s V = 0.327, p < 0.001).
Students expected that the JFDA has a better implementation of innovative website design (48.8%) compared with the Ministry of Health (30.2%) and the Pharmacists Association (24.2%). A similar pattern was also perceived by pharmacists; their evaluation was 36.8%, 23.6%, and 20.9%, respectively. It is noteworthy that the most common reported answer was “not sure” when responders were asked about the innovative design of the three national institutions, as illustrated in Table 2.
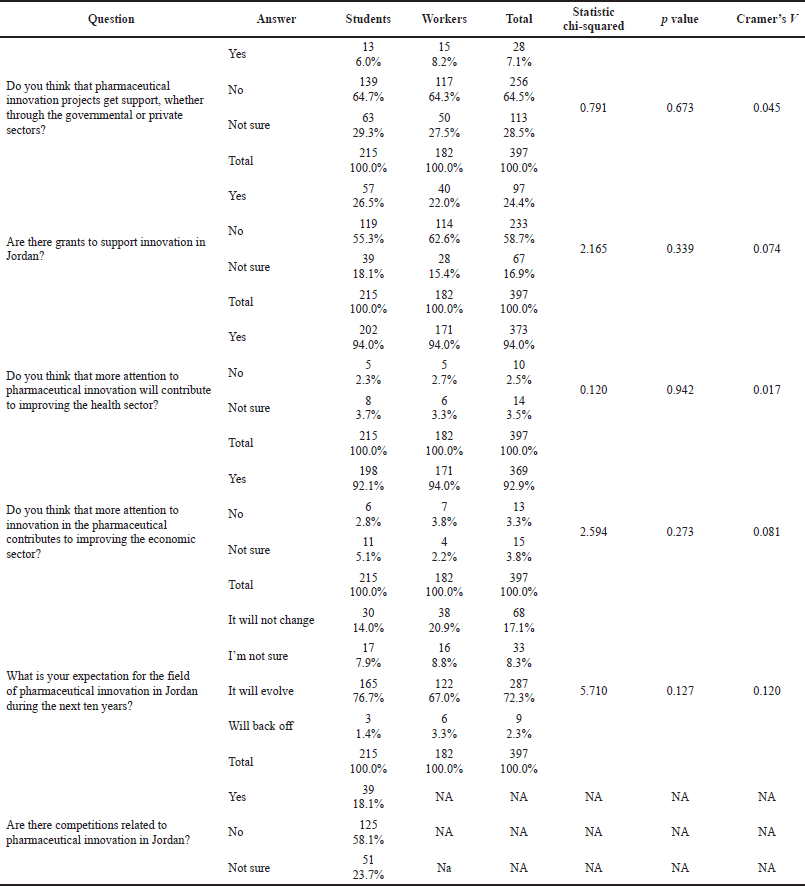
Interestingly, both students and pharmacists showed a similar distribution of their perception of governmental or private support to pharmaceutical innovation projects, where 64.7% of the students answered with “no support is provided to innovation projects” and 64.3% of the pharmacists had the same answer. Similarly, both groups were not familiar with available grants to support innovative projects; only 26.5% of the students and 22.0% of the pharmacists knew about relevant funding bodies in Jordan. Despite students’ interest in innovation, only 18.1% were aware of the annual national competition for pharmaceutical innovation, as further detailed in Table 3.
Both groups demonstrated positive and comparable perceptions toward evolving innovations over the next 10 years; 76.7% of the students and 67.0% of the pharmacists thought that it would evolve in that period. Almost all students (94%) and pharmacists (94%) thought that pharmaceutical innovation would contribute to improving health sector services with a similar attitude found for expected economic benefits. Details are illustrated in Table 3.
Participants were asked about their opinion about pharmaceutical innovation in Jordan compared to the Middle East region and the extent to which the Jordanian market accepts pharmaceutical innovations compared to the Middle East region. The assessment of Jordanian pharmaceutical innovation compared to the Middle East was almost similar across the 10-point ranking scale for both the pharmacists and students. The vast majority in both groups showed neutral assessment, ranging from 4 to 7. Similar findings were illustrated again in the acceptance of the Jordanian market for pharmaceutical innovations when compared to the markets of the Middle East region, as shown in Figures 1 and 2.
Attitudes: student-tailored questions
Students were asked about their interest in pharmaceutical innovation, whether they had studied pharmaceutical innovation during their university studies, and whether they knew of competitive funding bids related to innovation in Jordan. Most of the students were interested in the field of pharmaceutical innovation (81.9%). Despite this, 76.3% of the responders did not study pharmaceutical innovation during their bachelor’s program, where 55.8% of them denied any studies and 20.5% were not sure. Only 18.1% of the students confirmed their knowledge about the innovation competition.
Pharmacist-tailored questions
Approximately 32% of the pharmacists considered their work unrelated to the field of innovation, but almost one-quarter planned to implement innovative practices in the next 5 years.
Those who worked in innovation were in a variety of roles, including pharmaceutical services and tools (18.1%), medication marketing (34.6%), patents (0.5%), and research and development in the pharmaceutical industry (2.7%). New pharmaceutical services were the dominant area (34.6%) in which pharmacists hope to innovate over the next 5 years. Results are shown in Table 4. Even though most pharmacists (76.4%) were thinking of adopting innovation into their field of work, only 30% of them had a solid plan for such adoption (Table 5). The majority of the pharmacists believed financing pharmaceutical innovation was an impediment to progress in the area of innovation (89%). Two-thirds (66.5%) of the workers did not believe that there was a problem with the workforce and tools, but they believed there were difficulties in making changes to the system. Most pharmacists believed that laws and regulations were an impediment to progress in this area (75.8%). About 70% of the pharmacists believed that fears about investment in Jordan hinder progress in the field of pharmaceutical innovation. High taxes were perceived by 83% of the pharmacists as a factor preventing pharmaceutical innovation development. Surprisingly, almost 73.5% of the students and 78% of the pharmacists were not aware of the funding bodies in Jordan and that such bodies may support innovation and entrepreneurship.
DISCUSSION
The present research aimed to assess knowledge, attitude, and perceptions about pharmaceutical innovation in Jordan. The methodology was based on investigating and comparing results between working pharmacists and final-year bachelor of pharmacy students. The current study is the first in Jordan to assess such aspects.
Pharmaceutical innovations play a vital role in improving the health level and positively impacting the lives of individuals, as discussed in a study by Lichtenberg (2014b). In another study, Berger et al. (2016) indicated the significant role that pharmaceutical innovation plays in the economic growth of economically vulnerable African countries. Another study by Lichtenberg (2013) emphasized that increased life expectancy as a result of pharmaceutical innovation is an integral part of the economic growth of a country. The results of the current study are consistent with global attitudes that participants have a positive perception of pharmaceutical innovation and its potential for economic benefits.
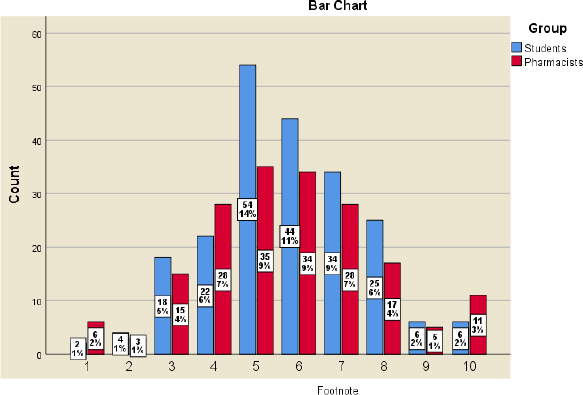 | Figure 1. Perceived assessment of the development of Jordanian pharmaceutical innovation, where scale 1 means very poorly developed and 10 means excellent development. X2(9) = 8.651, p = 0.470, Cramer’s V = 0.148.
[Click here to view] |
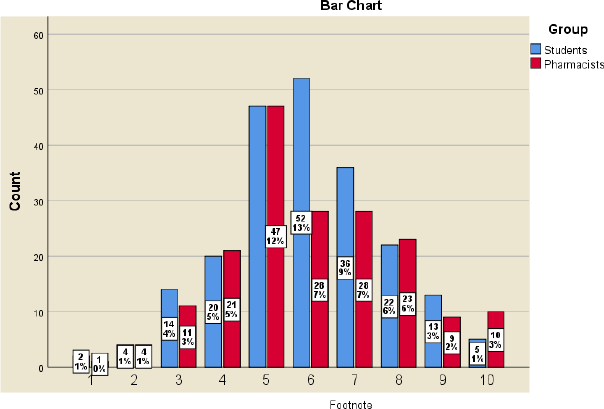 | Figure 2. Perceived acceptance of the Jordanian market for pharmaceutical innovations, where 1 means very poor and 10 means excellent. X2(9) = 9.462, p = 0.396, Cramer’s V = 0.154.
[Click here to view] |
A gap in knowledge around innovative terminology was identified as most respondents did not know the differences among the terms “innovation,” “invention,” “discovery,” and “entrepreneurship.” Moreover, most workers who participated in the study wanted to introduce innovation in their field of work, but 70% did not have a clear plan for that purpose. This may be attributed to insufficient content about innovation during university studies and the lack of a program of continuous learning after graduation.
The present study confirmed the importance and challenges with regard to the financial investment needed for pharmaceutical innovations; such perception is consistent with the literature (Berger et al., 2016; Sampat and Lichtenberg, 2011). The high cost of pharmaceutical innovation is one of the most critical problems (Dubois et al., 2015). The results of the current study are consistent with this conclusion and confirm that the high financial cost prevents pharmaceutical innovation in Jordan. Surprisingly, only 26.5% of the students and 22.0% of the pharmacists knew about funding bodies in Jordan to support innovative entrepreneurship. This could be addressed through strategic advertisement and awareness campaigns.
Lack of experience, lack of qualified workforce, legislation, and laws were perceived to hinder progress in innovation (Atkinson & Wial, 2008). The results of this study confirm that laws and legislation can hinder progress in the field of pharmaceutical innovation. Moreover, only 23.7% of the students in the present study claimed to receive education about innovation, and almost 70% of the pharmacists wanted to adopt innovation but had no plan for such adoption.
In addition to the crucial need for training at the undergraduate and postgraduate levels, it seems that amending legislation and laws to facilitate the innovation process, providing governmental and societal support for pharmaceutical innovations, and using experts to avoid the risk of failure—as much as possible—are among the leading and fundamental solutions to problems of hindering progress in the field of pharmaceutical innovation (Rovira, 2009).
A few limitations may have an impact on the study outcomes. First, using a self-reported questionnaire in data collection means that answers by participants could not be accurately estimated due to personal bias, which may limit the generalizability of the findings. Second, due to digital means of distributing the survey, many questions had been modified or canceled to keep the questionnaire acceptable and avoid being too long. Also, we could not estimate the response rate as the study questionnaire was distributed via open social media platforms. Third, the sample was only final-year pharmacy students and pharmacists who were able to respond by digital means. Accordingly, pharmacists who had limited access to the Internet might generate different results. Fourth, the survey did not have a question to specify the qualification of the pharmacists; different results may be related to a specific qualification as postgraduate levels or Pharm.D.
REFERENCES
Akkari ACS, Munhoz IP, Tomioka J, Santos N, Santos RD. Pharmaceutical innovation: differences between Europe, USA and ‘pharmerging’countries. Gestão & Produção, 2016; 23:365–80. CrossRef
Alsharif NZ. The need for disruptive innovation in pharmacy. Am J Pharm Educ, 2019; 83(10), 837719; doi:10.5688/ajpe837719 CrossRef
Al-Wazaify M, Albsoul-Younes A. Pharmacy in Jordan. Am J Health Syst Pharm, 2005; 62:2548–51. CrossRef
Al Bawab AQ, AlQahtani F, McElnay J. Health care apps reported in newspapers: content analysis. JMIR mHealth uHealth, 2018; 6:e10237. CrossRef
Alawi R, Alabbadi I. Investigating the effect of data exclusivity on the pharmaceutical sector in Jordan. Jordan J Pharm Sci, 2015; 8:70–81. CrossRef
Arruda A. An ethical obligation to use artificial intelligence: an examination of the use of artificial intelligence in law and the model rules of professional responsibility. Am J Trial Advoc, 2016 40:443.
Atkinson R, Wial H. Boosting productivity, innovation, and growth through a National Innovation Foundation. The Information Technology and Innovation Foundation, Washington, DC, 2008. CrossRef
The Arab Innovation Network, Be the solution competition. The Arab Innovation Network (AIN), England and Wales, UK, 2020.
Baregheh A, Rowley J, Sambrook S. Towards a multidisciplinary definition of innovation. Manag Decis, 2009; 47(8). CrossRef
Berchtold A. Test–retest: agreement or reliability? Methodol Innov, 2016; 9:205979911667287. CrossRef
Berger M, Murugi J, Buch E, IJsselmuiden C, Moran M, Guzman J, Devlin M, Kubata B. Strengthening pharmaceutical innovation in Africa. Council on Health Research for Development (COHRED), New Partnership for Africa’s Development (NEPAD) 2009. 2010 Council on Health Research for Development (COHRED) New Partnership for Africa’s Development (NEPAD), Geneva, Switzerland, 2016.
Bolarinwa O. Principles and methods of validity and reliability testing of questionnaires used in social and health science researches. Niger Postgrad Med J, 2015; 22:195. CrossRef
Hakeem Academy. Hakeem academy annual competition (HAAC), Amman, Jordan, 2020.
Dandan MM. Government expenditures and economic growth in Jordan, vol. 4. In International Conference on Economics and Finance Research, Singapore. City. pp 467–71, 2011.
Degroot AM, Dannenburg L, Vanhell JG. Forward and backward word translation by bilinguals. J Memory Lang, 1994; 33:600–29. CrossRef
Dubois P, De Mouzon O, Scott-Morton F, Seabright P. Market size and pharmaceutical innovation. RAND J Econ, 2015; 46:844–71. CrossRef
The Crown Prince Foundation. The Crown Prince Award for Best Government Service Application, Crown Prince Foundation, Amman, Jordan, 2018.
Ghannajeh AM, AlShurideh M, Zu’bi M, Abuhamad A, Rumman GA, Suifan T, Akhorshaideh AHO. A qualitative analysis of product innovation in Jordan’s pharmaceutical sector. Eur Sci J, 2015; 11:474–503.
Gubbins PO, Micek ST, Badowski M, Cheng J, Gallagher J, Johnson SG, Karnes JH, Lyons K, Moore KG. Innovation in clinical pharmacy practice and opportunities for academic–practice partnership. Pharmacotherapy, 2014; 34:e45–54. CrossRef
Heale R, Twycross A. Validity and reliability in quantitative studies. Evid Based Nurs, 2015; 18:66–7. CrossRef
Kreishan FM. Tourism and economic growth: the case of Jordan. Eur J Soc Sci, 2010; 15:63–8.
Lichtenberg FR. Pharmaceutical innovation, mortality reduction, and economic growth. Measuring the gains from medical research: an economic approach. The University of Chicago Press, Chicago, IL, 1980, p 74, 2010. CrossRef
Lichtenberg FR. The effect of pharmaceutical innovation on longevity: patient level evidence from the 1996–2002 medical expenditure panel survey and linked mortality public-use files. Forum for Health Economics and Policy, De Gruyter, Berlin, Germany, vol. 16, pp 1–33, 2013. CrossRef
Lichtenberg FR. The impact of pharmaceutical innovation on disability days and the use of medical services in the United States, 1997–2010. J Hum Capital, 2014a; 8:432–80. CrossRef
Lichtenberg FR. Pharmaceutical innovation and longevity growth in 30 developing and high-income countries, 2000–2009. Health Policy Technol, 2014b; 3:36–58. CrossRef
Lichtenberg FR, Tatar M, Çal??kan Z. The impact of pharmaceutical innovation on health outcomes and utilization in Turkey: a re-examination. Health Policy Technol, 2017; 6:226–33. CrossRef
McHugh ML. The chi-square test of independence. Biochem Med, 2013; 23:143–9. CrossRef
Morgan S, Lopert R, Greyson D. Toward a definition of pharmaceutical innovation. Open Med, 2008; 2:e4.
Obeidat BY, Al-Suradi MM, Tarhini A. The impact of knowledge management on innovation. Manag Res Rev, 2016; 39(10):1214–38. CrossRef
Rovira J. Intellectual property rights and pharmaceutical development. The Economics of New Health Technologies: Incentives, organization, and financing, Ney York, NY, p 219, 2009. CrossRef
Sampat BN, Lichtenberg FR. What are the respective roles of the public and private sectors in pharmaceutical innovation? Health Affairs, 2011; 30:332–9. CrossRef
Sams-Dodd F. Optimizing the discovery organization for innovation. Drug Discov Today, 2005; 10:1049–56. CrossRef
Shneiderman B, Leavitt M. Research-based web design & usability guidelines. Department of Health and Human Services, Washington DC, 2006.
Soriano DR, Huarng K-H. Innovation and entrepreneurship in knowledge industries. J Bus Res, 2013; 66:1964–9. CrossRef
Strobel N, Kratzer J. Obstacles to innovation for SMEs: evidence from Germany. Int J Innov Manag, 2017; 21:1750030. CrossRef
Taherdoost H. Validity and reliability of the research instrument; how to test the validation of a questionnaire/survey in a research. How to test the validation of a questionnaire/survey in a research, Jaipur, India, 2016. CrossRef
Taherdoost H. Determining sample size; how to calculate survey sample size. Int J Econ Manag Syst, 2017; 2.
Toner M, Tompkins RG. Invention, innovation, entrepreneurship in academic medical centers. Surgery, 2008; 143:168–71. CrossRef