INTRODUCTION
Intracoronary physiological assessments [fractional flow reserve (FFR) and resting pressure-derived physiologic indices] and intravascular imaging [intravascular ultrasound (IVUS) and optical coherence tomography (OCT)] have been developed as adjuncts to coronary angiography (CA) to provide better diagnostic information and improvise diagnostic and treatment choices (De Bruyne et al., 1995; Mintz et al., 1996; Pijls et al., 1995; Prati et al., 2012). These technologies have been available in Western healthcare systems for many years although uptake remains disappointingly low, despite increasingly convincing data.
Intracoronary imaging technologies to guide percutaneous coronary intervention (PCI) procedures have emerged as one of the key factors influencing precision during the procedure and optimization of outcomes. Imaging can become integral to every stage of PCI procedure, and can facilitate assessment of lesion severity, preprocedural planning (selection of appropriate stenting strategy, stent size, and landing zones), PCI optimization (PCIO) (detection of stent expansion, malapposition, and lumen gain), and management of immediate complications (presence of dissection, thrombus, tissue prolapse, and side-branch compromise). Furthermore, imaging can also help in the identification of and management of stent failure mechanisms (restenosis and thrombosis) during follow-up after the index intervention (Neumann et al., 2019; Waksman et al., 2013).
The adoption of PCIO techniques is highly variable, and generally low in routine clinical practice in India. Per the recent National Intervention Council Registry data, less than 5% of the total PCI done in India was under imaging guidance. Furthermore, while there are several American (Levine et al., 2011) and European (Johnson et al., 2019; Neumann et al., 2019; Räber et al., 2018) guidelines, and consensus statements from the Netherlands (IJsselmuiden et al., 2018), China (Johnson et al., 2019; Räber et al., 2018), Hong Kong, Australia, and New Zealand (Johnson et al., 2019) that provide guidance on appropriate use of PCIO techniques, guidelines, or consensus statements applicable in an Indian setting is lacking.
Considering the upward trends in the prevalence of coronary artery disease (CAD) in both urban and rural settings in India (Sharma et al., 2014) and the tendency of CAD to occur at a younger age in India (with over 50% of cardiovascular mortality occurring in individuals aged <50 years) (Dalal et al., 2016; Nag and Ghosh 2013), there is an urgent unmet need to optimize PCI procedures with adoption of novel imaging and physiological assessment techniques to ensure better patient experiences and clinical outcomes.
This position statement briefly provides an overview of the practical adoption of PCIO techniques in the Indian healthcare setting, by compiling the views of interventional cardiologists expressed during a survey and scientific meeting (conducted after the survey). This document also reflects important practical messages to the Indian interventional cardiology fraternity on when and how to use PCIO in Indian settings. Furthermore, the authors also aim to promote physician education on adoption of intravascular imaging at different stages of PCI procedures in their clinical practice settings.
METHODOLOGY
Survey
An online survey was conducted between November and December 2019 to evaluate the usage and views about PCIO techniques among interventional cardiologists from Tier I cities in India with experience of (a) ≥5 years in performing PCI procedures (b) ≥200 angioplasty procedures and (c) ≥1,000 diagnostic cardiovascular procedures. The survey questionnaire (Appendix 1) was designed based on the European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) clinical practice survey (Koskinas et al., 2018)
The scope of the survey was to determine:
- The availability and use of intravascular imaging (FFR, resting indices, OCT and/or IVUS).
- Clinicians’ views on clinical evidence pertaining to the use of intravascular imaging.
- Clinical situations (indications) where intravascular imaging is currently used in Indian settings.
- Factors that would support the use of intravascular imaging and impact specific techniques.
- Factors preventing the utilization of the combined use of both physiology and imaging assessments in the same case.
- Health-economic considerations affecting the optimization of PCI in Indian healthcare settings.
Scientific meeting
The survey was followed by a scientific meeting in February 2020 in New Delhi, involving the interventional cardiologists who were part of the survey. The results of the survey were presented during the meeting, which was followed by an interactive discussion. The goal of the meeting was to share and discuss the clinical usage patterns of intravascular imaging and techniques used in PCI guidance and develop a position statement on the appropriate use of intravascular imaging for PCIO in Indian healthcare settings.
During the meeting, clinical evidence, tips and techniques and clinical indications for the use of intravascular imaging were reviewed to propose key recommendations. Importantly, the group discussed the below key issues pertaining to intravascular imaging:
- Lesion and patient subsets suitable for intravascular imaging.
- Current global evidence, evidence gaps, and areas for further research.
- Trends in differential utilization of intravascular imaging.
- Learning curve and barriers to routine adoption of intravascular imaging.
- Impact of appropriate usage criteria on PCI in Indian healthcare settings.
RESULTS
Survey outcomes
The participants of the survey included 25 high-volume interventional cardiologists (60% from corporate hospitals) with 92% (n = 23/25) having more than 10 years clinical experience in performing intravascular imaging guided PCI. The median number of PCI procedures performed annually (self-reported) was 450 (range: 200–2,000). Additionally, 52% (n = 13/25) of the survey participants reported performing 1,500 diagnostic procedures or more per year. Intravascular imaging with IVUS only, OCT only and both IVUS and OCT, was available or used by 12% (n = 3), 20% (n = 5), and 65% (n = 15) of the respondents, respectively. The average number of OCT- and IVUS-guided PCI performed per month was 6.7 ± 4.8 and 6.3 ± 5.5, respectively.
The majority of respondents (76%, n = 18) opined that intravascular imaging with IVUS or OCT was associated with improved clinical outcomes compared with CA. Clinicians’ preferences for the use of OCT or IVUS during PCI are listed in Table 1. Moderate preference was noted for both OCT and IVUS for evaluating calcified lesions, bifurcations, and stent thrombosis while IVUS was more preferred for rotational atherectomy.
The availability of comparative clinical studies, ease of use, being trained in the imaging technique and less procedural time were found to be the main factors influencing the use of OCT and IVUS; contrast-free option was the reason for IVUS preference. Better cost and facilitation of reimbursement were the other factors driving utilization of both OCT and IVUS. The principal barriers to adoption of intravascular imaging techniques were safety concerns, lack of time for catheterization procedures, and cost for both OCT and IVUS; lack of adequate experience was cited as an additional barrier for IVUS uptake.
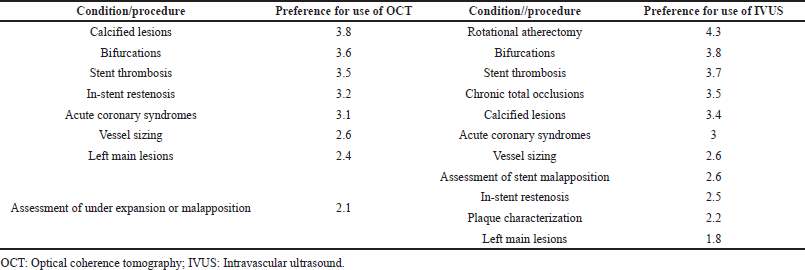 | Table 1. Clinicians’ preferences for use of intravascular imaging techniques as per the survey. Data indicates mean score from survey-recorded preferences, recorded on a scale of 1 to 5, where 1 was least preferred versus 5 most preferred. [Click here to view] |
All the respondents opined that the use of intravascular imaging would increase in the future. They also agreed that evaluation of coronary physiology along with imaging could be carried out if hospital policies are updated, related hardware is available and there is adequate time to conduct both. The participants noted that further research is required for optimization of PCI with intravascular imaging and compare clinical outcomes of CA versus OCT, CA versus IVUS, and IVUS versus OCT.
DISCUSSION
Use of intravascular imaging
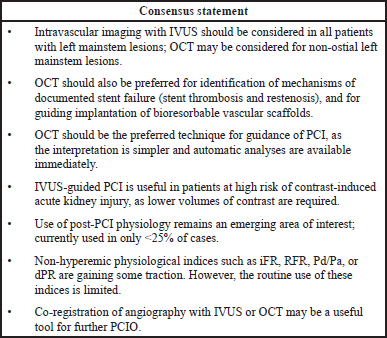
Several guidelines and consensus statements have recommended the use of intravascular imaging (IVUS and OCT) in varied clinical settings for PCIO (IJsselmuiden et al., 2018; Johnson et al., 2019; Levine et al., 2011; Neumann et al., 2019; Räber et al., 2018). The 2011 American College of Cardiology Foundation/American Heart Association Task Force/Society for Cardiovascular Angiography and Interventions guidelines recommend IVUS as a reasonable option for the assessment of angiographically indeterminant left mainstem stenoses (Recommendation Class II a, Level of evidence B) and to determine the mechanism of in-stent restenosis (Class II a, Level C). The guidelines also recommend that use of IVUS may be reasonable for the assessment of non-left main coronary arteries with angiographically intermediate coronary stenoses (50% to 70% diameter stenosis) (Class II b, Level B), for guidance of coronary stent implantation, particularly in cases of left main coronary artery stenting (Class II b, Level B), and to determine the underlying mechanism of stent thrombosis (Class II b, Level C) (Levine et al., 2011). The 2018 European Society of Cardiology and the European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularizations recommend IVUS for assessing the severity of unprotected left main lesions and for selected patients to optimize stent implantation (Recommendation Class II a, Level of evidence B). Furthermore, guidelines recommend that IVUS and/or OCT should be considered to detect stent-related mechanical problems leading to restenosis (Class II a, C) (Neumann et al., 2019).
According to majority of participating cardiologists in the current study, intravascular imaging with IVUS or OCT improved clinical outcomes.
 | [Click here to view] |
IVUS versus OCT
Both IVUS and OCT provide valuable information to help optimize stent implantation, minimize stent-related complications, and assess stent failure mechanisms such as thrombosis and restenosis. However, OCT provides ten-fold higher resolution compared with IVUS imaging. It can also detect unique details such as edge dissections, malapposition, and tissue coverage of stent struts with clarity, all of which may be missed by IVUS guidance (Prati et al., 2012; Räber et al., 2018; Waksman et al., 2013). Furthermore, OCT enables better tissue characterization and thrombus detection than IVUS, with the additional bonus that interpretation of images is perceptually easier with OCT than IVUS (Räber et al., 2018). However, the possible need for pre-dilatation to facilitate visualization and the additional contrast load to purge the vessel lumen for better visualization, adds time and may result in contrast-associated complications, limiting the use of OCT in patients with poor kidney function (Räber et al., 2018; Waksman et al., 2013).
There is a rich and growing body of evidence supporting the use of intravascular imaging in the current era of drug-eluting stents (DES). A recent large meta-analysis of randomized controlled trials (RCTs) and observational studies in over 27,000 patients have revealed that IVUS-guided PCI was associated with a significant relative risk (RR) reduction (33%) for cardiovascular death and lower risk of post-procedural myocardial infarction (RR: 0.71), target lesion revascularization (TLR) (RR: 0.81), and stent thrombosis (RR: 0.57) compared with CA alone (Darmoch et al., 2020). Several smaller meta-analyses of randomized clinical trials in over 6,000 patients have revealed a significantly lower incidence of myocardial infarction, major adverse cardiac events (MACE), stent thrombosis, TLR, cardiovascular death, and all-cause death with IVUS-guided PCI with DES implantation versus CA-guided DES implantation (Elgendy et al., 2019; Kumar et al., 2019; Tan et al., 2019). In a systematic review and Bayesian network meta-analysis of 17,882 patients, IVUS guidance was associated with significant reduction in all-cause death, TLR, and stent thrombosis compared to angiography guidance. In addition, both IVUS and OCT were similar in terms of clinical efficacy; IVUS- or OCT-guided PCI significantly reduced MACE and cardiovascular death (Buccheri et al., 2017). In a recent updated meta-analysis of RCTs in over 5,000 patients with stable ischemic heart disease or acute coronary syndrome, routine use of IVUS-guided DES implantation was found effective in reducing cardiovascular mortality, TLR, target vessel revascularization, and MACE (Malik et al., 2020). Similar findings have been noted in patients with left main CAD undergoing DES implantation; IVUS guidance was associated with a significant reduction in myocardial infarction, MACE, cardiac death, all-cause mortality, and stent thrombosis versus angiography guidance (Wang et al., 2018). Although evidence comparing OCT versus angiography guidance during PCI are limited, a meta-analysis of RCTs and observational comparative studies by Kuku et al. (2018) in over 2,500 patients revealed a significantly lower rate of MACE and cardiac death with OCT versus angiography guidance (Kuku et al., 2018). A retrospective analysis of 87,166 patients showed that OCT-guided PCI was associated with improved procedural outcomes, in-hospital events, and long-term survival compared to standard angiography-guided PCI (Smilowitz et al., 2018). However, direct randomized controlled clinical trial data are currently limited. In a recent meta-analysis of 10 RCTs of 7,822 patients IVUS-guided PCI was significantly superior to angiography, FFR, instantaneous wave-free ratio (iFR), OCT in terms of all-cause death and significantly superior to angiography in terms of MACE (Pang et al., 2020). Direct randomized controlled studies comparing the outcomes of IVUS versus OCT guidance during PCI are limited. In the ILUMIEN III study (n = 450), the primary endpoint of minimum stent area achieved non-inferiority after OCT- versus IVUS-guided PCI. While mean stent expansion was comparable between the two groups, untreated major dissections and major malappositions were significantly less frequent in the OCT group compared with IVUS group (14% vs. 26%, p = 0.009; and 11% vs. 21%, p = 0.02, respectively) (Ali et al., 2016). The 12-month outcomes from ILUMIEN II have also recently been published and did not show any differences in outcome between OCT, IVUS, and angiographic guidance alone, but the study was neither designed nor powered to demonstrate such differences (Ali et al., 2021); however, the pivotal ILUMIEN IV study (NCT03507777, 2018) has recently finished enrollment and is powered for clinical outcomes in a population randomized to OCT versus angiographic guidance for complex PCI (Ali et al., 2021). This large-scale, multicenter, randomized trial is designed to demonstrate the superiority of OCT- versus angiography-guided stent implantation in patients with high-risk clinical characteristics (diabetes) and/or complex angiographic lesions in achieving larger post-PCI lumen dimensions and improving clinical outcomes. Furthermore, in the OPINION RCT (n = 829), OCT was non-inferior to IVUS for TLR at 12 months post-PCI (p for non-inferiority <0.05). The rate of angiographic binary restenosis was also comparable between the two groups at 8 months post-PCI (Kubo et al., 2017). Current guidelines, therefore, suggest IVUS and OCT as equivalent intravascular imaging modalities, with superior outcomes versus angiography in guiding and optimizing PCI (Levine et al., 2011). Two ongoing large registries, iOPTICO and COMPLEX Lesion Registry are evaluating the use of OCT in determining the treatment strategy and the impact on minimum stent area in complex lesions, respectively (CTRI, 2018; Mathew et al., 2020). Interim results (n = 487) of the iOPTICO study revealed change in PCI strategy among 76% of the lesions following pre-procedural OCT. The treatment strategy was further altered in 23% lesions with the use of ACR (Mathew et al., 2020). The results of COMPLEX Lesion Registry are awaited. The outcomes of these studies are expected to highlight the clinical benefit of using an OCT guided PCI approach in an Indian setting.
Bioresorbable stents (BRS) which are often used for temporary scaffolding of coronary vessels require careful sizing to avoid cracking of the stent during deployment. CA has limited sensitivity owing to limited spatial resolution inability to visualize non-radioopaque structures. IVUS or OCT can help in better visualization of the BRS. Additionally, OCT enables accurate evaluation of the stent and vessel wall interaction (van Ditzhuijzen et al., 2013).
 | [Click here to view] |
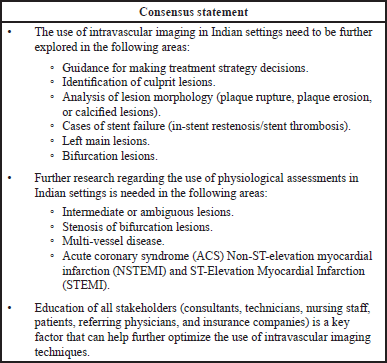
Although the interest in post-PCI physiology to determine optimal results and reduce post-PCI ischemia is increasing, the uptake of physiology seems limited compared with the visual cues obtained from imaging (De Bruyne et al., 1995; Jeremias et al., 2019). This has been attributed to lack of supporting data and specific guideline recommendations (Jeremias et al., 2019). In the current study, the participants opined that the combined approach of physiology and imaging could be practiced if hospital policies allow such practice, along with the availability of necessary hardware and time. Only a few studies conducted in India have assessed the benefits of OCT guided PCI. In an initial experience from India, use of OCT was associated with change in management plan in 65% of the cases, while stent length and diameter were altered based on OCT in 56% and 36% cases, respectively (Rath et al., 2014). Although international guidelines endorse the superiority of intravascular imaging with IVUS or OCT versus angiography guidance during PCI and DES implantation, and these technologies have been made available for many years, their use has been restricted mainly to the research setting in India. The use of imaging in daily practice in India is limited due to various factors including limited access, cost issues, steep learning curve, lack of technician training, and difficulty in image interpretation, as revealed from the responses of the pre-meeting survey. The utilization of intravascular imaging is also limited owing to reimbursement issues. Similar unmet needs have also been reported in surveys published by the EAPCI and Japanese Association of CVIT web-based clinical practice survey that included opinions from 1,010 interventional cardiologists (754 with >10 years of clinical experience) (Koskinas et al., 2018). While 34.7% of the survey respondents were from Europe, 52.0% were from Asia, (including 45.4% from Japan). Remaining respondents were from Americas and Australia. Of those surveyed, 96% reported having experience in performing intravascular imaging (95.5% in IVUS, 69.8% in OCT); optimization of stenting was rated as the most common indication for use of intravascular imaging (88.5%), followed by procedural or strategy guidance (79.6%) and guidance for left main interventions (77%). The most common factors limiting the use of intravascular imaging were high cost (65.9%) and additional duration of the procedure (35%). The use of intravascular imaging for guiding more than 15% of cases (frequent use) was significantly more common in Japan (96.6%) when compared with Europe (10.4%) (Koskinas et al., 2018). Amongst other Asian countries, imaging guided PCI is widely adopted in Hong Kong and to a lesser extent in Korea. Intervention cardiologists in other countries do not use intravascular imaging in daily clinical practice but rather in unique clinical situations or mainly as a research tool. Lack of reimbursement in some countries is one of the key factors in limiting the adoption. Similar outcomes were noted in the survey conducted.
Reimbursement programmes are hence important to decrease the economic and societal burden of CAD among Indians. The healthcare reimbursement systems in India include commercial, social, union-sponsored, state- and central-level insurance programs. There are about 30 private insurers and 17 government health insurance schemes active in India providing a coverage for about 65% of the Indian population. Among these, the center-state sponsored Ayushman-Bharat Pradhan Mantri Jan Arogya Yojana (AB-PMJAY) is the largest program, which covers 45% of the total Indian population.
 | [Click here to view] |
Cardiac interventions top the list of high-end medical procedures covered under the AB-PMJAY. According to the available data, angioplasty was the most commonly covered/reimbursed high-end procedure since the inception of AB-PMJAY. While simple procedures are covered under this scheme, complex lesions requiring further assessment with OCT or IVUS are not reimbursed. Such disparities in the reimbursement status warrant the need for appropriate utilization criteria, especially in the public insurance sector.
This is especially important as improved diagnostic procedures can help reduce the number of cardiac surgeries. This was evident in a study by Karthikeyan et al. (2017) which explored the feasibility of settling reimbursements for appropriate criteria-based PCI or CABG surgeries conducted at tertiary centers in Maharashtra as part of Rajiv Gandhi Jeevandayee Arogya Yojana. The number of PCI procedures performed reduced by 12.3% within a year after the introduction of this scheme; reduction was similar for both public and private centers. Overall, 783 [95% confidence interval (CI) 483–1,099] PCI procedures were avoided resulting in a potential annual saving of about INR 57 million (US$0.93 million; 95% CI 0.57–1.3) in the government schemes (Karthikeyan et al., 2017). Therefore, inclusion of advanced diagnostic procedures under government schemes including AB-PMJAY could reduce the economic burden in developing countries like India.
The NIC data suggest the number of imaging guided PCI is on rise in the last few years, from a low of < 1% to ~5%. Though a healthy and encouraging trend, imaging guided PCI is still in its early stage in India. There are several factors that will support the adoption of imaging in daily clinical practice. Some of these include:
- Improved access of the technology through training of intervention cardiologists and other catheterization laboratory staff.
- Adoption/endorsement by leading cardiology societies and inclusion in local guidelines.
- Availability of local and disease-specific data along with reimbursement and health economics data specific to India.
- Education of all stakeholders.
Impact of COVID-19 on imaging-guided PCI
The ongoing pandemic has changed the way intervention cardiologists are approaching the entire cardiovascular disease treatment algorithm globally, and India is not any different. As evident during the early stages of pandemic in India (March–May 2020), the hospitals could only perform emergency procedures. The patients with chronic stable angina were being managed medically and consultation was done virtually as well. For those requiring an urgent intervention, there were additional challenges and considerations such as guideline recommended time goals for the treatment. These were as such difficult to achieve even prior and pandemic further challenged the infrastructure. A few retrospective studies from Western India and South India (Choudhary et al., 2020) reported an overall reduction of admissions even for ACS. Even amongst patients who ended up in the hospital following a chest pain, there was an overall delay in seeking care and risk factors for poor outcomes on arrival were common. In many centers, a pharmaco-invasive strategy was preferred over PCI contrary to the guidelines. The preferred approach was to “get in and get out” thus significantly impacting the use of adjunct technologies like imaging or physiology.
Based on an anecdotal secondary survey done at few high-volume centers, the use of intravascular imaging significantly reduced in the first phase of the lockdown and was limited to a few cases like complex bifurcation or a left main PCI. The trend continued even during the first few months of the un-lockdown from May to September 2020 period though more patients were being treated now for stable disease compared to the lockdown phase. According to anecdotal feedback, imaging returned back to the Cath labs for those who already believed in the benefits.
Some of the factors which influenced adoption of imaging post lockdown were:
- Virtual training: A lot of physicians engaged in the virtual learning and collaboration during the lockdown period and benefitted from the experience of global experts while sitting in their own clinics/offices. Many intervention cardiologists reaffirmed their believe in the imaging through these interactions and continued Imaging guided PCI in the un-lockdown phase.
- Technical support from MedTech Companies: Due to the restrictions, an in-person support was limited, thus impacting the training of the Cath lab technicians, hands-on, etc. So, while new centers were limited by the lack of in-person training, the experienced centers had no such difficulty.
- Capital infrastructure limitations: During the pandemic, majority of funds were directed towards building additional infrastructure to cater to COVID-19 patients, thus limiting the capital availability for upgrading Cath labs with imaging.
The pandemic did not have a major impact in physicians’ preference either on the choice of imaging technology or patient/lesion selection. The anecdotal data from few hospitals also suggest a return to normalcy in imaging guided PCI in October–December 2020 time frame which has sustained till the beginning of second wave in April 2021. Situation is just beginning to ease in the last few months again.
Limitations
The survey period is till December 2019. The use of PCIO significantly declined during the pandemic. Procedures were done more often in ACS patients and shortening of the procedure time and minimizing the presence of technicians in the catheterization labs was recommended.
CONCLUSION
A detailed overview of the role of intravascular imaging in optimizing PCI and improving clinical outcomes along with the recommendations of subject experts provided in this position statement may help encourage cardiologists adopt PCIO in their routine practice. The use of PCIO in an Indian healthcare setting may be further enhanced by generation of real-world evidence in local settings, as the CAD patient population in India is different when compared to the West, both with respect to characteristics and clinical presentation. Targeted education of interventional cardiologists and development of validated appropriate use criteria for intravascular imaging applicable to local settings may help in further optimization of PCI in India.
ACKNOWLEDGMENTS
We would like to thank BioQuest Solutions Pvt Ltd for editorial support.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ali ZA, Maehara A, Genereux P. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomized controlled trial. Lancet, 2016; 388(10060):2618–28. CrossRef
Ali ZA, Karimi Galougahi K, Maehara A, Shlofmitz RA, Fabbiocchi F, Guagliumi G, Alfonso F, Akasaka T, Matsumura M, Mintz GS, Ben-Yehuda O, Zhang Z, Rapoza RR, West NEJ, Stone GW. Outcomes of optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation: one-year results from the ILUMIEN IV: OPTIMIZE PCI trial. EuroIntervention, 2021; 16:1085–91. CrossRef
Buccheri S, Franchina G, Romano S. Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and Bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc Interv, 2017; 10:2488–98. CrossRef
Choudhary R, Gautam D, Mathur R, Choudhary D. Management of cardiovascular emergencies during the COVID-19 pandemic. Emerg Med J, 2020; 37:778–80.CTRI. A study to check the safety and effect of optical coherence tomography-guided percutaneous coronary intervention in patients with complex lesions in India. Available via http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=29676&EncHid=&userName=Praveen%20chandra (Accessed 07 April 2021). CrossRef
Dalal J, Hiremath MS, Das MK. Vascular disease in young Indians (20–40 years): role of ischemic heart disease. J Clin Diagn Res, 2016; 10:OE08–12.
Darmoch F, Alraies MC, Al-Khadra Y, Moussa Pacha H, Pinto DS, Osborn EA. Intravascular ultrasound imaging-guided versus coronary angiography-guided percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc, 2020; 9:e013678. CrossRef
De Bruyne B, Bartunek J, Sys SU, Heyndrickx GR. Relation between myocardial fractional flow reserve calculated from coronary pressure measurements and exercise-induced myocardial ischemia. Circulation, 1995; 92:39–46. CrossRef
Elgendy IY, Mahmoud AN, Elgendy AY. Intravascular ultrasound-guidance is associated with lower cardiovascular mortality and myocardial infarction for drug-eluting stent implantation - insights from an updated meta-analysis of randomized trials. Circ J, 2019; 83:1410–13. CrossRef
IJsselmuiden AJJ, Zwaan EM, Oemrawsingh RM. Appropriate use criteria for optical coherence tomography guidance in percutaneous coronary interventions: Recommendations of the working group of interventional cardiology of the Netherlands Society of Cardiology. Neth Heart J, 2018; 26:473–83. CrossRef
Jeremias A, Davies JE, Maehara A, Matsumura M, Schneider J, Tang K, Talwar S, Marques K, Shammas NW, Gruberg L, Seto A, Samady H, Sharp A, Ali ZA, Mintz G, Patel M, Stone GW. Blinded physiological assessment of residual ischemia after successful angiographic percutaneous coronary intervention: the DEFINE PCI study. JACC Cardiovasc Interv, 2019; 12:1991–01. CrossRef
Johnson TW, Räber L, di Mario C. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J, 2019; 40:2566–84. CrossRef
Karthikeyan G, Shirodkar U, Lochan MR, Birch S. Appropriateness-based reimbursement of elective invasive coronary procedures in low- and middle-income countries: preliminary assessment of feasibility in India. Natl Med J India, 2017; 30:11–4. CrossRef
Koskinas KC, Nakamura M, Räber L. Current use of intracoronary imaging in interventional practice - results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) clinical practice survey. EuroIntervention, 2018; 14:e475–84. CrossRef
Kubo T, Shinke T, Okamura T. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J, 2017; 38:3139–47. CrossRef
Kuku KO, Ekanem E, Azizi V. Optical coherence tomography-guided percutaneous coronary intervention compared with other imaging guidance: a meta-analysis. Int J Cardiovasc Imaging, 2018; 34:503–13. CrossRef
Kumar A, Shariff M, Adalja D. Intravascular ultrasound versus angiogram guided drug eluting stent implantation. A systematic review and updated meta-analysis with trial sequential analysis. Int J Cardiol Heart Vasc, 2019; 25:100419. CrossRef
Levine GN, Bates ER, Blankenship JC. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation, 2011; 124:e574–651.
Malik AH, Yandrapalli S, Aronow WS, Panza JA, Cooper HA. Intravascular ultrasound-guided stent implantation reduces cardiovascular mortality - updated meta-analysis of randomized controlled trials. Int J Cardiol, 2020; 299:100–5. CrossRef
Mathew R, Abdullakutty J, Patel T, Sivakumar R, Singh B, Chouhan N, Nesa Malik FT, Hiremath MS, Gunasekaran S, Mathew S, Kumar V, Subban V. TCT CONNECT-410 impact of real-time optical coherence tomography-angio co-registration (OCT-ACR) on physician decision making during percutaneous coronary intervention: a multicenter, prospective study (iOPTICO study). J Am Coll Cardiol, 2020; 76:B176. CrossRef
Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, DeFalco RA, Leon MB. Limitations of angiography in the assessment of plaque distribution in coronary artery disease: a systematic study of target lesion eccentricity in 1446 lesions. Circulation, 1996; 93:924–31. CrossRef
Nag T, Ghosh A. Cardiovascular disease risk factors in Asian Indian population: a systematic review. J Cardiovasc Dis Res, 2013; 4:222–8. CrossRef
NCT03507777, 2018. Available via https://clinicaltrials.gov/ct2/show/NCT03507777. (Accessed 01 May 2021).
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J, 2019; 40:87–165. CrossRef
Pang J, Ye L, Chen Q. How to guide PCI? A network meta-analysis. Medicine (Baltimore), 2020; 99:e20168. CrossRef
Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ, El Gamal MI. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation, 1995; 92:3183–93. CrossRef
Prati F, Guagliumi G, Mintz GS. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J, 2012; 33:2513–20. CrossRef
Räber L, Mintz GS, Koskinas KC. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J, 2018; 39:3281–300. CrossRef
Rath PC, Reddy K, Agarwal MK, Purohit BV, Deb T, Reddy AM. Optical coherence tomography guided PCI - initial experience at Apollo Health City, Jubilee Hills, Hyderabad. Indian Heart J, 2014; 66:31–7. CrossRef
Sharma R, Bhairappa S, Prasad S. Clinical characteristics, angiographic profile and in hospital mortality in acute coronary syndrome patients in south Indian population. Heart India, 2014; 2:65–9. CrossRef
Smilowitz NR, Mohananey D, Razzouk L. Impact and trends of intravascular imaging in diagnostic coronary angiography and percutaneous coronary intervention in inpatients in the United States. Catheter Cardiovasc Interv, 2018; 92:E410–5. CrossRef
Tan YY, Man XX, Liu LY. Comparison of clinical outcomes between intravascular ultrasound-guided and angiography-guided drug-eluting stent implantation: a meta-analysis of randomised control trials and systematic review. Int Wound J, 2019; 16:649–58. CrossRef
van Ditzhuijzen NS, Ligthart JM, Bruining N, Regar E, van Beusekom HM. Invasive Imaging of bioresorbable coronary scaffolds - a review. Interv Cardiol, 2013; 8:23–35. CrossRef
Waksman R, Kitabata H, Prati F, Albertucci M, Mintz GS. Intravascular ultrasound versus optical coherence tomography guidance. J Am Coll Cardiol, 2013; 62:S32–40. CrossRef
Wang Y, Mintz GS, Gu Z. Meta-analysis and systematic review of intravascular ultrasound versus angiography-guided drug eluting stent implantation in left main coronary disease in 4592 patients. BMC Cardiovasc Disord, 2018; 18:115. CrossRef
APPENDIX 1 - SURVEY QUESTIONNAIRE
1. Age
a. <40
b. 40–50
c. 50–60
2. Professional status (years of clinical experience)
a. <5 years
b. >10 years
3. Geographical representation
a. Central India
b. East
c. North
d. South
e. West
4. Type of institution
a. Corporate Hospital
b. Government Academy/University Hospital
c. Private (Non-Corporate) Multi Speciality
d. Private Single Owner.
5. Approx. how many diagnostic angiograms are you preferring in a year? ______
6. Approx. how many PCIs do you perform per year? _____________
7. What % of your PCI cases are left main artery assessment? _______________
8. What % of your PCI cases are Bifurcation Lesions? ____________________
9. Do you believe that IVUS or OCT guidance for coronary interventions improves clinical outcomes compared with angiography-only guidance?
a. Yes, I am convinced that intracoronary imaging has the potential to improve clinical outcomes even if evidence is not definitive
b Yes, there is evidence that intracoronary imaging has the potential to improve clinical outcomes.
10. Which of the following systems do you have available to use?
a. IVUS
b. OCT
c. Both
11. Which of the following systems do you currently use?
a. IVUS
b. OCT
c. Both
12. On average, how many OCT cases do you perform per month? ________
13. Please indicate the top 5 situations where you personally use OCT, with 1 = most common situation.
i. Assessing stent malapposition / under-expansion
ii. Plaque characterization
iii. Vessel sizing
iv. Stent thrombosis
v. ISR
vi. Left main
vii. ACS
viii. Calcified lesion
ix. Bifurcation
x. Other
14. Please indicate the top 5 factors that would help you to use OCT more often, with 1-most common factor
i. Clinical evidence / more data
ii. Reimbursement
iiii. Discounted catheter price
iv. Training on interpretation of images
v. Evidence versus IVUS
vi. Less time consuming
vii. Ease of use
viii. Greater Familiarity
15. For current non-users, for what reasons, situations and patient types would you consider using OCT?
i. Assessing stent malapposition / under-expansion
ii. Plaque characterization
iii. Vessel sizing
iv. Stent thrombosis
v. ISR
vi. Left main
vii. ACS
viii. Calcified lesion
ix. Bifurcation
x. Other
16. What are the main barriers preventing you from using OCT currently?
i. Cost
ii. Lack of familiarity
iii. Not enough cath time / too busy
iv. Safety with current system
v. No reason to use
17. Do you have access to at least one IVUS machine?
i. Yes
ii. No
18. On an average, how many IVUS cases do you perform per month? _____
19. What is the main IVUS system that you use? ____________
20. Please indicate the top 5 situations where you personally use OCT, with 1 = most common situation
i. Assessing stent malapposition / under expansion.
ii. Plaque characteristics
iii. Vessel sizing
iv. Stent thrombosis
v. ISR
vi. Left main
vii. ACS
viii. Calcified lesion
ix. Bifurcation
x. Rotational atherectomy
xi. CTO
xii. Other
21. Please indicate the top 5 factors that would help you to use IVUS more often, with 1 = most common factor
i. Clinical evidence / more data
ii. Reimbursement
iii. Discounted catheter price
iv. Training on interpretation of images
v. Evidence versus OCT
vi. Less time consuming
vii. Ease of use
viii. No contrast
22. For current non-users, for what reasons, situations and patient types would you consider using IVUS?
i. Assessing stent malapposition
ii. Plaque characteristics
iii. Vessel sizing
iv. Stent thrombosis
v. ISR
vi. Left main
vii. ACS
viii. Calcified lesion
ix. Bifurcation
x. Other
23. What are the main barriers preventing you from using IVUS currently?
i. Cost
ii. Lack of familiarity
iii. Not enough cath time / too busy
iv. Safety with current system
v. No reason to use
24. Please indicate the top 5 reasons or situations where you would choose to use IVUS in place of OCT, with 1 = most common situation
i. All left main
ii. Ostial left main
iii. Don’t need contrast
iv. Renal impairment
v. Greater depth of penetration
vi. Greater familiarities with IVUS
vii. Assessing malapposition
viii. CTO
25. Please indicate the top 5 reasons or situations where you would choose to use OCT in place of IVUS, with 1 = most common situation
i. Higher resolution / clearer picture quality
ii. Easier to interpret (Use of Co-registration)
iii. Automation
iv. Future will be OCT
v. Easier to set up
vi. Color pictures
vii. Catheter less rigid and bulky
26. Which of the following imaging systems would you consider while upgrading your cath lab in the next 1 year?
a. OCT with ACR (Co-registration)
b. IVUS
c. None
27. How do you foresee the adoption of Imaging for guiding and optimizing coronary interventions in the future? (Choose one only)
a. Will greatly increases
b. Will slightly increases
28. In your opinion, what should be the focus of future clinical research in Imaging guided PCI and PCIO?
a. Determination of specific criteria for corrective measures in case of abnormal imaging findings
b. Head-to-head comparison of angiography-guided versus IVUS-guided PCI focusing on clinical outcomes
c. Head-to-head comparison of angiography-guided versus OCT-guided PCI focusing on clinical outcomes
d. Head-to-head comparison of IVUS versus OCT is specific patient and lesion subsets
29. What would prevent you from utilizing Physiology and Imaging in the same case in an appropriate clinical scenario?
a. Availability of hardware
b. Hospital rules/purchase department
c. Time