INTRODUCTION
In January 2020, the severe acute respiratory syndrome coronavirus (SARS-CoV-2) was identified as the source of a pneumonia outbreak in Wuhan, and it quickly spread throughout China (Pascarella et al., 2020). Notably, the highest morbidity–mortality of COVID-19 was observed among the elderly (Wu and McGoogan, 2020). COVID-19 causes a wide variety of symptoms, from mild (asymptomatic) to severe sickness and death. The most frequent symptoms are cough, fever, and shortness of breath (Lovato et al., 2020). Clinical signs, viral genome detection by reverse transcription polymerase chain reaction, CT scan or chest X-ray, serological blood tests, and a few additional laboratory procedures are used to diagnose COVID-19 (Paranjpe et al., 2020; Zhu et al., 2020). A large nonstructural polyprotein (ORF1a/b) is encoded by the SARS-CoV-2 genome which is split by proteolytic activity into 15/16 proteins, 5 accessory proteins, and 4 structural proteins (Chan et al., 2020; Ramaiah and Arumugaswami, 2020; Wu et al., 2020). In the United States, influenza and other etiologies of pneumonia are considered the eighth cause of death. In addition, many respiratory viruses have been identified as common pathogens that infect hospitalized patients with community-acquired pneumonia (Jain et al., 2015). Although influenza and COVID-19 possess similar signs and symptoms, huge causalities have been reported due to the novel COVID-19 infection. In some circumstances, the coinfections, including both the infections, can have some serious consequences or result in death (Hashemi et al., 2020; Ma et al., 2020). Unfortunately, both COVID-19 and seasonal influenza individually carry significant risks for elders and those having chronic comorbidities (Kostas et al., 2020; Petrilli et al., 2020). Nonspecific immunity caused by spontaneous influenza infection may reduce the risk of infection with other respiratory viruses, while the influenza vaccine protects against influenza infection. Not being infected with influenza, as a result of the vaccine, may lead to an increase in the incidence of other respiratory diseases, according to the so-called phenomenon of viral interference, due to the lack of specific immunity acquired from spontaneous infection (Cowling et al., 2012; Cowling and Nishiura, 2012; Feng et al.; Suzuki et al., 2014). Importantly, limited evidence supports the hypothesis that the virus interference process may be associated with the influenza vaccine (Cowling et al., 2012; Rikin and Stockwell, 2018). On the other hand, the influenza vaccination may boost influenza immunity at the expense of SARS-CoV-2 immunity due to some unknown biological mechanism for the noninfluenza respiratory virus (Cowling et al., 2012). Many other studies do not show the correlation between increased respiratory virus risk and influenza vaccination (Cowling and Nishiura, 2012; Sundaram et al., 2013). The influenza vaccine, on the other hand, was found to have cross-protective effects against heterologous illnesses. The long-term enhancement of innate immunity (trained immunity) was the underlying mechanism. Several epidemiological research studies have shown that influenza vaccination and COVID-19 have a cross-protective impact during the current epidemic. However, the mechanism responsible for this effect is undetermined (Debisarun et al., 2020). Previous studies have also found a link between influenza vaccination and COVID-19-related mortality, which might be attributable to heterologous immunity or alterations in innate immunity. Heterologous immunity can cause immunizations to have nonspecific effects that impact other illnesses and disorders, such as boosting the vaccine’s protective impact (Agrawal, 2019; Goodridge et al., 2016). Even with the broad use of influenza vaccines, information about the role of the influenza vaccine on the outcome of COVID-19 and other infections is contradictory. The current study intends to investigate if there is a link between influenza vaccination and the incidence of COVID-19 infection and illness severity; nevertheless, a negative link was predicted.
PATIENTS AND METHODS
People who are working in the Iraqi healthcare sector and some medical students were selected as the sample population for the study. It involved physicians, dentists, pharmacists, and medical faculties, including students, who were previously cured of COVID-19 infections during the last few weeks. Because no electronic data about the COVID-19 patients were available in Iraqi hospitals and special centers for COVID-19 management, we depended on the questionnaire to get information from the participants. A total of 1,476 subjects were included in the current study. The age of the participants who were included in the study ranged between 20 and 55 years. The participants was divided into two main groups; group one included 194 participants who had received the influenza vaccination during the previous flu season and group two included 1,281 participants who had not received the influenza vaccine. The primary outcome of interest was COVID-19 incidence (diagnosed by nasopharyngeal swab), with rates compared between the first and second groups of patients, depending on vaccination status. To compare the effect of the influenza vaccine on the severity of COVID-19 infection, 102 patients from the whole sample study were selected: from group one, 52 patients who had taken the influenza vaccine compared with 50 patients from group two who did not receive the influenza vaccine. They were chosen because they were recently cured of COVID-19 infection and were very cooperative and accessible to get more accurate information about the severity of the infection. The clinical outcomes, including hypoxia [who have saturation of oxygen (SpO2) <90% on room air measured by a pulse oximeter on the finger by clinician 2–3 times a day], depended on the WHO definition and the need for hospitalization or mechanical ventilation. All the participants who had been positive for COVID-19 were completely cured at the time of the study and nasopharyngeal swabs had been negative. The current study tried to evaluate to what extent receiving the influenza vaccine would affect the COVID-19 infection rate and severity.
Statistical analysis
Chi-squared test was used to measure p-value; Z-test was used to compare the significant differences between the percentage of different groups; odds ratio was used to determine the effect of influenza vaccination on the COVID-19 infection rate and severity between the study groups. A two-sided p-value ≤ 0.05 was used to indicate significance.
RESULTS
The results showed that there was a high percentage (86.8%) of medical staff and medical students who had not received the influenza vaccine compared to a very low percentage (13.15%) of subjects from the same population who received the vaccine (Fig. 1).
Comparing the percentage of COVID-19 patients in the vaccinated group with the unvaccinated group did not show statistical significance (p-value > 0.05) (Fig. 2).
The findings also revealed that there was no statistically significant difference in COVID-19 infection rates between the vaccinated and unvaccinated groups, and that COVID-19 incidence was nonsignificantly higher in patients who got the influenza vaccination compared to those who did not (odds ratio = 1.20, 95% CI = 0.890–1.630, p-value > 0.05) (Fig. 3 and Table 1).
Within the influenza vaccinated group, the COVID-19 patients were nonsignificantly lower than the noninfected odds, while within the unvaccinated group, the COVID-19 patients were significantly lower than the noninfected odds (Fig. 3).
Interestingly, the outcome of the current study showed no significant association between gender and influenza vaccination status on the percentages of COVID-19 incidence (odds ratio = 1.294, 95% CI = 0.837–2.001, p-value > 0.05). A significant (p < 0.05) decrease had been seen only in the odds of male patients compared to females in the unvaccinated group (Fig. 4).
Importantly, the results determined that hypoxia and need for hospitalization were significantly less among influenza vaccinated patients [1 out of 52 patients (1.9%)] in comparison with unvaccinated patients [9 out of 50 patients (18%)] (odds ratio = 0.08, 95% CI = 0.01–0.7, p-value < 0.05) (Fig. 5).
DISCUSSION
The medical staff and students are the subjects of the present study and have been chosen as they represent the first line of defense against COVID-19. On the other hand, this segment of the society is more susceptible to getting infected due to direct contact with COVID-19 patients. Our results showed that a very small percentage of the participants received the influenza vaccine. Influenza is not considered a critical illness in Iraq due to lack of proper awareness, in addition to the hot climatic nature of Iraq and short winter season in which the temperature rarely reaches zero. Also, the availability of the influenza vaccine reduces the percentage of vaccinated subjects. Our findings, contrary to predictions, do not support earlier research which found a negative relationship between COVID-19 incidence and influenza vaccination in several clinical investigations. The results showed that the incidence of COVID-19 infection was nonsignificantly higher in the influenza vaccinated group than in the unvaccinated group. This could be because many influenza vaccinated subjects were given the vaccine due to medical problems or chronic diseases that required vaccination. In addition, many of them were highly susceptible to the influenza virus and other respiratory viruses. Those subjects may be also susceptible to COVID-19 because of the similarity between type and route of transmission of the two viral diseases. Although a nonsignificant difference was reported in the infection rate between the study groups, outcomes of the current study are in line with the clinical investigation carried out by Lisewki (2020), which showed that there was an increase in COVID-19 risk in 28 OECD countries due to influenza vaccination. In Europe and the United States, a recent study found a clear positive link between vaccination rate and the risk of COVID-19 infection among the elderly (65 years of age) (Consortium, 2020). Influenza vaccinations give protection against influenza infection but not against other viral illnesses in patients who have received the vaccination because nonspecific immunity fades with time (Cowling et al., 2012), perhaps due to virus interference (Isaacs and Lindenmann, 1957; Seppälä et al., 2011; Wolff, 2020). Even though the vaccine adjuvant has a high degree of safety, particularly the influenza vaccine adjuvant should be examined for adverse responses in COVID-19 patients, such as rise in inflammatory markers, which are already high with COVID-19 (Petrovsky, 2015; Qin et al., 2020). According to Pawlowski et al. (2020), acquired immunity from general vaccines, such as measles, rubella, mumps, varicella, hepatitis A and B, geriatric flu, pneumococcal conjugate (PCV13), polio, Haemophilus influenzae type B 1–5 years, prior to the pandemic resulted in lower rates of infection with SARS-CoV-2. Results from the present study determined that influenza vaccination significantly decreases the need for oxygen therapy and hospitalization. Interestingly, these results indicate that the influenza vaccination may have a role in reducing disease severity. Importantly, many clinical studies support the beneficial impacts of influenza vaccination to reduce mortality and morbidity of COVID-19, indicated by decreasing hospitalization and progression of the disease to severely critical stages. SARS-CoV-2 and influenza viruses share a property, which is enveloped RNA with similar entrance and transmission mechanisms and clinical characteristics that overlap. Recent studies (Arokiaraj, 2020) in Italy (Marin-Hernández et al., 2020) and the United States have found an adverse link between influenza vaccine and COVID-19 mortality at the country level. Several linked or separate causes for the influenza vaccine’s putative protection against COVID-19 infection have been shown. This may happen if another respiratory virus such as SARS-CoV-2 triggers enough trained innate immune memory to prepare the local immune system of the lung for a rapid response, which might impact the recovery of the COVID-19 infection course. A study by Marín-Hernández et al. (2020) showed the epidemiological evidence of an association between increased influenza vaccination rate in elderly individuals and reduced COVID-19-related mortality in Italy. High morbidity and mortality have been linked to an adverse connection between influenza vaccination rates and COVID-19. Furthermore, earlier research has connected the influenza vaccination’s potential to reduce COVID-19 infection severity to the cessation of influenza-SARS-CoV-2 coinfections (Arokiaraj, 2020) and tendency to shift in innate immunity (Netea et al., 2020). Similarly, research in OECD countries found a negative relationship between immunization rate and COVID-19-linked morbidity and death in those over 65 years (Arokiaraj, 2020). An Italian study found that the influenza vaccination decreased hospitalization rates, intensive care admissions, and mortality of COVID-19 in the elderly (Amato et al., 2020). Zanettini et al. (2020) found that the mortality rate of the COVID-19 disease decreased by 28% after recording a 10% increase in vaccination. Another study carried out in Brazil found that those at risk of severe COVID-19 illness may benefit greatly from influenza immunization and have a higher probability of surviving and requiring less extensive hospital care than those who did not receive the vaccine recently. Late vaccination boosts the innate immune response, which may contribute to quick and powerful SARS-CoV-2 clearance, avoiding lower lung tissue invasion and/or suppressing the detrimental proinflammatory host response that occurs at the crucial stage of COVID-19 sickness (Fink et al., 2020). Furthermore, in an established in vitro model, it has been demonstrated that the tetravalent inactivated influenza vaccination develops trained immunity and improves immune cell responsiveness to SARS-CoV-2 activation (Kleinnijenhuis et al., 2012). An increase in cytokines has been reported after the activation of white blood cells by the influenza vaccine (Debisarun et al., 2020). This response by cytokines was more specific compared to the response generated by BCG. It has been shown to prevent systemic inflammation and reduce the viral load associated with the microactivation of cytokines (Tanaka et al., 2014).
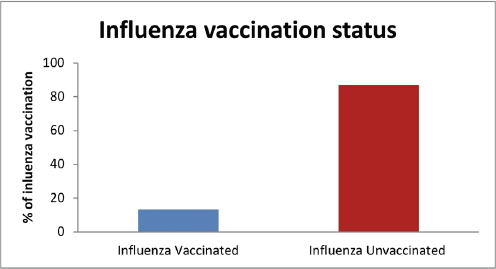 | Figure 1. Percentages of vaccinated and unvaccinated members of medical staff and students included in the study. [Click here to view] |
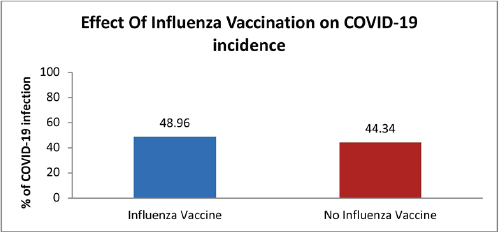 | Figure 2. COVID-19 infection incidence in influenza vaccinated and unvaccinated subjects (p- value > 0.05). [Click here to view] |
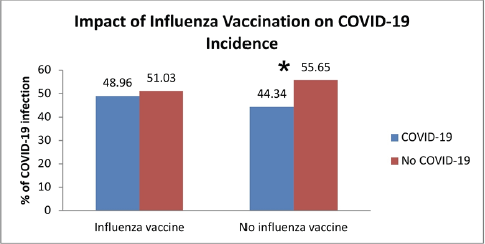 | Figure 3. Comparison of COVID-19 infection rates in influenza vaccinated and unvaccinated subjects. [Click here to view] |
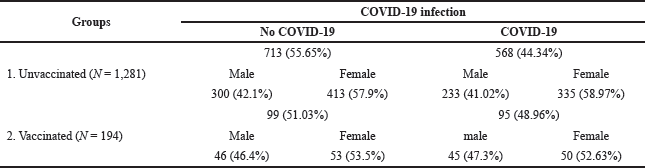 | Table 1. Percentages of vaccinated and unvaccinated subjects who have COVID-19. [Click here to view] |
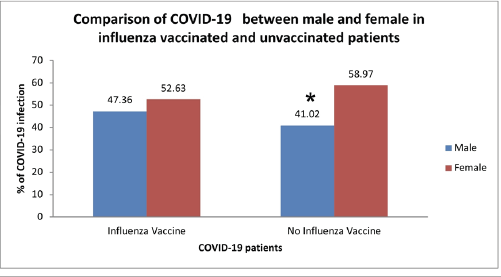 | Figure 4. Comparison between males and females in the influenza vaccinated and unvaccinated groups. [Click here to view] |
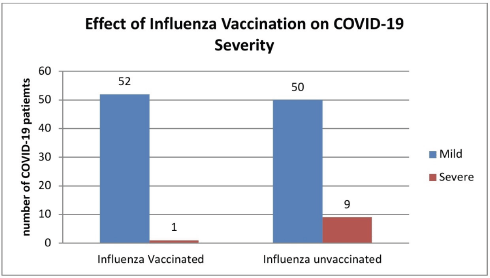 | Figure 5. The effect of influenza vaccine on COVID-19 severity. [Click here to view] |
Anti-inflammatory cytokines, like interleukin (IL)-1Ra, are critical for regulating and preventing inflammation. When influenza vaccination and BCG were given, clinical research found that significant levels of IL-6 and IL-1Ra were produced. This supports the idea that these cytokines have a role in keeping the participants’ inflammatory condition in check (Moorlag et al., 2018). Inactivated influenza vaccination stimulates the innate immune system by activating the toll-like receptor 7, which increases IL-6 and tumor necrosis factor production in peripheral white blood cells in response to antigen challenge and causes functional changes in the natural killer cell compartment for several weeks to months (Fink et al., 2020). In conclusion, influenza immunization may reduce the severity of COVID-19 and the negative results, but it does not reduce the occurrence.
CONCLUSION
In this short study, nonsignificant differences in the odds infected with COVID-19 who got the influenza vaccine compared to those who did not get the vaccine are demonstrated. The results also showed no significant association between gender and influenza vaccination status on the percentages of COVID-19 incidence. In addition, the COVID-19 patients who previously received the influenza vaccine had significantly better clinical outcomes; lower complication rates and less hospitalization and hypoxia were noted. The influenza vaccination may have a role in reducing the burden of the COVID-19 disease severity.
ACKNOWLEDGMENT
The authors acknowledge the participants in this study.
ETHICAL APPROVAL
The present study was approved by the Medical Ethical Committee of the College of Medicine in Wasit University. The ethical number is A/123.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conceptualization, study design, data acquisition and interpretation, took part in writing-original draft, revising, and final approval for the version to be published and agree to be accountable for all aspects of the work.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agrawal B. Heterologous immunity: role in natural and vaccine-induced resistance to infections. Front Immunol, 2019; 10:164. CrossRef
Amato M, Werba JP, Frigerio B, Coggi D, Sansaro D, Ravani A, Ferrante P, Veglia F, Tremoli E, Baldassarre D. Relationship between influenza vaccination coverage rate and COVID-19 outbreak: an Italian Ecological Study. Vaccines (Basel), 2020; 8(3). CrossRef
Arokiaraj MC. Correlation of influenza vaccination and influenza incidence on COVID-19 severity and other perspectives. SSRN, Rochester, NY, 2020. CrossRef
Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect, 2020; 9:221–36; doi: 10.1080/22221751.2020.1719902 CrossRef
Consortium E. COVID-19 Severity in Europe and the USA: Could the seasonal influenza vaccination play a role? SSRN, Rochester, NY, 2020. CrossRef
Cowling B.J., Nishiura H. Virus interference and estimates of influenza vaccine effectiveness from test-negative studies. Epidemiology, 2012; 23(6):930–1. CrossRef
Cowling BJ, Fang VJ, Nishiura H, Chan KH, Sophia Ng, Dennis K M Ip, Chiu SS, Leung GM, Peiris JSM. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis, 2012; 54:1778–83. CrossRef
Debisarun PA, Struycken P, Domínguez-Andrés J, Moorlag SJCFM, Taks E, Gössling KL, Ostermann PN, Müller L, Schaal H, ten Oever J, van Crevel R, Netea MG. The effect of influenza vaccination on trained immunity: impact on COVID-19. medRxiv; doi:10.1101/2020.10.14.20212498
Feng S, Fowlkes A, Steffens A, Finelli L, Cowling B. Assessment of virus interference in a test-negative study of influenza vaccine effectiveness. Epidemiology, 2017; 28(4):514–24. CrossRef
Fink G, Orlova-Fink N, Schindler T, Grisi S, Ferrer AP, Daubenberger C, Brentani A. Inactivated trivalent influenza vaccine is associated with lower mortality among COVID-19 patients in Brazil. BMJ Evid Based Med, 2020; doi: 10.1136/bmjebm-2020-111549 CrossRef
Goodridge HS, Ahmed SS, Curtis N, Kollmann TR, Levy O, Netea MG, Pollard AJ, Van Crevel R, Wilson CB. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol, 2016; 16(6):392–400. CrossRef
Hashemi SA, Safamanesh S, Ghafouri M, Taghavi MR, Mohajer Zadeh Heydari MS, Namdar Ahmadabad H. Co-infection with COVID-19 and influenza A virus in two died patients with acute respiratory syndrome, Bojnurd. Iran. J Med Virol, 2020; doi: 10.1002/jmv.26014 CrossRef
Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond Ser B Biol Sci, 1957; 147(927):258–67. CrossRef
Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L, CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med, 2015; 373:415–27. CrossRef
Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, Jacobs C, Loenhout J, Jong D, Stunnenberg HG, Xavier RJ, van der Meer JWM, van Crevel R, Netea MG. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA, 2012; 109(43):17537–42. CrossRef
Kostas D, Laure F, Scarlett G, Côme D, Sibylle BS, Lisa D. High impact of COVID-19 in long-term care facilities, suggestion for monitoring in the EU/EEA, May 2020. Euro Surveill, 2020; 25(22); doi: 10.2807/1560-7917.ES.2020.25.22.2000956 CrossRef
Lisewski A M, Association between Influenza Vaccination Rates and SARS-CoV-2 Outbreak Infection Rates in OECD Countries. SSRN, 2020. https://ssrn.com/abstract=3558270 CrossRef
Lovato A, de Filippis C, Marioni G. Upper airway symptoms in coronavirus disease 2019 (COVID-19). Am J Otolaryngol, 2020; 102474. CrossRef
Ma S, Lai X, Chen Z, Tu S, Qin K. Clinical characteristics of critically ill patients co-infected with SARS-CoV-2 and the influenza virus in Wuhan. China. Int J Infect Dis, 2020; 96:683–7. CrossRef
Marín-Hernández D, Schwartz RE, Nixon DF. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J Med Virol, 2020; doi: 10.1002/jmv.26120 CrossRef
Moorlag S, Röring RJ, Joosten LAB, Netea MG. The role of the interleukin-1 family in trained immunity. Immunol Rev, 2018; 281(1):28–39. CrossRef
Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis TJ, Divangahi M, Fuchs E, Joosten LAB, Van der Meer JWM, Mhlanga MM, Mulder WJM, Riksen NP, Schlitzer A, Schultze JL, Benn CS, Sun JC, Xavier RJ, Latz E. Defining trained immunity and its role in health and disease. Nat Rev Immunol, 2020; 20(6):375–88. CrossRef
Paranjpe I, Russak A, De Freitas JK, Lala A, Miotto R, Vaid A, Johnson KW, Danieletto M, Golden E, Meyer D, Singh M, Somani S, Manna S, Nangia U, Kapoor A, O’Hagan R, O’Reilly PF, Huckins LM, Glowe P, Kia A, Timsina P, Freeman RM, Levin MA, Jhang J, Firpo A, Kovatch P, Finkelstein J, Aberg JA, Bagiella E, Horowitz CR, Murphy B, Fayad ZA, Narula J, Nestler EJ, Fuster V, Cordon-Cardo C, Charney DS, Reich DL, Just AC, Bottinger EP, Charney AW, Glicksberg BS, Nadkarni GN. Clinical characteristics of hospitalized COVID-19 patients in New York City. medRxiv. 2020; doi: 10.1101/2020.04.19.20062117 CrossRef
Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R,Costa F, Scarlata S, Agrò FE. COVID-19 diagnosis and management: a comprehensive review. J Intern Med, 2020; 288(2):192–206. CrossRef
Pawlowski C, Puranik A, Bandi H, Venkatakrishnan AJ, Agarwal V, Kennedy R, O’Horo JC, Gores GJ, Williams AW, Halamka J. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci Rep, 2021; 11(1):4741. CrossRef
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’ Donnell L, Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. Br Med J; 2020; 22(369):m1966. CrossRef
Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf, 2015; 38(11):1059–74. CrossRef
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis, 2020; 71(15):762–8. CrossRef
Ramaiah A, Arumugaswami V. Insights into cross-species evolution of novel human coronavirus 2019-nCoV and defining immune determinants for vaccine development. bioRxiv. 2020 Jan 30; doi: 10.1101/2020.01.29.925867 CrossRef
Rikin S, Jia H, Stockwell M. Assessment of temporally-related acute respiratory illness following influenza vaccination. Vaccine, 2018; 36:1958–64. CrossRef
Seppälä E, Viskari H, Hoppu S, Honkanen H, Huhtala H, Simell O, Ilonen J, Knip M, Hyöty H. Viral interference induced by live attenuated virus vaccine (OPV) can prevent otitis media. Vaccine, 2011; 29(47):8615–8. CrossRef
Sundaram ME, McClure DL, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis, 2013; 57:789–93. CrossRef
Suzuki M., Camacho A., Ariyoshi K. Potential effect of virus interference on influenza vaccine effectiveness estimates in test-negative designs. Epidemiol Infect, 2014; 142:2642–6. CrossRef
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring HarbPerspect Biol, 2014; 6(10):a016295. CrossRef
Wolff GG. Influenza vaccination and respiratory virus interference among Department of Defense personnel during the 2017–2018 influenza season. Vaccine, 2020; 38(2):350–4. CrossRef
Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe, 2020; 27:325–8; doi: 10.1016/j.chom.2020.02.001. CrossRef
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for disease control and prevention. JAMA, 2020; doi:10.1001/jama.2020.2648 CrossRef
Zanettini, C. Omar M, Dinalankara W, Imada EL, Colantuoni E, Parmigiani G, Marchionni L. Influenza vaccination and COVID-19 mortality in the USA: an Ecological study. Vaccines, 2021; 9(5):427. CrossRef
Zhu J, Zhong Z, Ji P, Li H, Li B, Pang J, Zhang J, Zhao C. Clinicopathological characteristics of 8697 patients with COVID-19 in China: a meta-analysis. Fam Med Commun Health, 2020; 8(2):e000406. CrossRef