INTRODUCTION
Snake envenomation is a neglected life-threatening public health issue. In June 2017, the World Health Organization (WHO) listed snake envenomation as the highest priority neglected tropical disease (The Lancet, 2017; WHO, 2021). The WHO has also launched a global initiative to reduce mortality and disabilities caused by snakebites to 50% by 2030 (WHO, 2021, Williams et al., 2019).
The death rate due to snake envenomation in tropical countries is alarming, with estimated deaths of around 58,000 per year alone in India (Suraweera et al., 2020; WHO, 2021). The average rural population, farm laborers, and snake rescuers are the categories most exposed to this threat. However, many of them remain unreported even today, as villagers consult unregistered traditional healers (Punde, 2005).
The spectacled cobra (Naja naja), common krait (Bungarus caeruleus), Russell’s viper (Daboia russelii), and saw-scaled viper (Echis carinatus) are the four ubiquitous venomous snake species found within the Indian subcontinent and are termed as the “Big Four”; out of which, cobra and krait (elapid) venoms are neurotoxic, while both viper (viperid) venoms are hemotoxic. Administration of antiserum is the only approved and highly effective treatment available for snake envenomation. Commonly available antiserum in India is polyvalent antiserum, which is active against venoms of these four snake species (Whitaker, 2006). However, anaphylactic reactions and administration difficulties are the major challenges associated with antiserum. In addition, complications like nephrotoxicity, myonecrosis, and hemorrhage caused by venom cannot be managed with available antiserum (Alam and Gomes, 2003; Ode and Asuzu, 2006). Traditional Indian literature enlists various therapies for the management of snake envenomation (Pade, 1893a, 1893b; Pade et al., 2010; Sathe, 2003). Most of these therapies are plant-based and have been described by traditional medical practitioners based on their experiences and observations. Various herbs have been listed in the literature, which are effective against snake envenomation, and many of them have been investigated pharmacologically (Aslam et al., 2021; Ghag-Sawant et al., 2016; Gomes et al., 2010; Houghton and Osibogun, 1993; Khyade et al., 2011; Martz, 1992; Mors et al., 2000; Samy et al., 2008).
Owing to the popular preference for such therapies from most of the rural Indians, a systematic investigation of these plants developing stable and easy to administer antivenom molecules with lesser side effects is highly desired. The investigation would be worthwhile even if such plant-derived agents prove to slow down the clinical ill effects of venom and offer valuable time for a snake bite victim to reach the nearest hospital. The development of such promising molecules is of immense importance in India, where healthcare centers for the most vulnerable population are less in the neighborhoods (Mohapatra et al., 2011).
Our study of the traditional literature included therapies described for the treatment of snake envenomation and agents used therein. This provided the basic clues for probable treatment effectivity. Chemical constituents of the plants were checked for possible anti-snake venom action from literature references. From the list of more than 135 plant species found in the traditional literature, we selected five species, i.e., Sapindus laurifolius Vahl., Spondias pinnata (L.f.) Kurz., Plumeria lutea Ruiz., Woodfordia fruticosa (L.) Kurz., and Croton roxburghii Balakr., and investigated their anti-snake venom potential (Pade, 1893a, 1893b; Pade et al., 2010; Sathe, 2003).
MATERIALS AND METHODS
Animals
Swiss albino mice of either sex (20 ± 2 g) were procured from Haffkine Biopharmaceutical Corporation, Mumbai, India, and maintained under standard laboratory conditions (Ode and Asuzu, 2006; Samudrala et al., 2015). The animals had free access to food and water unless specified. All protocols involving animal experimentation were approved by the Institutional Animal Ethics Committee (IAEC-962/c/06/CPCSEA) of Sinhgad Institute of Pharmaceutical Sciences, Lonavala, Maharashtra, India, vide protocol numbers, SIPS/IAEC/2011-12/03, SIPS/IAEC/2012-13/04, and SIPS/IAEC/2013-14/07.
Venoms
Lyophilized samples of N. naja and D. russelii venoms, as representatives of neurotoxic and hemotoxic categories, respectively, were procured from Irula Snake Catchers’ Industrial Co-operative Society, Kancheepuram, Tamil Nadu, India. The venoms were stored desiccated at 4°C. While reconstituting into a solution, the venoms were dissolved in physiological saline in predetermined concentrations, just before use. The venom concentrations were expressed in terms of dry weight.
Snake venom antiserum (SVA)
A lyophilized polyvalent antiserum manufactured by Vins Bioproducts Limited, India, with the potential of neutralizing venoms of the Big Four, was used as a reference standard. The antiserum was reconstituted into a solution as per the manufacturer’s guidelines and was expressed in terms of reconstituted volume. As per the label claim, 1 ml of the reconstituted antiserum was capable of neutralizing 0.6 mg (each by dry weight) of N. naja and D. russelii venoms. This SVA was used as a positive control comparator.
Collection of plant parts
Dried fruits of S. laurifolius Vahl. (Sapindaceae) were purchased from the local market of Pune, Maharashtra, India. Barks of S. pinnata (L.f.) Kurz. (Anacardiaceae) and P. lutea Ruiz. (Apocynaceae) were collected from the Erandawane area of Pune, Maharashtra, India. Leaves of W. fruticosa (L.) Kurz. (Lythraceae) were collected from the hillside near Kusgaon village, Lonavala, in the Western Ghats region of Maharashtra, India. Roots of C. roxburghii Balakr. (Euphorbiaceae) were collected near Parpoli village, Sawantwadi, in the Western Ghats region of Maharashtra, India. These plant samples and/or whole plants were submitted to the Botanical Survey of India, Western Regional Center, Pune, Maharashtra, India and were authenticated vide voucher numbers ADDSAL1, ADDSPP2, ADDPLL5, ADDWOF3, and ADDCRR4, respectively. The plant parts were subjected to shade drying and stored in desiccated condition at 20°C–25°C.
Preparation of extracts
The plant parts were converted to a coarse powder with a pulverizer (Gem Pharma, Navi Mumbai, India). 25 g of each powder sample was subjected to soxhlation (Borosil Soxhlet extractor coupled with Allihn condenser) at 100°C, using 300 ml distilled water as an extractant in one batch. The extracts, thus obtained, were concentrated, lyophilized (Alpha 1-2 LDplus, Martin Christ Gefriertrocknungsanlagen GmbH, Germany), and stored in a desiccated condition at 4°C until further use. The yield of dried extracts was, 3.57 ± 0.01 g for S. laurifolius, 2.10 ± 0.02 g for S. pinnata, 1.92 ± 0.02 g for P. lutea, 2.66 ± 0.02 g for W. fruticosa, and 2.32 ± 0.01 g for C. roxburghii per 25 g coarse powder. During experimentation, the dried extracts were reconstituted by dissolving in a physiological saline solution in predetermined concentrations, just before use. Extract concentrations were expressed in terms of dry weight.
Acute toxicity of plant extracts
Acute toxicity of the plant extracts was performed by subcutaneous injection of reconstituted extracts in experimental mice by following the OECD 423 guidelines for testing of chemicals with suitable modifications (OECD, 2001a). Mice were fasted for around 4 hours before dosing. Groups of 3 mice were used for every level of dose and 300 mg/kg was the starting dose administered. The mice were observed critically for the initial 6 hours for any symptoms of toxicity or mortality and continued to be monitored for up to 48 hours. If two or three animals were found dead in step 1, a subsequent lower dose from the next level was administered in a group of three mice (n = 3); and if one or zero animals were found dead in step 1, the same dose was repeated for step 2 (steps 1 + 2, n = 6) and a subsequent higher dose from the next level was administered in another group of three mice. This was continued until the safety level was determined. For the conclusive dose, both the steps were conducted (n = 6) as per guidelines. The available literature on these plants was used as a guideline for determining lethal doses (LD50) (Arul et al., 2004; Arya et al., 2012; Elnour et al., 2013; Gupta et al., 2004; Pal et al., 2013; Panda et al., 2014; Tessou et al., 2013; Vijayalakshmi et al., 2011).
Inhibition of venom lethality
Median LD50 of individual venoms in mice were determined by observing death within 24 hours postsubcutaneous administration of venom concentrations. OECD guidelines for acute toxicity testing with suitable modifications for microgram doses were used and precise LD50 values were determined (OECD, 2001a, 2001b). Groups of three mice were used for every level of the dose and the starting doses and dose ranges were designed considering earlier experimentation on particular venoms (Jayanthi and Gowda, 1988; Mukherjee et al., 2000; Shashidharamurthy et al., 2002). If two or three animals were found dead in step 1, a subsequent lower dose from the next level was administered in a group of three mice (n = 3); and if one or zero animals were found dead in step 1, the same dose was repeated for step 2 (steps 1 + 2, n = 6) and a subsequent higher dose from the next level was administered in another group of three mice. This was continued until LD50 was determined. For the conclusive dose, both the steps were conducted (n = 6) as per guidelines.
To assess the in-vitro inhibition of venom lethality, 10 mg of the investigational plant extracts were mixed with 1 of 1–40 LD50 doses of venoms, incubated at 37°C for 1 hour, and clear supernatants after centrifugation were injected in mice subcutaneously and LD50 values were determined (Alam and Gomes, 2003). To assess the in-vivo inhibition of venom lethality, 1 of 1–6 LD50 doses of either venom was administered in mice subcutaneously, followed by administration of 1 dose of 10–20 mg of either plant extract at a different subcutaneous site.
The doses of venoms were incremental and an initial dose taken was LD50 of the respective venom. In one group of animals, only one dose of venom and one dose of extract were administered. Each extract was tested against both venoms in different groups. SVA was used as a positive control comparator in a dose proportionate to the amount of snake venom used as per the manufacturer’s guideline.
Inhibition of hemorrhagic activity
Fertilized galline eggs procured from a local hatchery were used to perform hemorrhagic activity for D. russelii venom and the standard protocol was followed (Ode and Asuzu, 2006; Sells et al., 1997). Whatman filter paper no. 1 was cut into disks of 2 mm diameter. These disks were saturated with reconstituted venom. 0.1× LD50 and 0.5× LD50 dose/ml were the initial venom doses used. Based on the results, further doses were designed at the midpoints of the earlier doses. Fertilized eggs were cracked on day 4 into cling film hammocks and were further incubated till day 6. The saturated paper disk was placed over the major vein on the yolk sac. After 3 hours, the diameter of the hemorrhagic corona was measured. The dose of venom yielding a hemorrhagic corona of 2 mm diameter was considered the minimum hemorrhagic dose (MHD).
To assess the inhibition of hemorrhagic activity, similar steps were repeated as above but the filter paper disks were saturated with a mixture of venom and plant extract. 1× MHD of venom was mixed with 1 and 10 mg/ml of plant extracts initially, and based on the results, further extract doses were designed at midpoints of the earlier doses. The minimum dose of the plant extract that could neutralize the hemorrhagic activity completely was termed as the minimum hemorrhage neutralizing dose (MHND). Individual plant extracts without mixing with venoms were also tested in the same way to assess any hemorrhagic activity possessed by plant extracts. SVA was used as a positive control comparator in a dose proportionate to the amount of snake venom used as per the manufacturer’s guideline.
Inhibition of coagulant activity
Citrated human plasma solution was used for this activity and clotting time determination was done by the standard method (Theakston and Reid, 1983). For N. naja venom, 1× LD50 and 5× LD50 dose/ml and for D. russelii venom, 0.1× LD50 and 0.5× LD50 dose/ml were the initial venom doses used. Based on the results, further doses were designed at the midpoints of the earlier doses. While determining these venom doses, earlier literature (Suntravat et al., 2010) available was considered and as D. russelii venom has much potent procoagulant activity than Elapid venoms, lower doses were formulated. 0.1 ml of different venom concentrations were added to 0.4 ml citrated human plasma solution maintained at 37°C, mixed thoroughly and the clotting time was recorded. The minimum clotting dose (MCD) for the venoms was estimated by adding reconstituted venom doses to plasma solutions that coagulated plasma at the end of 60 seconds at 37°C.
To assess the inhibition of coagulant activity, equal volumes of a 1× MCD of venom and plant extract were mixed; the mixtures were incubated at 37°C for 1 hour. The clear supernatant was added to the plasma solutions and the clotting times were recorded. 1 and 10 mg/ml doses of plant extracts were used initially and based on the results, further extract doses were designed at midpoints of the earlier doses. The minimum dose of plant extract which could neutralize the clotting activity completely was termed as the minimum clotting neutralizing dose (MCND). Individual plant extracts without mixing with venoms were also tested in the same way to assess any alterations in coagulation by plant extracts. SVA was used as a positive control comparator in a dose proportionate to the amount of snake venom used as per the manufacturer’s guideline.
Enzyme inhibition
Quantitative estimation of phospholipase A2 (PLA2) and acetylcholinesterase (AChE) from 1.0 mg/ml snake venoms was performed spectrofluorometrically. EnzChek® Phospholipase A2 Assay Kit (Molecular Probes®, OR) was used to assay PLA2 and fluorescence emission was detected at 515 nm by excitation at 450 nm. Amplex® Red Acetylcholine/Acetylcholinesterase Assay Kit (Molecular Probes®, OR) was used to assay AChE and fluorescence emission was detected at 590 nm by excitation at 530 nm. Assaying of the enzymes was done according to the manufacturer’s guidelines using black 96-well microplates by a SpectraMax® M5 Microplate Reader (Molecular Devices, CA). Actual quantitative determination was performed by using the standard curves obtained for the respective enzymes.
To assess enzyme inhibition, 1.0 mg/ml of either venom was mixed with 1.0–3.5 mg/ml of either plant extract and the mixtures were incubated at 37°C for 1 hour. Quantitative estimation of PLA2 and AChE was done by repeating the steps mentioned earlier and alterations in the quantities of enzymes were determined. SVA was used as a positive control comparator in a dose proportionate to the amount of snake venom used as per the manufacturer’s guideline.
RESULTS
Acute toxicity of plant extracts
Aqueous extracts of the plants did not present significant toxicity for up to 48 hours of observation postsubcutaneous administration. Extracts of S. pinnata, P. lutea, W. fruticosa, and C. roxburghii were found safe up to 2,000 mg/kg dose, whereas S. laurifolius was found safe up to 1,000 mg/kg. Dose designing for further experiments was done considering these safety margins and solubility for individual plant extracts.
Venom lethality
LD50 values of N. naja and D. russelii venoms in mice were found to be 0.625 and 4.0 mg/kg by subcutaneous administration, respectively. 10 mg/kg extract doses were evaluated for neutralization of venom lethality both in vitro and in vivo. As the venom can be neutralized by the extract in direct contact in vitro, only one dose of extract was evaluated. During in-vivo experimentation, because many factors affect the neutralization of snake venom systemically, a higher 2× concentration of extracts, i.e., 20 mg/kg, was evaluated additionally. As hypothesized, the extracts acted in a dose-dependent manner in vivo and higher neutralization was exhibited by the higher dose of extract. The results of in-vitro and in-vivo inhibition of venom lethality are presented in Table 1. Woodfordia fruticosa neutralized both the venoms in the highest fold (p < 0.005 in vivo) and was followed by C. roxburghii (p < 0.05 in vivo), whereas P. lutea exhibited the least neutralization in vivo. As a positive control, 1 ml SVA was used for both in-vitro and in-vivo interactions. While injecting the SVA, as the volume was higher, two subcutaneous administrations were carried out at different sites. As indicated in Table 1, the fold of protection exhibited by SVA seems lower; however, it should be noted that the amount of SVA is not comparable to the amount of plant extracts used in the experimentation. Since a definitive dose of SVA capable of neutralizing a specific amount of snake venom is known, only 1 ml dose was tested, which yielded expected results.
Hemorrhagic activity
Daboia russelii venom was investigated for hemorrhagic activity and its MHD was found to be 1.9 mg/ml, which produced a distinct hemorrhagic lesion of 2 mm diameter. MHNDs for S. laurifolius, S. pinnata, P. lutea, W. fruticosa, and C. roxburghii were found to be 1.25, 1.5, 10.5, 0.95, and 4.5 mg/ml, respectively (Table 2). 3.4 ml of reconstituted SVA was used as a positive control comparator which neutralized the hemorrhagic activity of 1.9 mg/ml dose of D. russelii venom completely. Individual plant extracts without venom did not present any hemorrhagic activity. As a technique of experimentation, there are a few specific requirements where the researchers have to be very careful. Maintenance of a high level of sterility in the experimental setup is the key to success and once the technique is established, a large batch of experiments can be run at a time.
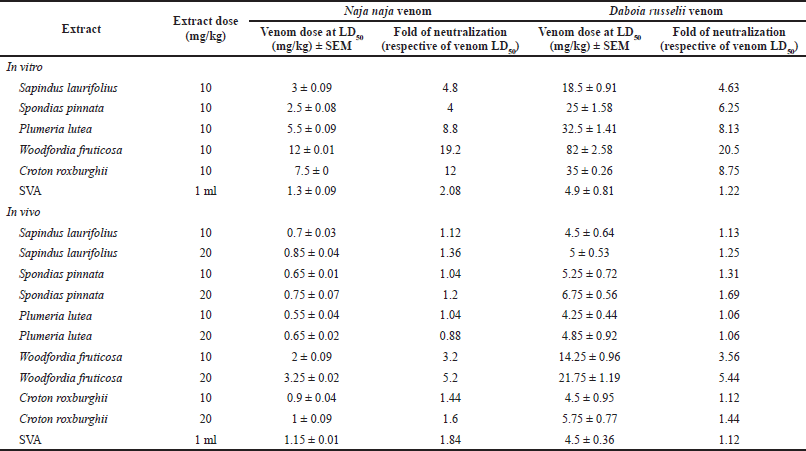 | Table 1. Inhibition of N. naja and D. russelii venoms’ lethality by plant extracts. [Click here to view] |
Coagulant activity
MCDs for N. naja and D. russelii venoms were found to be 0.85 and 1.3 mg/ml, respectively. Woodfordia fruticosa extract was capable of neutralizing the coagulation induced by both N. naja and D. russelii venoms significantly (p < 0.005) and exhibited potent action. Spondias pinnata exhibited second-best results and P. lutea exhibited the least activity against coagulation. Deviations in MCDs of venoms in the presence of plant extracts and MCNDs of individual extracts are listed in Table 3. In the positive control comparator group, 1.6 and 3.4 ml of reconstituted SVA neutralized the coagulant activity of 0.85 mg/ml dose of N. naja venom and 1.3 mg/ml dose of D. russelii venom, respectively. Individual plant extracts without venoms did not produce any coagulant activity. The technique used in this experiment is simpler to run but is manual and time-consuming.
Enzyme inhibition
Content and in-vitro inhibition of PLA2 was determined in both the venoms, whereas the determination of AChE was done only in the N. naja venom, as the D. russelii venom is known not to contain AChE (Cousin and Bon, 1996). The D. russelii venom has a relatively higher quantity of PLA2 than the N. naja venom. Spectrofluorometric assays revealed that preincubation of extracts with venoms reduced the quantities of the enzymes from venoms in a dose-dependent manner. Higher doses of W. fruticosa and S. pinnata completely neutralized PLA2 and AChE from N. naja venom (p < 0.005). Croton roxburghii also presented a significant reduction (p < 0.05) of PLA2 from both venoms and AChE from N. naja venom. Tables 4 and 5 indicate enzyme concentrations for the respective treatments. SVA was used as positive control comparator and 1.8 and 2 ml of SVA was capable of neutralizing PLA2 from 1 mg/ml dose of N. naja and D. russelii venom respectively. Similarly, 1.7 ml of SVA was capable of neutralizing AChE from 1 mg/ml dose of N. naja venom. With a proper set of apparatus and microinstruments and a lower margin of error, this experiment has high repeatability and consistency.
DISCUSSION
While collecting information on traditionally observed therapies for snake envenomation in India, 135 remedies that exhibited some promise to act against snake venom surfaced. Depending on the nature of the remedy, the route of administration of the therapeutic agent, and multiple references about a single plant, five plant species were selected and investigated. Sapindus laurifolius, P. lutea, and W. fruticosa are largely found throughout India, especially in the Western Ghats. Spondias pinnata is a sparsely found tree, whereas C. roxburghii is a rarely found plant that occurs in specific patches within the Western Ghats of Maharashtra, India. Croton roxburghii was earlier known as Croton oblongifolius and is also known as Croton virbalae (Almeida and Almeida, 2005). The roots of this plant carry a characteristic pleasant aroma. It is a known remedy against Ghonas (Russell’s viper) venom in the Konkan region of Maharashtra, India. This plant has not been investigated much pharmacologically. None of these five plants has been screened systematically for their snake venom neutralization potential in vivo and only S. laurifolius has been evaluated in vitro for enzymatic activity neutralization in D. russelii venom (Ghag-Sawant et al., 2016).
After a venomous snake bite, if the victim does not receive medical help in a short time, chances of survival or living without permanent disabilities reduce with passing time. In such cases, safer drugs that can be administered via oral or sublingual routes, which are developed out of chemical leads from plant origin, might prove to be lifesavers.
As the remedies that were discovered from the traditional literature do not make use of any organic solvent, it was considered that the active ingredients were possibly water-soluble and only aqueous extracts were used in the present investigation. During experimentation, plant extracts were prepared by soxhlation at 100°C, where there are chances of a formation of artifacts by a combination or breakdown of naturally occurring chemicals in the plants (Ebrahimi Majdar et al., 2019; Inouye et al., 1969). A systematic investigation with the separation of compounds from plant extracts and their characterization would shed light on the formation of such artifacts.
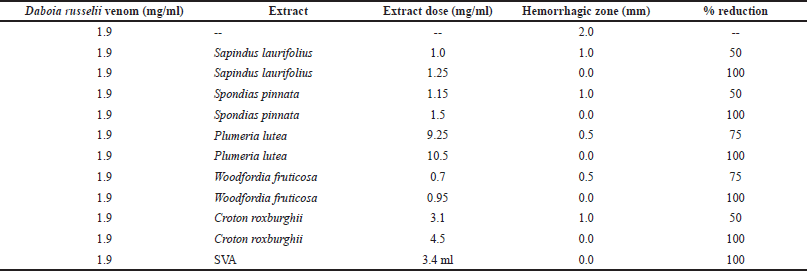 | Table 2. Inhibition of D. russelii venom hemorrhagic activity by plant extracts. [Click here to view] |
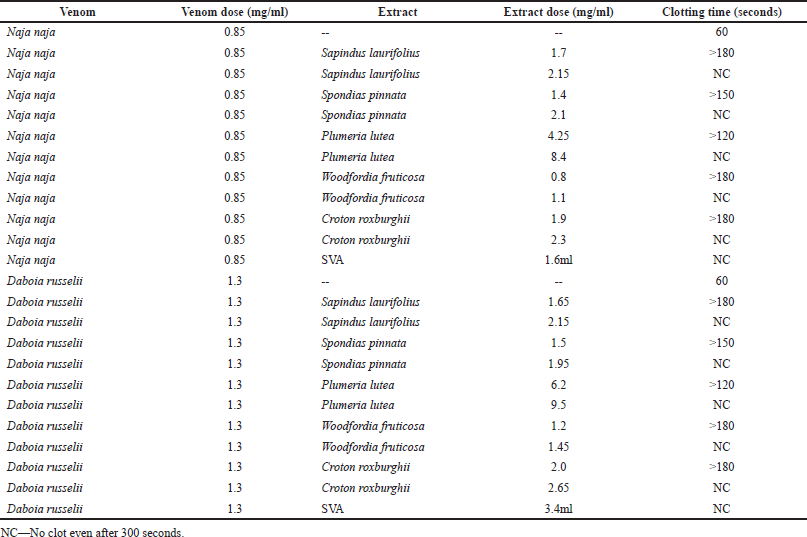 | Table 3. Inhibition of venom coagulant activity by plant extracts. [Click here to view] |
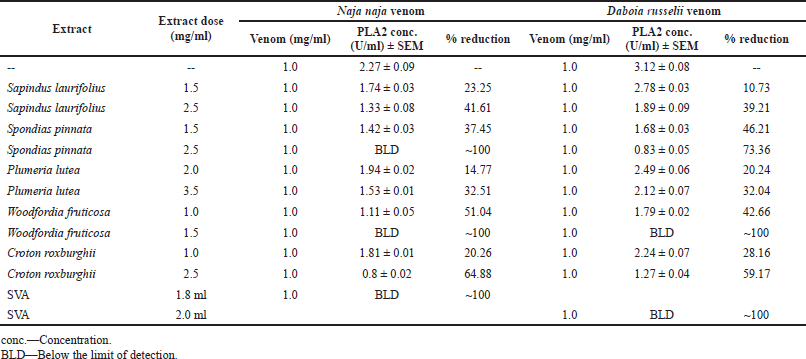 | Table 4. In-vitro inhibition of PLA2 from N. naja and D. russelii venoms by plant extracts. [Click here to view] |
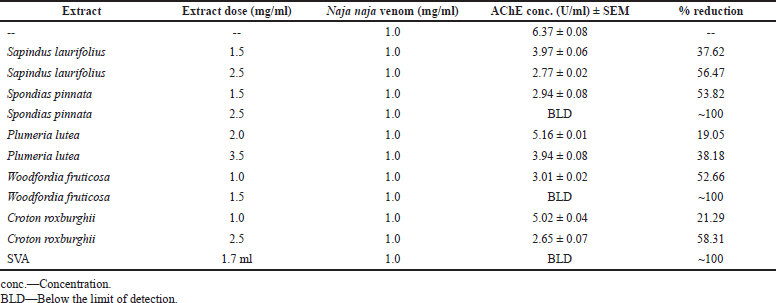 | Table 5. In-vitro inhibition of AChE from N. naja venom by plant extracts. [Click here to view] |
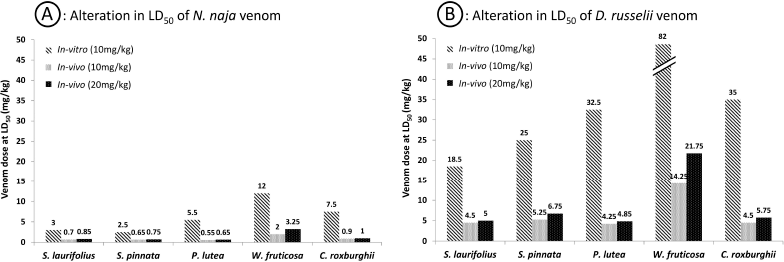 | Figure 1. Alterations in LD50 of venoms in the presence of plant extracts in vitro and in vivo. [Click here to view] |
Five plant species tested against neurotoxic and hemotoxic venoms presented some degree of protection when investigated in vitro. Promising in-vivo and in-vitro activities were presented by W. fruticosa. Acute toxicity of both venoms was reversed by W. fruticosa significantly in experimental mice. Croton roxburghii presented significant activity in vitro against cobra venom, but limited protection in vivo. Figure 1 shows the comparative action of plant extracts against the venoms. In-vivo neutralization of snake venoms is the most important activity tracked in the current experimentation as it represents the actual clinical scenario after a snakebite. The whole venom when injected into the animal body creates various pathological conditions that lead to temporary or permanent disabilities due to tissue necrosis or interfering with the normal functioning of the affected tissue. Plant extracts administered without mixing with the venoms exhibit the near actual pharmacological action in these animals. The action of W. fruticosa extract in this experimental model is a direct indicator of its pharmacological action as a snake venom neutralizer. Whole snake venom, being a mixture of proteins, can be denatured easily in vitro when mixed with plant derivatives; thus, although in-vitro activity might not prove to be a sure representation of pharmacological activity, it provides a basis to knowing the possible mechanism of action of the agents under investigation. Croton roxburghii is known to act against the venom of Russell’s viper in folklore; however, it did not present strong evidence of viper venom neutralization in the current experimental setup. Possibly the active ingredient might be water-insoluble and did not get extracted which, in turn, did not produce any pharmacological activity.
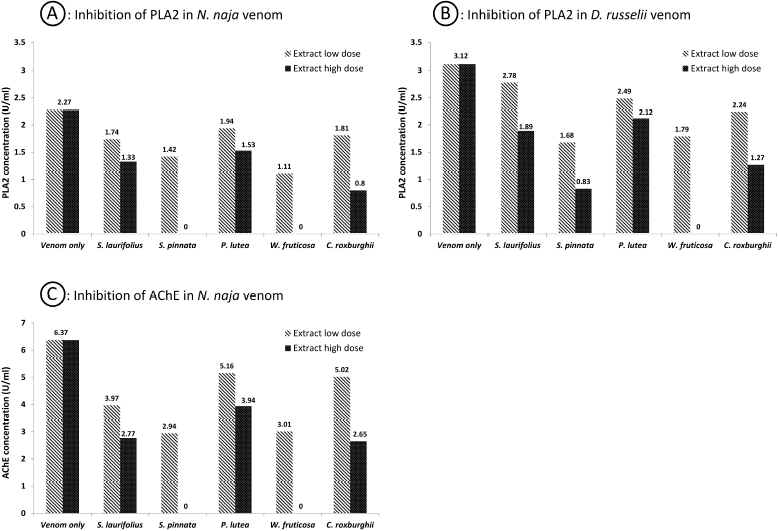 | Figure 2. Inhibition of PLA2 and AChE enezymes from venoms in the presence of plant extracts. [Click here to view] |
Performing hemorrhagic activity by using galline eggs is simpler as compared to the conventional animal skin method. Its results are consistent and the method is more humane and ethical considering the animal rights perspective (Sells et al., 1997). The dose of W. fruticosa needed to neutralize the hemorrhagic potential of D. russelii venom was the least among the plants under investigation. This result is sacrosanct with the neutralization of venom lethality and might be a prominent contributor to the same. With high potency to neutralize venom enzymes, W. fruticosa is a promising candidate for further molecular investigation. In terms of antihemorrhagic activity, S. pinnata and S. laurifolius followed W. fruticosa, whereas, P. lutea exhibited the least activity among plant species under investigation (Table 2).
Coagulant activity studies revealed that cobra venom has more blood coagulating ability than viper venom. While investigating the anticoagulant activity of plant extracts against venoms, W. fruticosa was found to exhibit the highest activity, followed by S. pinnata and S. laurifolius. The study also overruled the possibility of plant extracts solitarily exhibiting pro- or anticoagulant activity.
Enzymes play a vital role in the spread and toxicity of venoms. One of the vital enzymes of snake venoms, PLA2, primarily causes inflammation and pain at the bite area and shows vivid pharmacological effects (Kini, 2003). In our present findings, we observed that the quantity of PLA2 in viper venom was higher than that in cobra venom. Woodfordia fruticosa showed the highest ability to inhibit PLA2, followed by S. pinnata. AChE, which is a synaptic transmission terminator, is found in large quantities in cobra venom. Woodfordia fruticosa and S. pinnata were found to inhibit this enzyme. Figure 2 shows the relative inhibition of enzymes from the snake venoms in the presence of plant extracts. Later, it was understood that AChE plays almost no role in the toxicity of neurotoxic venoms (Cousin and Bon, 1996).
From the researchers’ parallel experimentation with High performance thin layer chromatography (HPTLC), W. fruticosa extract confirmed the presence of phenolic compounds, flavonoids, and tannins (unpublished data). These compounds might have contributed to the denaturation of venom proteins as revealed by the alterations in hemorrhagic and coagulant activity of venoms estimated by in-vitro methods. Various researchers have already established the role of phenolic compounds, flavonoids, and tannins in the inhibition of PLA2 and AChE (Chethankumar and Srinivas, 2008; Deepa and Gowda, 2002; Khan et al., 2018, Lattig et al., 2007; Pereanez et al., 2011; Roseiroa et al., 2012; Ticli et al., 2005; Türkan et al., 2019; Zhang and Li, 2017).
SVA was used as a positive control during experimentation. Since the doses of test products and SVA were adjusted till significant positive results were seen, a quantitative comparison between SVA and test products for the superiority of results was not carried out.
Snake venoms are mixtures of a variety of structurally and functionally different peptides eliciting various actions on various body parts of the prey. While studying an activity against any whole snake venom, it becomes difficult to identify exactly which peptides were deactivated by the test chemical(s), unless fractionated peptides are tested. At the same time, it is necessary to test the whole venom for possible antivenom activity of chemical(s) as venom peptides may behave synergistically in vivo and produce more severe effects than individual components.
CONCLUSION
From our experimental findings, it is clear that the plants under investigation exhibited potential anti-snake venom activity and can be significant lead candidates for further investigation. Woodfordia fruticosa has shown good antivenom potential consistently in all experiments of the present study and needs to be investigated further for active compound(s). However, a much more detailed study is needed to use these agents as anti-snake venom therapeutics on a commercial scale.
ACKNOWLEDGMENTS
The authors are thankful to Dr M R Almeida (Blatter Herbarium, St. Xavier’s College, Mumbai, India) and the residents of the Parpoli village (Sawantwadi, MS, India) for helping to identify and procure C. roxburghii; Ms S Ullal (Erandawane, Pune, India) for helping to get S. pinnata; Dr B G Kulkarni (Ex-scientist, Botanical Survey of India, Pune, India) to identify plant samples; Dr D Mitra (Scientist ‘G’, National Center for Cell Science, Pune, India) for help with spectrofluorometry; Mr Laxmikant Deshpande (Environmental Consultant, Mumbai, India); Dr Ninad Mungi (Wildlife Institute of India, Dehradun, India); Dr Vishnu Thakare (SIPS, Lonavala) for language proofreading; and the management of Sinhgad Institute of Pharmaceutical Sciences, Lonavala, for research facilities.
CONFLICT OF INTEREST
All authors declare that they do not have any conflict of interest related to this work.
FUNDING
This study was not financially supported by any agency.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
All protocols involving animal experimentation were approved by the Institutional Animal Ethics Committee (IAEC-962/c/06/CPCSEA) of Sinhgad Institute of Pharmaceutical Sciences, Lonavala, Maharashtra, India, vide protocol numbers, SIPS/IAEC/2011-12/03, SIPS/IAEC/2012- 13/04, and SIPS/IAEC/2013-14/07.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alam MI, Gomes A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J Ethnopharmacol, 2003; 86(1):75–80. CrossRef
lmeida SM, Almeida MR. Dictionary of generic names of flowering plants and ferns in Maharashtra. Satyam Enterprises, Mumbai, India, 2005.
Arul B, Kothai R, Jacob P, Sangameswaran B, Sureshkumar K. Anti-inflammatory activity of Sapindus trifoliatus Linn. J Herb Pharmacother, 2004; 4(4):43–50. CrossRef
Arya A, Abdullah MA, Haerian BS, Mohd MA. Screening for hypoglycemic activity on the leaf extracts of nine medicinal plants: in-vivo evaluation. E J Chem, 2012; 9(3):1196–205. CrossRef
Aslam N, Fatima S, Khalid S, Hussain S, Qayum M, Afzal K, Afzal K, Asad MHHB. Anti-5-Nucleotidases (5-ND) and acetylcholinesterase (AChE) activities of medicinal plants to combat Echis carinatus venom-induced toxicities. Biomed Res Int, 2021; 2021:10. CrossRef
Chethankumar M, Srinivas L. New biological activity against phospholipase A2 by Turmerin, a protein from Curcuma longa L. J Biol Chem, 2008; 389:299–3303. CrossRef
Cousin X, Bon C. Acetylcholinesterase from snake venoms. C R Seances Soc Biol Fil, 1996; 191(3):381–400.
Deepa M, Gowda TV. Purification and characterization of a glycoprotein inhibitor of toxic phospholipase from Withania somnifera. Arch Biochem Biophys, 2002; 408:42–50. CrossRef
Ebrahimi Majdar R, Ghasemian A, Resalati H, Saraeian A, Crestini C, Lange H. Facile isolation of LCC-fraction from organosolv lignin by simple soxhlet extraction. Polymers, 2019; 11(2):225. CrossRef
Elnour EA, Abdmageed MAM, Shyoub ME, Mohammed RR. Evaluation of toxicity of some plants having traditional uses in Sudan on brine shrimp. Glob J Tradit Med Syst, 2013; 2(1):19–23.
Ghag-Sawant M, More TV, Samant LS, Chowdhary AS. Study of neutralization of enzymatic activity of Daboia russelii venom by various plant extracts and their combinations using in vitro methods. Int J Pharm Sci Res, 2016; 7(6):2531–6.
Gomes A, Das R, Sarkhel S, Mishra R, Mukherjee S, Bhattacharya S, Gomes A. Herbs and herbal constituents active against snake bite. Indian J Exp Biol, 2010; 48:865–78.
Gupta M, Mazumder UK, Vamsi MLM, Sivakumar T, Kandar CC. Anti-steroidogenic activity of the two Indian medicinal plants in mice. J Ethnopharmacol, 2004; 90(1):21–5. CrossRef
Houghton PJ, Osibogun IM. Flowering plants used against snakebite. J Ethnopharmacol, 1993; 39(1):1–29. CrossRef
Inouye H, Okigawa M, Shimokawa N. Studies on monoterpene glucosides VIII artefacts formed during extraction of Asperuloside and Paederoside. Chem Pharm Bull, 1969; 17(9):1949–54. CrossRef
Jayanthi GP, Gowda VT. Geographical variation in India in the composition and lethal potency of Russell’s viper (Vipera russelli) venom. Toxicon, 1988; 26(3):257–64. CrossRef
Khan H, Marya, Amin S, Kamal MA, Patel S. Flavonoids as acetylcholinesterase inhibitors: current therapeutic standing and future prospects. Biomed Pharmacother, 2018; 101:860–70. CrossRef
Khyade MS, Takate YA, Divekar MV. Plants used as an antidote against snakebite in Akole Taluka of Ahmednagar District (MS), India. J Nat Remedies, 2011; 11(2):182–92.
Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon, 2003; 42(8):827–40. CrossRef
The Lancet. Snake-bite envenoming: a priority neglected tropical disease. Lancet, 2017; 370(10089):P2. CrossRef
Lattig J, Bohl M, Fischer P, Tischer S, Tietbohl C, Menschikowski M, Gutzeit HO, Metz P, Pisabarro MT. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: rationale for lead design. J Comput Aided Mol Des, 2007; 21:473–83. CrossRef
Martz W. Plants with a reputation against snakebite. Toxicon, 1992; 30(10):1131–42. CrossRef
Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, Rodriguez PS, Mishra K, Whitaker R, Jha P, Million Death Study Collaborators. Snakebite mortality in India: a nationally representative mortality survey. PLOS Negl Trop Dis, 2011; 5(4):e1018. CrossRef
Mors WB, Célia do Nascimento M, Ruppelt Pereira BM, Pereira NA. Plant natural products active against snake bite—the molecular approach. Phytochemistry, 2000; 55(6):627–42. CrossRef
Mukherjee AK, Ghosal SK, Maity CR. Some biochemical properties of Russell’s viper (Daboia russelli) venom from Eastern India: correlation with clinico-pathological manifestation in Russell’s viper bite. Toxicon, 2000; 38(2):163–75. CrossRef
Ode OJ, Asuzu IU. The anti-snake venom activities of the methanolic extract of the bulb of Crinum jagus (Amaryllidaceae). Toxicon, 2006; 48(3):331–42. CrossRef
OECD. 423 Guidelines for the testing of chemicals: acute oral toxicity—acute toxic class method. Organisation for economic co-operation and development, 2001a. Available via https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf (Accessed 22 May 2022).
OECD. 425 Guidelines for the testing of chemicals: acute oral toxicity—up-and-down procedure. Organisation for economic co-operation and development, 2001b.
Pade SD. Aryabhishak arthat hindusthancha vaidyaraj. Rajesh Prakashan, Pune, India, 1893a.
Pade SD. 1893b. Vanaushadhi Gunadarsha. Shri Gajanan Book Depot, Pune, India, 1893b.
Pade SD, Patil PB, Gadre DV, Padhye-Gurjar AB. Aushadhi Baad. Rajesh Prakashan, Pune, India, 2010.
Pal R, Mukherjee A, Saha A. Exploring post-coital anti-fertility activity with toxicological and hormonal profiling of Sapindus trifoliatus Linn. Int Res J Pharm Appl Sci, 2013; 3(5):53–60.
Panda BK, Patro VJ, Mishra US. Evaluation of diuretic activity of acetone and ethanol stem barks extracts of Spondias pinnata (Linn. F) Kurz in Rats. Int J Pharm Chem Sci, 2014; 3(2):589–93.
Pereanez JA, Nunez V, Patino AC, Londono M, Quintana JC. Inhibitory effects of plant phenolic compounds on enzymatic and cytotoxic activities induced by a snake venom phospholipase A2. Vitae, Revista De La Facultad De Química Farmacéutica, 2011; 18(3):295–304.
Punde DP. Management of snake-bite in rural Maharashtra: a 10-year experience. Natl Med J India, 2005; 18(2):71.
Roseiroa LB, Rauter AP, Mourato Serralheirob ML. Polyphenols as acetylcholinesterase inhibitors: structural specificity and impact on human disease. Nutr Aging, 2012; 1:99–111. CrossRef
Samudrala PK, Augustine BB, Kasala ER, Bodduluru LN, Barua C, Lahkar M. Evaluation of antitumor activity and antioxidant status of Alternanthera brasiliana against Ehrlich ascites carcinoma in Swiss albino mice. Pharmacogn Res, 2015; 7(1):66–73. CrossRef
Samy RP, Thwin MM, Gopalakrishnakone P, Ignacimuthu S. Ethnobotanical survey of folk plants for the treatment of snakebites in Southern part of Tamilnadu, India. J Ethnopharmacol, 2008; 115(2):302–12. CrossRef
Sathe KN. Gharguti Aushadhe. 16th edition, Shailaja Anil Sathe, Mumbai, India, 2003.
Sells PG, Richards AM, Laing GD, Theakston RDG. The use of hens’ eggs as an alternative to the conventional in vivo rodent assay for antidotes to haemorrhagic venoms. Toxicon, 1997; 35(9):1413–21. CrossRef
Shashidharamurthy R, Jagadeesha DK, Girish KS, Kemparaju K. Variations in biochemical and pharmacological properties of Indian cobra (Naja naja naja) venom due to geographical distribution. Mol Cell Biochem, 2002; 229(1-2):93–101.
Suntravat M, Nuchprayoon I, Pérez JC. Comparative study of anticoagulant and procoagulant properties of 28 snake venoms from families Elapidae, Viperidae, and purified Russell’s viper venom-factor X activator (RVV-X). Toxicon, 2010; 56:544–53. CrossRef
Suraweera W, Warrell D, Whitaker R, Menon G, Rodrigues R, Hang Fu S, Begum R, Sati P, Piyasena K, Bhatia M, Brown P, Jha P. Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. ELife, 2020; 9:e54076. CrossRef
Tessou KZ, Lawson-Evi P, Metowogo K, Diallo A, Eklu-Gadegkeku K, Aklikokou K, Gbeassor M. Acute and sub-acute toxicity studies of Plumeria alba Linn. (Apocynaceae) hydroalcoholic extract in rat. Int J Biomed Sci, 2013; 9(4):255–9.
Theakston RDG, Reid HA. Development of simple standard assay procedures for the characterization of snake venoms. Bull World Health Organ, 1983; 61(6):949–56.
Ticli FK, Hage LI, Cambraia RS, Pereira PS, Magro AJ, Fontes MR, Stábeli RG, Giglio JR, França SC, Soares AM, Sampaio SV. Rosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): antiserum action potentiation and molecular interaction. Toxicon, 2005; 46:318–27. CrossRef
Türkan F, Taslimi P, Saltan FZ. Tannic acid as a natural antioxidant compound: discovery of a potent metabolic enzyme inhibitor for a new therapeutic approach in diabetes and Alzheimer’s disease. J Biochem Mol Toxicol, 2019; 33:e22340. CrossRef
Vijayalakshmi A, Ravichandiran V, Velraj M, Hemalatha S, Sudharani G, Jayakumari S. Anti-anaphylactic and anti-inflammatory activities of a bioactive alkaloid from the root bark of Plumeria acutifolia Poir. Asian Pac J Trop Biomed, 2011; 1(5):401–5. CrossRef
Whitaker R. Common Indian snakes—a field guide. Macmillan Publishers India Ltd, Chennai, India, 2006.
WHO. Snakebite envenoming. World Health Organization, Geneva, Switerzland, 2021. Available via https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming. (Accessed 5 September 2021).
Williams DJ, Abul Faiz M, Abela-Ridder B, Ainsworth S, Bulfone TC, Nickerson AD, Habib AG, Junghanss T, Fan HW, Turner M, Harrison RA, Warrell DA. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLOS Negl Trop Dis, 2019; 13(2):e0007059. CrossRef
Zhang Y, Li CM. The detoxifying effects of structural elements of persimmon tannin on Chinese cobra phospholipase A2 correlated with their structural disturbing effects well. J Food Drug Anal, 2017; 25:731–40. CrossRef