INTRODUCTION
Remifentanil is a novel short-acting μ-opioid receptor agonist with a similar analgesic and sedative potency as fentanyl (Burkle et al., 1996). However, remifentanil is rapidly metabolized and has a very short duration of action (Egan et al., 1993; Joshi et al., 2002). Remifentanil has a rapid onset of action (about 1 minute) and a rapid offset of action following discontinuation (about 3–10 minutes) (Battershill and Keating, 2006). Remifentanil is approximately 70% bound to plasma proteins, and its average clearance of remifentanil is about 40 ml/minute/kg (Kimia Farma, 2019). Around the world, remifentanil is mainly used for induction and maintenance in general anesthesia (Scott and Perry, 2005) and as an analgesic and sedative in the intensive care unit setting (Battershill and Keating, 2006). Remifentanil’s rapid peak impact in the operating room makes it easier for users to perceive the anesthetic effect’s dose-effect relationship. The quick onset of remifentanil also aids in detecting the presence of an excessive dose, which manifests as hypotension, hypoventilation, and bradycardia shortly after administration (Scott and Perry, 2005).
Based on a large post-marketing phase IV study in the US, adverse events following the administration of remifentanil are similar to those after fentanyl (Joshi et al., 2002). Compared to fentanyl, remifentanil has organ independent mode of metabolism, which makes a good choice of opioid for use in renal-impaired patients (Muellejans et al., 2004). Furthermore, a meta-analysis of the randomized controlled trial (RCT) suggested that remifentanil can benefit cardiac surgery with a decreased mechanical ventilation duration, cardiac biomarker release, and hospitalization duration. Hence, remifentanil can be used as an alternative to fentanyl with the additional advantages of short-acting potency and organ independent mode of metabolism (Greco et al., 2012). However, it was suggested that remifentanil has a higher risk of hypotension compared to fentanyl (Joshi et al., 2002), and the safety profile of remifentanil in the Indonesian population through a post-marketing study has not been evaluated yet. Therefore, this study aims to evaluate the safety profiles of remifentanil in a multicenter pharmacovigilance study in Indonesia.
MATERIALS AND METHODS
A post-marketing study of remifentanil was conducted in six referral hospitals in Indonesia (three hospitals in Jakarta, two in Surabaya, and one in Banjarmasin) during the period of June–September 2019. The use of remifentanil and its adverse events were observed until discharge. The remifentanil used in this study was in a concentrate powder for a solution with vial packages of 1, 2, and 5 mg of remifentanil hydrochloride. Remifentanil must be further diluted after reconstitution. The administration route was via a continuous infusion recommended with an infusion device (Kimia Farma, 2019).
The design of this study is a single-arm cohort study that targeted two types of hospitalized patients for whom remifentanil was indicated, which include patients on intubation or invasive mechanical ventilation and patients on noninvasive ventilation (NIV). For patients who are intubated or on invasive mechanical ventilation, remifentanil is used for its sedative and analgesic properties and as an alternative to fentanyl. The dose used for this type of indication is 0.05–0.5 mg/kg/minute with a 100 mg/ml concentration solution and close monitoring due to potential reduced respiratory drive. Patients on NIV and patients with Richmond Agitation-Sedation Scale (RASS) >0 and who are non-compliant with NIV can have remifentanil. This may help with compliance with therapy and prevent the need for intubation. The dose used for this indication is 0.01–0.15 mg/kg/minute with a 50 mg/ml concentration. The absolute contraindication of the use of remifentanil includes previous anaphylaxis/allergy to remifentanil or fentanyl analogs. The relative contraindications among patients on intubation or invasive mechanical ventilation include hypotension and bradycardia (pulse rate < 50 bpm), while the relative contraindications among patients on NIV include RASS <0, reduced respiratory rate (RR<10 times/minute), hypotension, and bradycardia. Investigators observed any adverse event that occurred within five consecutive days. Study nurses monitored the vital signs closely while patients were asked to report any symptoms. Provided with the details of health records and concomitant medications, the expert panel team assessed the causality of adverse events using the modified World Health Organization-Uppsala Monitoring Center system that consists of six categories: certain, probable, possible, unlikely, unclassified, and unassessable.
Using a Poisson distribution, power of 80%, and assumed risk ratio of occurring adverse events of 1.5, we calculated the sample size of a minimum of 36 patients. The baseline characteristics, including comorbidity events, were collected, and then the drug administration details (dose, route of administration, and device use) were collected during follow-up. Finally, all adverse events and management from health records and patient reports were recorded as the endpoints. We also collected concomitant medications during the follow-up to address potential sources of bias or confounding factors. All missing data were recorded and reported as missing and were not included in the analysis. At the same time, all lost-to-follow-up patients were still analyzed and reported for any available data. No sensitivity and subgroup analyses were conducted in this study.
RESULTS
Baseline characteristics
There were 42 patients between June and September 2019 included in this Post Marketing Surveillance (PMS) of remifentanil. The patients were mostly adult, with mean age 43.9 years, at a balance between male and female, mostly recorded from Pusat Otak Nasional (PON) Hospital (41%), with mostly neurological and malignancy main diagnosis (57%). No lost-to-follow-up patients were reported. Comorbidities were present in up to one-third of all participants, with the highest proportion being those with diabetes and cardiovascular diseases such as hypertension, heart disease, and arterial diseases (55%). The characteristics of patients who used remifentanil in this post-marketing study can be seen in Table 1.
Drug administration
The characteristics of remifentanil used during this post-marketing study were mostly with dosage 2 mg (64.3%), using it with a device (90.5%), and administering it through injection or infusion. The dose package used by the hospitals was mostly one dose (66.7%). The characteristic use of remifentanil can be seen in Table 2.
Concomitant medications
There were 11 types of anesthetic agents concomitantly used with remifentanil during the PMS, which are included into five subclasses of agents. A total of 114 drugs have been administered. There were 71 (62.3%) of these drugs used with a device and 43 (37.7%) without, with 107 drugs (94%) administered parenterally and seven drugs (6%) given by inhalation. Duration of use (in minutes) of these anesthetic drugs was 1 minute (minimum-maximum: 1–335 minutes). The type of the anesthetic drugs used during surgery can be seen in Table 3.
There were 24 types of concomitant medications used during the PMS, which are included in nine classes of agents. A total of 84 drugs have been administered and were mostly administered parenterally (94%), and only five drugs (6%) were given orally. The duration of use of these comedications was 1 day (minimum-maximum: 1–2 days). The type of other concomitant medications used during surgery can be seen in Table 4.
Safety profile
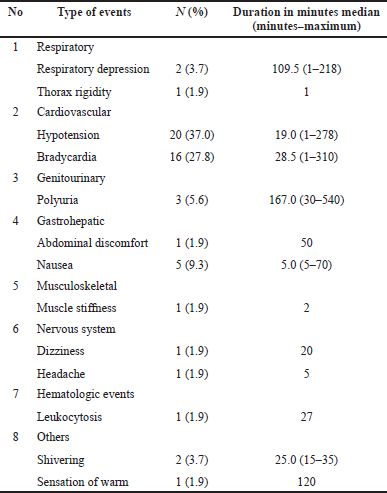
There were 54 adverse events recorded in 35 out 42 patients (83%) during the observation period, or 1.3 adverse events per observed patient. There were a total of 13 types of adverse events. In this study, the severity of the adverse events was not recorded. The types of adverse events during the use of remifentanil can be seen in Table 5.
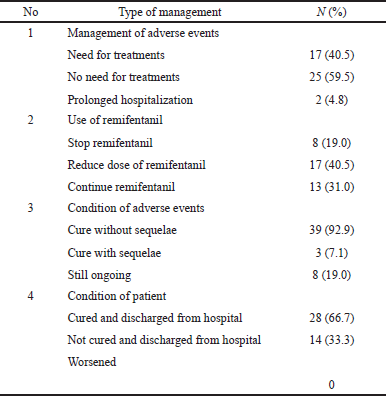
Table 6 explains how the anesthetist/physician managed the adverse events that occurred during the use of remifentanil. Most patients with adverse events were not receiving additional treatment and others were receiving additional treatment or prolonged hospitalization. Most of the physicians reduced the dose during surgery, and one out five patients stopped using remifentanil. There is no information on how much the dosages have been reduced for each patient with this adverse event (AE). Almost all patients were cured of these adverse events without any sequelae, and 67% of patients were discharged from the hospital.
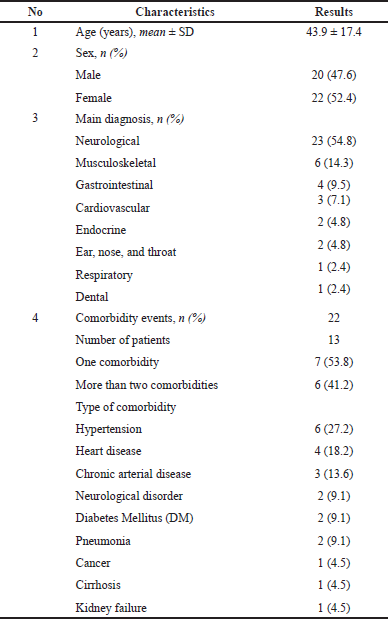 | Table 1. Baseline characteristics of patients. [Click here to view] |
DISCUSSION
Among 42 patients who used remifentanil within the 3 months in 6 hospitals, we found 35 patients (83%) have experienced at least one adverse event. This proportion was higher than the one was found in another phase IV study by Joshi et al. (2002), where about 52% of the inpatients using remifentanil had at least one adverse event (Joshi et al., 2002). There were 54 adverse events recorded during the observations, with hypotension and bradycardia being the most common adverse events, followed by nausea, respiratory depression, dizziness, shivering, warm sensation, and muscle rigidity. Despite a long list of adverse events, the risk of serious adverse events which need further management appears to be low in this study, with less than 5% of the total incidence. Most of the adverse events (59.5% of patients) that occurred did not need further treatment/management in this study. However, there were eight (19%) patients who needed to stop remifentanil. The expert panel meeting concluded that, from those adverse events, 98% were possibly related to remifentanil and only one adverse event was unlikely related.
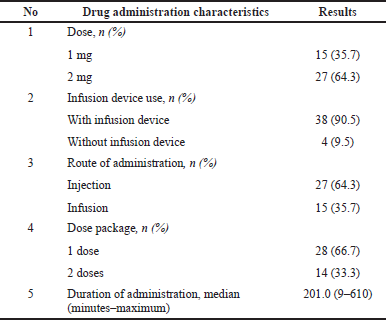 | Table 2. Drug administration characteristics. [Click here to view] |
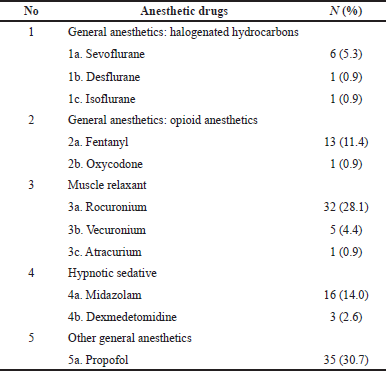 | Table 3. Concurrent anesthetic drugs. [Click here to view] |
Among these adverse events, hypotension and bradycardia have the commonest occurence in this study, contributing 37.0% and 27.8%, respectively. These proportions are higher than what Joshi et al. (2002) found, where hypotension and bradycardia occurred among 477 inpatient surgeries using remifentanil at percentages of only 18% and 3%, respectively (Joshi et al., 2002). However, a study by Myles et al. (2002) with a small number of patients also found higher proportions, where 17% of patients had bradycardia and 66% patients had hypotension following remifentanil administration intraoperatively (Myles et al., 2002). Hypotension and bradycardia are actually more common in remifentanil than in other kinds of anesthesia. Komatsu et al. (2007) found that when remifentanil is used as a general anesthetic, the risk of bradycardia and hypotension is doubled, compared to other short-acting opioids such as fentanyl, alfentanil, or sufentanil (Komatsu et al., 2007). However, these hemodynamic events seem to be dose-dependent (Joshi et al., 2002). Therefore, close monitoring with the higher and lower doses is recommended during the administration of remifentanil. There is no difference in the occurrence of mild adverse events such as nausea and vomiting between remifentanil and other agents (Komatsu et al., 2007).
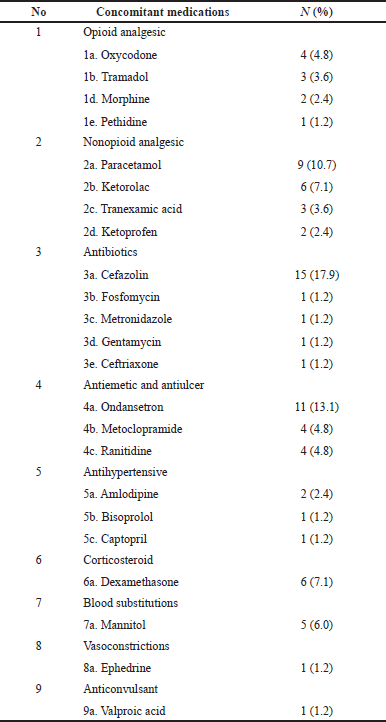 | Table 4. Concomitant medications recorded during observation. [Click here to view] |
 | Table 5. The adverse events reported. [Click here to view] |
In this study, thorax and muscle rigidity occurred in two (3.8%) remifentanil-treated patients. The concern of thorax and muscle rigidity is lower based on another large multicenter phase IV study where four (0.3%) remifentanil-treated patients experienced thorax and muscle rigidity compared to none among patients treated with fentanyl (Joshi et al., 2002). Post-marketing surveillance is important because of the range of observed patients and the capability to detect even rare adverse events (Joshi et al., 2002). Remifentanil seems to have potency as an alternative to fentanyl as long as the known negative event risks can be detected and managed properly.
Most of the adverse events recorded during the use of remifentanil have been known as part of adverse drug reactions. The clinical trial of remifentanil has reported the following adverse drug reactions, including common adverse drug reactions, important identified risks, and important potential risks (Joshi et al., 2002; Myles et al., 2002). Common adverse drug reactions include bradycardia, muscle rigidity, nausea, vomiting, shivering, hypotension, nausea and vomiting, drowsiness, and dizziness. For important identified risks, remifentanil showed cardiovascular effects such as hypotension and bradycardia, respiratory depression, drug dependence, and withdrawal syndrome. There are drug abuse/misuse and cardiac arrhythmia under important potential risks. However, another multicenter phase IV study suggested that reducing the initial dose of remifentanil might prevent hemodynamic side effects (Joshi et al., 2002).
 | Table 6. The management of adverse events. [Click here to view] |
Though remifentanil has potential adverse events, it has some benefits. Compared to other anesthesia drugs such as fentanyl, remifentanil demonstrates faster clearance, can deliver a stable intraoperative course (Twersky et al., 2001), and is safe for elective surgery such as a coronary artery bypass graft (Hillel et al., 1999). According to a RCT in Turkey, remifentanil can be used until the end of surgery, which benefits surgeries with high levels of stimulation or with unknown duration (Ozkose et al., 2002). Furthermore, compared to fentanyl, remifentanil has the advantage of better functional assessments (walking without being dizzy, clear thinking, concentrating, and good communication) within the first 24 hours after anesthesia based on a large RCT (Fleisher et al., 2001). A systematic review also adds up that remifentanil might be useful for patients at risk of intraoperative awareness or obstructive sleep apnea or patients with early ambulation (Komatsu et al., 2007).
This study observed the range of patients from six different hospitals in Indonesia. The limitations of this study include the minimal sample sizes that do not allow subgroup analysis and the considerable short period of follow-up. The results might differ from the results in populations other than Indonesians that were included in this study.
CONCLUSION
Based on this pharmacovigilance study, there are no additional safety concerns related to remifentanil as an analgesic and sedative drug indicated for patients on intubation, invasive mechanical ventilation, and NIV. No evidence emerged of previously unknown side effects. Nevertheless, further study with larger patient groups considering potential subgroup analysis and a longer period of observation is needed to observe the new rare and delayed adverse drug reaction of remifentanil.
ACKNOWLEDGMENT
The authors would like to thank all the hospital directors and research teams. Finally, they would like to show their gratitude to all the participants and families who participated in this study.
CONFLICT OF INTEREST
All authors declare no conflicts of interest regarding this article.
FUNDING
PT Kimia Farma Tbk, a state-owned pharmaceutical company in Indonesia, funded this study as part of the post-marketing activities.
ETHICAL APPROVAL
The study was held in compliance with the International Conference on Harmonization-Good Clinical Practice (ICH-GCP) guideline and the Declaration of Helsinki. Informed consent was obtained from all participants prior to study procedures. The protocol has been approved by the ethics committee (approval no. KE/FK/1155/EC/2017).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
AUTHOR CONTRIBUTIONS
Authors JAT, JHH, and LAC are responsible for original design, study conduct, causality assessment, data collection, data analysis, and article writing.
DISCLOSURE
Authors NH, RKD, RP, and DJP are PT Kimia Farma Tbk employees, which funded this study. However, the funder does not have any involvement in or influence on the study outcome. The funder was not involved during the process of study, and all the investigators are independent.
REFERENCES
Battershill AJ, Keating GIM. Remifentanil: a review of its analgesic and sedative use in the intensive care unit. Drugs, 2006; 66(3):365–85; doi:0012-6667/06/0003-0365/$44.95/0 © CrossRef
Burkle H, Dunbar S, Van Aken H. Remifentanil: a novel, short-acting, μ-Opioid. Anesth Analg, 1996; 83(3):646–51; Available via https://journals.lww.com/anesthesia-analgesia/fulltext/1996/09000/remifentanil__a_novel,_short_acting,_mu_opioid.38.aspx CrossRef
Egan TD, Lemmens HJM, Fiset P, Hermann DJ, Muir KT, Stanski DR, Shafer SL. The pharmacokinetics of the new short-acting opioid remifentanil (GI87084B) in healthy adult male volunteers. Anesthesioology, 1993; 79:881–92. CrossRef
Fleisher LA, Hogue S, Colopy M, Twersky RS, Warner DS, Jamerson BD, Tuman KJ, Glass PSA, Roizen MF. Does functional ability in the postoperative period differ between remifentanil- and fentanyl-based anesthesia? J Clin Anesth, 2001; 13(6):401–6; doi:10.1016/S0952-8180(01)00291-4 CrossRef
Greco M, Landoni G, Biondi-Zoccai G, Cabrini L, Ruggeri L, Pasculli N, Giacchi V, Sayeg J, Greco T, Zangrillo A. Remifentanil in cardiac surgery: a meta-analysis of randomized controlled trials. J Cardiothor Vasc Anesth, 2012; 26(1):110–6; doi:10.1053/j.jvca.2011.05.007 CrossRef
Hillel Z, Howie M, Hogue C, Haddow G, Fitch J, Bowdle A, Nielsen V, Bukenya D. A multicenter trial comparing the safety and efficacy of remifentanil and fentanyl in elective CABG surgery patients. Anesthesia & Analgesia. 1999 Apr 1;88(4S):76SCA. doi:10.1097/00000539-199904001-00076 CrossRef
Joshi GP, Warner DS, Twersky RS, Fleisher LA. A comparison of the remifentanil and fentanyl adverse effect profile in a multicenter phase IV study. J Clin Anesth, 2002; 8180(02):494–9. CrossRef
Kimia Farma. Remikaf powder for concentrate for solution for injection or infusion. Kimia Farma, 2019. Available via http://pionas.pom.go.id/sites/default/files/obat_baru/Remikaf Serbuk Injeksi 5 mg_Remifentanil Hidroklorida_DNI1849900144C1_2019.pdf
Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia, 2007; 62(12):1266–80; doi:10.1111/j.1365-2044.2007.05221.x CrossRef
Muellejans B, López A, Cross MH, Bonome C, Morrison L, Kirkham AJT. Remifentanil versus fentanyl for analgesia based sedation to provide patient comfort in the intensive care unit: a randomized, double-blind controlled trial [ISRCTN43755713]. Crit Care (Lond, Engl), 2004; 8(1):1–11; doi:10.1186/cc2398 CrossRef
Myles PS, Hunt JO, Fletcher H, Watts J, Bain D, Silvers A, Buckland MR. Remifentanil, fentanyl, and cardiac surgery: a double-blinded, randomized, controlled trial of costs and outcomes. Anesth Analg, 2002; 95(4):805–12; doi:10.1213/00000539-200210000-00004 CrossRef
Ozkose Z, Yalcin Cok O, Tuncer B, Tufekcioglu S, Yardim S. Comparison of hemodynamics, recovery profile, and early postoperative pain control and costs of remifentanil versus alfentanil-based total intravenous anesthesia (TIVA). J Clin Anesth, 2002; 14(3):161–8; doi:10.1016/S0952-8180(01)00368-3 CrossRef
Scott LJ, Perry CM. Remifentanil: a review of its use during the induction and maintenance of general anaesthesia. Drugs, 2005; 65(13):1793–823; doi:10.2165/00003495-200565130-00007 CrossRef
Twersky RS, Jamerson B, Warner DS, Fleisher LA, Hogue S. Hemodynamics and emergence profile of remifentanil versus fentanyl prospectively compared in a large population of surgical patients. J Clin Anesth, 2001; 13(6):407–16; doi:10.1016/S0952-8180(01)00292-6 CrossRef