INTRODUCTION
The liver is a principal organ in the human and animal body. It is responsible for the functions that help support metabolism, immunity, digestion, detoxification, storage of vitamins, and other functions. It accounts for about 2% of an adult’s body weight. The role of the liver in the blood supply to the body from the portal vein (about 75%) and the hepatic artery (about 25%) makes it a featured organ (Messina et al., 2020). However, many reasons may lead to the destruction of the liver, among which is exposure to mycotoxins produced by Aspergillus fungi (Vaz et al., 2020). Aflatoxin B1 (AFB1) is a carcinogenic, teratogenic, and immunosuppressive compound causing public issues. AFB1 submits to liver biotransformation and converts to aflatoxin M1 (AFM1), which is excreted in the milk, urine, tissues, and biological fluids after consuming a contaminated diet (Marchese et al., 2018). The AFM1 is nondegradable and resistant to various industrial treatments, such as heat treatments, sterilization, or pasteurization (Kamyar and Movassaghghazani, 2017) and is considered to be carcinogenic (Marchese et al., 2018), causing oxidative liver damage, which results in immunosuppression, mutagenic, teratogenic, and carcinogenic effects. It also leads to death in cases of severe poisoning (Benkerroum, 2020). Physical, chemical, and biological assays could be applied to remove AFM1 from foods (Kumar et al., 2017).
Encapsulating is an efficient technology to preserve the vitality of the probiotics and protect the peptidoglycan structure of the cell wall, which contains the binding sites of AFM1, from the harmful effects of heat (protein degradation) and acidity (breaking down the glycosidic bonds in polysaccharides) (Samedi and Charles, 2019). Zizyphus fruit has high nutritional value and contains active substances, including flavonoids, polyphenols, organic acids, pectin, carotenoids, oligo, and polysaccharides. These minor components are considered materials that support health benefits (Kao and Chen, 2015).
Synbiotics are a mixture of probiotics (Pro) and prebiotics (Pre) in a combination that work together (synergistically) and reflect health benefits (Pandey et al., 2015). Synbiotics allude to synergism where the components around bacterial cells improve and support their activity and their growth rate, which is thought to benefit the health, metabolism, and immune system (Cencic and Chingwaru, 2010). Synbiotics could be obtained as supplements or in foods. The health benefits claimed by synbiotics include the improvement of liver function, the improvement of immunomodulating ability, and prevention and reduced incidences of infections (Pandey et al., 2015).
Likewise, food toxins and emerging food pathogens are possibly available in both raw and final food products, posing a health threat. The improvement of public health requires different foods for healthy persons, distinguished by safety and healthy characteristics besides their benefits (Cencic and Chingwaru, 2010). Nonetheless, economic downturns and high food costs tend to promote imbalanced “cheap” food consumption (Cavalcante Caetano et al., 2012). This forces researchers to find new sources of natural additives to support public health. For that; structuring for a system consists of Pro and Pre components may reflect efficiency to treat toxin-hurtful.
Camel milk (CM) has many health benefits, including its antidiabetic and anti-inflammatory effects, due to its content of lactoferrin and immunoglobulins (Rasheed, 2017). However, unfortunately, it may also be contaminated with AFM1 (Yousof and El Zubeir, 2020). The authors hypothesized that the encapsulated probiotics and Zizyphusz might be a promising method for the elimination of AFM1 and thus reduce their harmful effect on the liver. Therefore, this study aimed to evaluate the role of encapsulated probiotics strains, encapsulated Zizyphusz, and their combination with CM in preventing oxidative liver damage and genotoxicity induced by AFM1 in rats.
MATERIALS AND METHODS
Materials
Starter cultures of Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium bifidum were obtained from Chr. Hansen’s Laboratory, Copenhagen, Denmark. The fruits of Ziziphus spina-christi (L) were purchased from a local market. CM samples were donated from healthy lactating camels in the arid and semiarid regions of Egypt. All used chemicals were of high-quality analytical grade.
Animals
Male Wistar rats (6 weeks old) weighing 132.48 ± 18.13 g as mean±SD were collected from the National Research Centre’s Animal House in Cairo, Egypt. Individual animals were kept in stainless steel cages under conventional laboratory settings (23°C–25°C, 12 hours light/dark cycle) with free access to food and water. This work was performed following the regulations and policies of the Medical Research Ethics Committee of the National Research Centre in Cairo, Egypt, as well as the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Animals’ diet
The AIN-93 balanced diet (12% protein supplemented from casein, 10% corn oil, 10% sucrose, 58.5% maize starch, 5% fiber, 3.5% AIN-93 salt mixture, and 1% AIN-93 vitamin mixture) was produced according to Reeves et al. (1993).
METHODS
Preparation of probiotics pellet and Zizyphus suspension
The probiotic strains were firstly reactivated using De-Man Rogoza and Sharp (MRS) media. Activated strains were centrifuged at 4,200 Xg/7°C/40 minutes to obtain the pellets, which further were utilized for the encapsulation process. The pellets were lyophilized using a freeze drier (Dura-Dry MP Freeze Drier FTS System, USA) and then were kept at 2°C. The Ziziphus. spina-christi fruits were washed and seeds carefully removed and then sun-dried, and their fine powder was suspended in warm water by the same methodology applied before (Abdel-Salam et al., 2020). The collected suspension was kept in an amber bottle (100 ml, 2°C) till further usage.
Encapsulation of probiotics and Zizyphus
Probiotics pellets (1 g of lyophilized pellets) and the suspension of Zi. spina-christi fruit (2 ml) were encapsulated individually using a wall material of soy protein concentrate and maltodextrin, prepared according to the methodology described by Badr et al. (2021a). Briefly, a maltodextrin solution (contains Arabic gum and Tween 80 as emulsifiers) was mixed with a soy protein solution to form wall materials. The Pro pellets and the suspension of Zi. spina-christi (core materials) were mixed during the wall material blending by the T18 Basic Ultra-Turrax Homogenizer (IKA, Wilmington, NC), at an operating speed of 18,000 rpm/minute. The core system was dropped in fine drops. The emulsion was blended for 20 minutes/22,000 rpm to form the oral administration dose for the animal experiment. The emulsion stability was determined using the following equation:
Percent of separation = (H1/H0) × 100, (1)
where H0 is the emulsion’s starting volume and H1 is the separation layer’s volume.
Determination of encapsulated probiotics, Zizyphus, and their combination with CM for protecting the liver against AFM1 in vivo
In the current investigation, 48 rats were employed. Rats were split into eight groups (n = 6 each) after 1 week of acclimatization as follows:
Group 1 (Control), control normal group.
Group 2 (Zizyphus), where rats were treated orally with 1 ml encapsulated Zizyphusz (1 mg).
Group 3 (Pro), where rats were treated orally with encapsulated probiotics (1 ml contained 2.7 × 1011 CFU).
Group 4 (Zizyphus + Pro+CM), where rats were treated orally with a mixture (1 ml) of encapsulated probiotics and Zizyphusz together along with CM.
Group 5 (AFM1), where rats were treated orally with AFM1 (100 μg/kg).
Group 6 (Zizyphus + AFM1), where rats were treated orally with encapsulated Zizyphusz (1 ml) along with AFM1 (100 μg/kg).
Group 7 (Pro + AFM1), where rats were treated orally with encapsulated probiotics (1 ml contained 2.7 × 1011 CFU) along with AFM1 (100 μg/kg).
Group 8 (Zizyphus + Pro + CM + AFM1), where rats were treated orally with a mixture (1 ml) of encapsulated probiotic, Zizyphusz, and CM along with AFM1 (100 μg/kg).
The treatment in Group 8 was designed to evaluate the presence or absence of a synergistic effect between probiotics, Zizyphusz, and CM. The experiment lasted 4 weeks, during which the rats were fed a balanced diet. The total food intake, body weight gain, and food efficiency ratio were calculated at the end of the experiment.
Biochemical parameters
Following the treatment period, all rats’ blood samples were taken following an overnight fast. A fraction of the entire blood was tested for hemoglobin (Hb) content according to Drabkin (1949). The rest of the blood was centrifuged, and the plasma was tested for iron and total iron-binding capacity (TIBC) according to Stookey (1970) and Betts and Stuart (1973), respectively. Serum ferritin was determined using Elisa kits (Glory Science Co., Ltd., China). Total protein and the activities of gamma-GT (γ-GT) and the activities of alkaline phosphatase (ALP), aspartate transaminase (AST), and alanine transaminase (ALT) were measured according to Rheinhold and Seligron (1953), Szasz (1969), Bessey et al. (1946), and Reitman and Frankel (1957), respectively. The levels of creatinine, urea, and albumin were determined depending on Larsen (1972), Fawcett and Scott (1960), and Doumas et al. (1997), respectively.
Oxidative markers of liver homogenate
Rats were slaughtered, and the livers and kidneys were promptly removed and weighed from each rat. A part of each liver was utilized in the comet assay, another fraction was immersed in a 10% buffered formaldehyde solution for histological examination, and the remaining portion was immediately homogenized and analyzed for malondialdehyde (MDA), nitric oxide (NO), glutathione peroxidase (Gpx), and catalase (CAT) and superoxide dismutase (SOD) activity according to Ohkawa et al. (1979), Montgomery and Dymock (1961), Paglia and Valentine (1967), Beers and Sizer (1952), and Nishikimi et al. (1972), respectively. Liver tumor necrosis factor-alpha (TNF-α) was determined using an Elisa kit (Sunlong Co., Ltd., China).
Estimation of DNA damage using the comet assay
The comet analysis was carried out following the demonstrated competence as described by Blasiak et al. (2004), with minor modifications. The liver cells from each treatment were mixed with low-melting-point agarose (ratio of 1:10 v/v) before being pipetted to precoated slides with normal-melting-point agarose. The slides were maintained flat in a dark atmosphere (at 4°C for 30 minutes). The third layer of the low-melting-point agarose was pipetted onto the slides and allowed to harden (at 4°C for 30 minutes). The slides were placed in a prechilled lysis solution and maintained (at 4°C for 60 minutes). After that, the slides were submerged in a newly made alkaline unwinding solution at room temperature (60 minutes) in the dark. Electrophoresis was performed on the slides (30 minutes/4°C/0.8 V/cm, 300 mAmps). The slides were washed in a neutralizing solution before being immersed in 70% ethanol and air-dried. Ethidium bromide was used to stain the slides, which were then examined with a 400 magnification Zeiss epifluorescence microscope (510–560 nm, barrier filter 590 nm). A quantity of 100 cells was scored for each animal and then examined using DNA damage analysis software (Comet Score, TriTek Corp., Sumerduck, VA 22742) (Collins et al., 1997).
Preparation of liver and kidney specimens for histopathological examination
The liver and kidney specimens were taken and were fixed in 10% buffered formalin for 24 hours, and then they were dehydrated in ascending grades of alcohol. Then the specimens were cleared in xylene, two changes half an hour each. After that, they were embedded in two changes of soft paraffin, 1 hour each in an oven at 58°C–60°C. Then paraffin blocks were obtained, and serial sections of 6 μm thickness were cut, deparaffinized, rehydrated, and stained with hematoxylin and eosin (H &E). Then slides were subjected to the photomicroscopic examination using a Leica DM3000 light microscope (Germany).
Statistical analysis
Utilizing SPSS version 20 for statistical analysis, the data were reported as mean and standard error (SE) and statistically evaluated using one-way analysis of variance followed by the Duncan test. The probability at p ≤ 0.05 was used to calculate the statistical significance of the difference.
RESULTS
The probiotic and Zizyphusz emulsions did not show any separation percent, which indicates the efficiency of the encapsulation process.
The results (Fig. 1) indicated that AFM1 significantly caused a reduction in body weight and food efficiency ratio when compared with the healthy control rats. However, relative liver weight increased significantly in rats treated with AFM1 compared with the healthy control rats. Body weight gain and food efficiency ratio of the rats treated with capsulated materials of Zizyphusz, probiotics, or a mixture of encapsulated materials (Zizyphus + Pro) dissolved in CM without exposure to AFM1 were relatively similar to those of the control group. Body weight gain and food efficiency ratio of the rats exposed to AFM1 and treated with encapsulated materials of Zizyphusz, probiotics, or a mixture (Zizyphus + Pro) dissolved in CM increased significantly compared to those of the AFM1 group.
As evident from the results (Fig. 2), Hb, ferritin, and iron levels of the rats exposed to AFM1 were decreased significantly compared with the healthy control rats. However, TIBC increased significantly in the rats treated with AFM1 compared with the healthy control rats. Hb, ferritin, iron, and TIBC levels of the rats treated with encapsulated Zizyphusz, encapsulated probiotics, or a mixture of encapsulated materials with CM without exposure to AFM1 were relatively similar to those of the control group. The rats exposed to AFM1 and treated with either encapsulated Zizyphusz or encapsulated probiotics recorded a significant increase in the levels of Hb, ferritin, and iron compared to that in the AFM1 group.
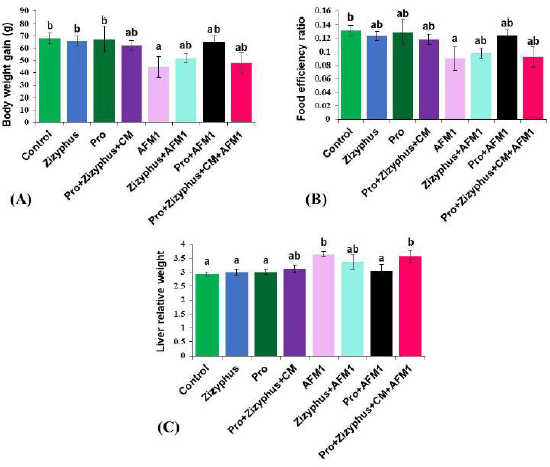 | Figure 1. (A) Body weight gain of studied groups; (B) Food efficiency ratio of studied groups; (C) Liver relative weight of studied groups. Control: normal rats; Zizyphus: rats received encapsulated Zizyphusz; Pro: rats received encapsulated probiotics; Pro+Zizyphus+CM: rats received mixture of encapsulated Zizyphusz, encapsulated probiotics, and camel milk; AFM1: rats received aflatoxin M1; Zizyphus+AFM1: rats received encapsulated Zizyphusz along with aflatoxin M1; Pro+AFM1: rats received encapsulated probiotics along with aflatoxin M1; Pro+Zizyphus+CM+AFM1: rats received mixture of encapsulated Zizyphusz, encapsulated probiotics, and camel milk along with aflatoxin M1. Same superscripts, on each bar, mean nonsignificant difference; different superscripts, on each bar, mean significance among the tested groups. [Click here to view] |
As shown in Table 1, liver functions (AST, ALT, γ-GT, and ALP) of the rats treated with encapsulated Zizyphusz, encapsulated probiotics, or a mixture of encapsulated materials (Zizyphus + Pro) with CM and without being exposed to AFM1 were relatively similar to those of the control group. The rats exposed to AFM1 recorded a significant increase in liver functions (AST, ALT, γ-GT, and ALP) compared with the healthy control rats. The rats exposed to AFM1 and treated with encapsulated Zizyphusz, encapsulated probiotics, or a mixture of encapsulated materials with CM recorded a significant reduction in liver functions (AST, ALT, γ-GT, and ALP) compared to the AFM1-treated rats. Encapsulated Zizyphusz or encapsulated probiotics were more promising in the suppression of liver functions increase.
As shown in Table 2, urea, creatinine, total protein, and albumin of the rats treated with either encapsulated Zizyphusz only, encapsulated probiotics only, or a mixture of encapsulated materials with CM (without being exposed to AFM1) were relatively similar to the control group. The rats exposed to AFM1 recorded a significant increase in urea, creatinine, total protein, and albumin compared with the healthy control rats. The rats exposed to AFM1 and treated with encapsulated Zizyphusz, encapsulated probiotics, or a mixture of encapsulated materials with CM recorded a significant reduction in urea, creatinine, total protein, and albumin compared to those of AFM1-treated rats.
The results (Fig. 3) exhibited that the MDA, CAT, NO, and TNF-α of the rats exposed to AFM1 were increased significantly compared with the control rats, while Gpx and SOD activities were decreased significantly in the group treated with AFM1. The MDA, CAT, NO, Gpx, SOD, and TNF-α of the rats treated with encapsulated Zizyphusz, encapsulated Pro, or a mixture of encapsulated materials with CM without being exposed to AFM1 were relatively similar to those of the control group. The rats treated with encapsulated probiotics recorded CAT and TNF-α significantly less and Gpx and SOD higher than the control group. The rats exposed to AFM1 and treated with the mixture of encapsulated Zizyphusz, encapsulated probiotics, and CM showed a decrease, although nonsignificant, in MDA, CAT, and NO compared to those of the group of AFM1. Moreover, MDA, CAT, NO, and TNF-α decreased significantly in rats exposed to AFM1 and treated with either encapsulated Zizyphusz or encapsulated probiotics compared to those in the group treated with AFM1. The rats exposed to AFM1 and treated with either encapsulated Zizyphusz or encapsulated probiotics recorded Gpx and SOD significantly higher than those of the AFM1 group.
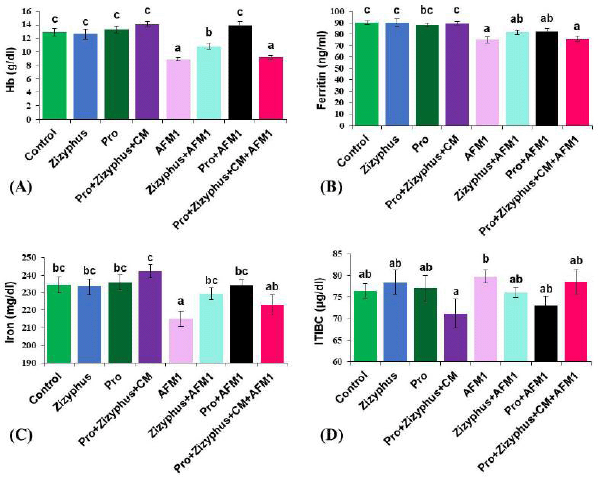 | Figure 2. (A) Hemoglobin concentration of the studied groups; (B) Ferritin concentration of the studied groups; (C) Iron concentration of the studied groups; (D) Total binding protein capacity of the studied groups. Control: normal rats; Zizyphus: rats received encapsulated Zizyphusz; Pro: rats received encapsulated probiotics; Pro+Zizyphus+CM: rats received mixture of encapsulated Zizyphusz, encapsulated probiotics, and camel milk; AFM1: rats received aflatoxin M1; Zizyphus+AFM1: rats received encapsulated Zizyphusz along with aflatoxin M1; Pro+AFM1: rats received encapsulated probiotics along with aflatoxin M1; Pro+Zizyphus+CM+AFM1: rats received mixture of encapsulated Zizyphusz, encapsulated probiotics, and camel milk along with aflatoxin M1. Same superscripts, on each bar, mean nonsignificant difference; different superscripts, on each bar, mean significance among the tested groups. [Click here to view] |
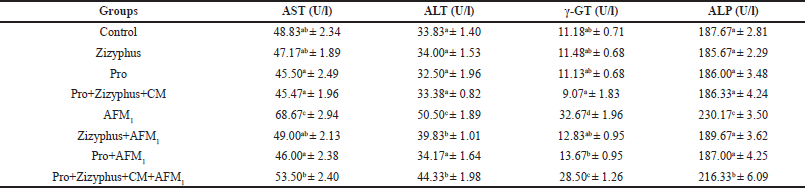 | Table 1. Liver functions in the studied groups. [Click here to view] |
Table 3 summarizes the evaluation of DNA damage in liver tissues for several groups of rats. The results showed that the rates of DNA damage in the liver tissues of the rats subjected to AFM1 were substantially higher (p < 0.001) than in healthy control rats. Conversely, DNA damage seen in the liver tissues of the rats treated with encapsulated materials of Zizyphusz, Pros, or their mixture with CM (without being exposed to AFM1) was relatively similar to the rate of DNA damage in the control group (7.26 ± 0.85). On the other hand, rats exposed to AFM1 and treated with the mixture of encapsulated Zizyphusz, encapsulated probiotics, and CM decreased significantly (p < 0.05) in the rate of DNA damage compared to that in the group of AFM1 (25.78 ± 1.03). Moreover, the rate of DNA damage in rats exposed to aflatoxin and treated with encapsulated Zizyphusz decreased significantly (p < 0.01) much more than that in the group of aflatoxin (25.78 ± 1.03). Additionally, the DNA damage decreased further in rats exposed to aflatoxin and treated with encapsulated probiotics (12.79 ± 1.31) compared to that in the group of the AFM1 treatment.
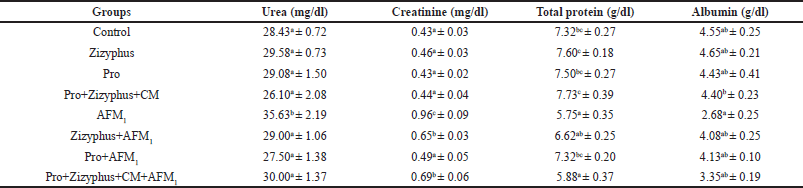 | Table 2. Urea, creatinine, total protein, and albumin in the studied groups. [Click here to view] |
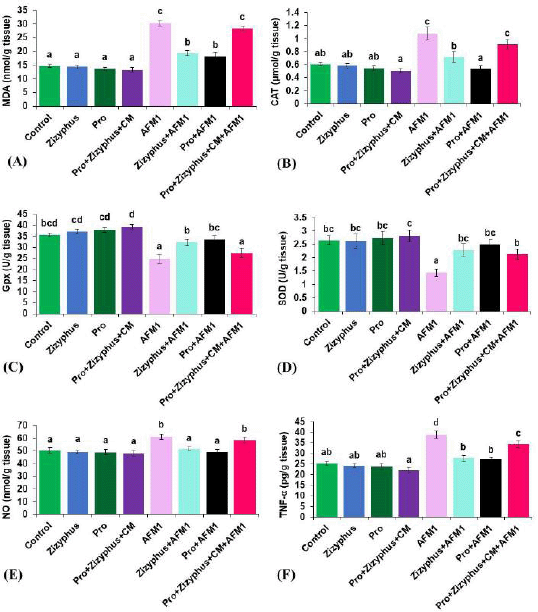 | Figure 3. (A) MalondialdehydeMDA of the studied groups; (B) CAT of the studied groups; (C) Gpxglutathione of the studied groups; (D) SOD of the studied groups; (E) NO of the studied groups; (F) TNF-α of the studied groups. Control: normal rats; Zizyphus: rats received encapsulated Zizyphusz; Pro: rats received encapsulated probiotics; Pro+Zizyphus+CM: rats received mixture of encapsulated Zizyphusz, encapsulated probiotics, and camel milk; AFM1: rats received aflatoxin M1; Zizyphus+AFM1: rats received encapsulated Zizyphusz along with aflatoxin M1; Pro+AFM1: rats received encapsulated probiotics along with aflatoxin M1; Pro+Zizyphus+CM+AFM1: rats received mixture of encapsulated Zizyphusz, encapsulated probiotics, and camel milk along with aflatoxin M1. Same superscripts, on each bar, mean nonsignificant difference; different superscripts, on each bar, mean significance among the tested groups. [Click here to view] |
 | Table 3. Rate of DNA damage in liver tissues using the comet assay. [Click here to view] |
Histopathological examination of the control rats shows the normal hepatic lobules and portal tracts. Each hepatic lobule is formed of cords of hepatocytes and blood sinusoids in between. The shape of hepatocytes appears as polyhedral cells with one or rarely two spherical nuclei and an abundant granular and strongly eosinophilic cytoplasm. The nuclei of the hepatocytes are large with peripherally dispersed chromatin and prominent nucleoli. The hepatic sinusoids are composed of a single row of endothelial cells, run radially, and converge at the center of the hepatic lobule to form the central vein (Fig. 4A). The portal tracts are a triangular mass of liver tissue that consists of the hepatic portal vein, hepatic artery, and bile ducts (Fig. 4B).
The results of the examination of liver sections from the rats supplemented with encapsulated Zizyphusz, encapsulated probiotics, or a mixture of encapsulated materials with CM (without being exposed to AFM1) showed no histological or morphometric difference between them and that of the control as manifested by the normal structure of the hepatic lobules (Fig. 4, respectively) and portal tracts ( respectively).
In the case of the rats administered with AFM1, a microscopic investigation showed disturbance of the hepatic lobule that was associated with massive hydropic degeneration of the hepatocytes and condensed hyperchromatic nuclei (Fig. 5A). Lymphocytic infiltration in the portal and periportal areas and degeneration of the hepatocytes surrounding the portal area were seen. The dilated and congested sinusoids were noticed (Fig. 5B). In addition, focal necrosis of hepatocytes was associated with lymphocytic infiltration and presence of cell debris in the blood sinusoids. Some nuclei showed hyperchromasia, and others showed pyknosis (Fig. 5C). Moreover, others showed congested dilated portal areas with degenerative hepatocytes surrounding the portal areas and severe proliferation of bile ducts (Fig. 5D)
Examination of micrographs of the liver sections from the AFM1-treated rats supplemented with encapsulated Zizyphusz or encapsulated probiotics showed that the hepatic lobules and portal tract appeared more or less like normal control (Fig. 6A–D). On the other hand, the liver sections from the AFM1-treated rats supplemented with the mixture of encapsulated Zizyphusz, encapsulated probiotics, and CM showed disturbance of the hepatic lobules associated with micro- and macrovesicular fatty change. The hepatocytes showed condensed hyperchromatic nuclei (Fig. 6E). In foci of hepatocytes necrosis associated with lymphocytic infiltration the dilated and congested sinusoids were noticed (Fig. 6F).
Microscopic examination of the cortex of the kidneys of the control rats and rats administered with encapsulated Zizyphusz, encapsulated probiotics, or their mixture with CM (without being exposed to AFM1) showed the normal structure of the nephron tissue that consists of two major components, the renal corpuscle and the renal tubules. The renal corpuscle is formed of two structures: Bowman’s capsule and the glomerulus. The space between the two layers is known as the urinary space. The main components of the renal tubules are convoluted renal tubules and the proximal convoluted tubule. The proximal convoluted tubule is lined with a simple cuboidal epithelium. The cells of this epithelium have an acidophilic cytoplasm and contain only 3–5 spherical nuclei, usually located in the center of the cells. The distal convoluted tubule is lined with a simple cuboidal epithelium. The cytoplasm of these cells is more acidophilic, and the nuclei appear more numerous. The lumina of the distal convoluted tubules are larger than those of the proximal convoluted tubules (Fig. 7).
Examination of kidney cortex sections from the rats administered with AFM1 showed congested blood vessels and inflammatory infiltration (Fig. 8A). On the other hand, hypercellularity and congestion in the glomeruli associated with wide urinary spaces and shrinkage of some glomeruli were noticed (Fig. 8B), and there was degeneration of the renal tubules (Fig. 8C). Moreover, inflammatory infiltration, hemorrhagic areas in the interstitium, necrosis of some of the proximal convoluted tubules, and hemorrhagic areas in the interstitium were found. The renal corpuscle exhibited lobulation of glomeruli and wide urinary spaces (Fig. 8D).
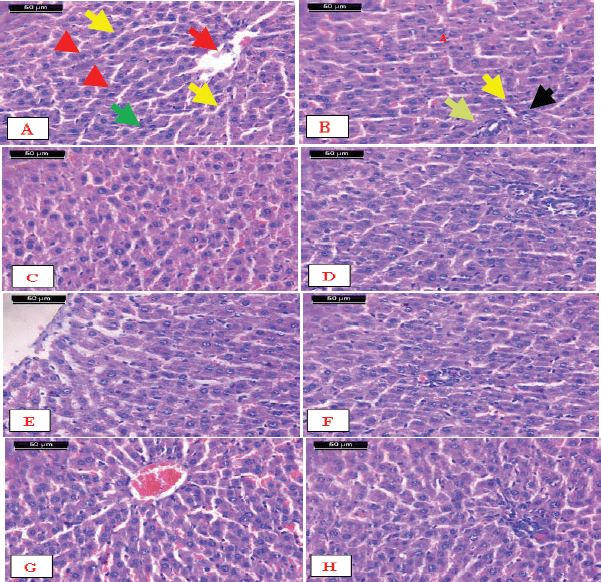 | Figure 4. Micrographs of sections of the liver. A) Control showing the normal structure of hepatic lobules. The central vein (arrow) lies at the center of the lobule surrounded by the hepatocytes with strongly eosinophilic granulated cytoplasm (arrowheads) and distinct nuclei (green arrow). Between the strands of hepatocytes, the hepatic sinusoids are shown (yellow arrows). B) Notice the normal structure of the portal tract that consists of the hepatic portal vein (green arrow), hepatic artery (yellow arrows), and bile ducts (arrow), C, D, E, F, G, and H) Liver of rats supplemented with encapsulated Zizyphuszizyphus, encapsulated probiotic, or the mixture of encapsulated Zizyphusz, encapsulated probiotics, and CM showing normal structure of the hepatic lobules and portal tract (H&E stain, scale bar 50 μm). [Click here to view] |
Histological investigation of kidney cortex specimens from the rats exposed to AFM1 and treated with encapsulated Zizyphusz, encapsulated probiotics, or their mixture with CM showed the normal structure of the cortex. The distal convoluted tubules, proximal convoluted tubules, and renal corpuscles appeared more or less the same as the control ones (Fig. 9).
DISCUSSION
The existence of AFM1 in the human diet represents a public health issue, which requires necessarily a strategy targeting to reduce the in vivo impacts. This is because AFM1 does not reflect changes in odor, taste, or color of contaminated products, which leads to blind consumption without consumer knowledge about contamination occurrence in their diets. Thence, the authors search for bioactive components that possess biological effects that dismiss or reduce the changes joined to AFM1 toxicity. Several strains of probiotics bacteria were reported to reduce the toxicity of AFM1 (Afshar et al., 2020; Kamyar and Movassaghghazani, 2017; Khadivi et al., 2020), as well as the natural extract rich in phytochemicals (Abdel-Fattah et al., 2018; Loi et al., 2020) known to limit the aflatoxins toxicity in dairy products. So, a mix of the probiotic strains and Zizyphusz fruit was chosen for examination in an encapsulated form to reduce the biological toxicity of AFM1 in rats. Besides, the mixture of encapsulated materials was applied in CM and evaluated for its impact on AFM1 toxicity in rats.
The present investigation stated that experimental exposure to AFM1 decreased the body weight gain and hemoglobin, increased liver functions, increased CAT and lipid peroxidation in the liver, increased inflammatory markers (TNF-α and NO) in the liver, and increased the rate of liver DNA damage. Liver dysfunction has a significant impact on the kidney so the rats exposed to the toxin had increased kidney function. Experimental exposure to AFM1 resulted in histological changes in the liver and kidney when compared to the normal rats. Ben Salah-Abbes et al. (2020) found that treatment of mice with AFM1 resulted in a considerable rise in ALT, AST, and ALP enzyme activity. Li et al. (2018) also found that, by altering the expression of proline dehydrogenase and L-proline, AFB1 and AFM1 activated oxidative stress and caused kidney damage synergistically.
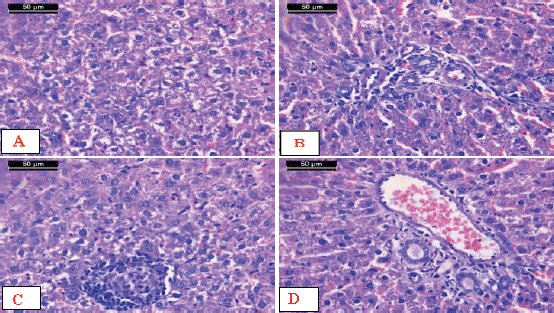 | Figure 5. Micrographs of sections of liver from AFM1-treated rats. A) Disturbance of the hepatic lobule associated with massive hydropic degeneration of the hepatocytes and condensed hyperchromatasic nuclei. B) Lymphocytic infiltration in the portal and periportal areas (arrow) and degeneration of the hepatocytes surrounding the portal area. Notice the dilated and congested sinusoids (arrowhead). C) Focal necrosis of hepatocytes associated with lymphocytic infiltration and the presence of cell debris in the blood sinusoids. Some nuclei showed hyperchromasia, and others showed pyknosis. D) Notice the congested dilated portal area with degenerative hepatocytes surrounding the portal area and severe proliferation of bile ducts (short arrow) (H&E stain, scale bar 50 μm). [Click here to view] |
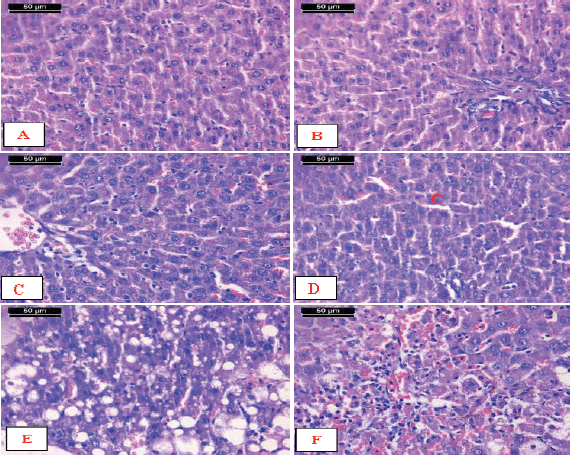 | Figure 6. Micrographs of sections of liver from AFM1-treated rats. A–D) Livers supplemented with encapsulated Zizyphusz or encapsulated probiotics showed that the hepatic lobules and portal tract appeared more or less like the normal control. E) AAFM1-treated rats supplemented with the mixture of encapsulated Zizyphusz, encapsulated probiotics, and CM showed disturbance of the hepatic lobule associated with micro- and macrovesicular fatty change. The hepatocytes show condensed hyperchromatasic nuclei (arrowhead). F) Livers showing foci of hepatocytes necrosis associated with lymphocytic infiltration (arrow). Notice the dilated and congested sinusoids (arrowhead) (H&E stain, scale bar 50 μm). [Click here to view] |
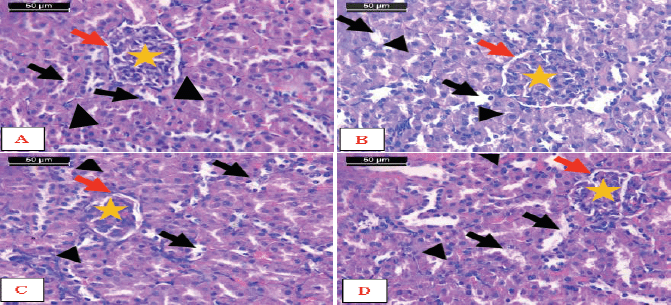 | Figure 7. Photomicrographs of sections of kidney. A) Control rats, B) Encapsulated Zizyphusz, C) Encapsulated probiotics, D) Mixture of encapsulated Zizyphusz, encapsulated probiotics, and CM administered rats showed the normal structure of the cortex. Notice that distal convoluted tubules (arrows) could be differentiated from the proximal convoluted tubules (arrowheads) as having a larger and well-defined lumen, with less affinity to staining. The renal corpuscles are formed of the glomerulus (asterisk) and urinary space (red arrows) (H&E stain, scale bar 50 μm). [Click here to view] |
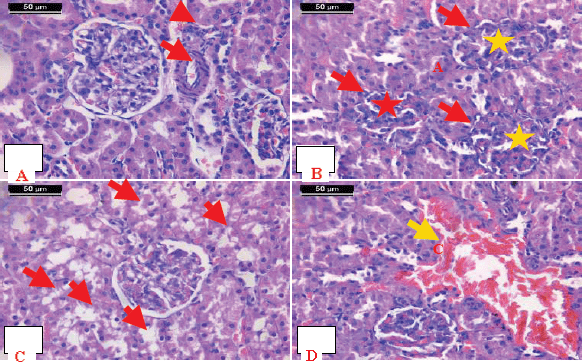 | Figure 8. Photomicrographs of sections of cortex from kidney of rats administered with AFM1. A) Congested blood vessel (arrow) and inflammatory infiltration (arrowhead). B) Hypercellularity and congestion in the glomeruli (asterisk) associated with a wide urinary space (arrowhead). Notice the shrinkage of some glomeruli (red asterisk) and wide urinary space. C) Degeneration of the renal tubules. D) Inflammatory infiltration and hemorrhagic areas in the interstitium (arrow). Notice necrosis of some of the proximal convoluted tubules (arrowhead). The renal corpuscle exhibited a lobulated glomerulus and wide urinary space (asterisk) (H&E stain, scale bar 50 μm). [Click here to view] |
Where AFM1 is a toxic metabolite like AFB1, it has been identified as a potential human carcinogen and induces DNA damage on its own by covalently attaching to it. Also, it has a direct hazardous impact on human cell lines, regardless of the metabolic AFB1 activation (Saha Turna and Wu, 2021). AFM1 has been shown to decrease both innate and adaptive immune responses in several in vivo experiments (Shirani et al., 2018, 2021). In agreement with the present results, Ben Salah-Abbes et al. (2015) reported a significantly less for body mass (BM), an elevation of white blood cells (WBCs), and differences in absolute and relative amounts of cell types in the thymus and spleen in mice given 100μg AFM1 /kg b.w. Aflatoxins’ ability to cause genotoxicity is heavily influenced by oxidative damage. The ability of AFM1 to cause damage by covalently binding in DNA only adds to its genotoxicity (Ben Salah-Abbès et al., 2015).
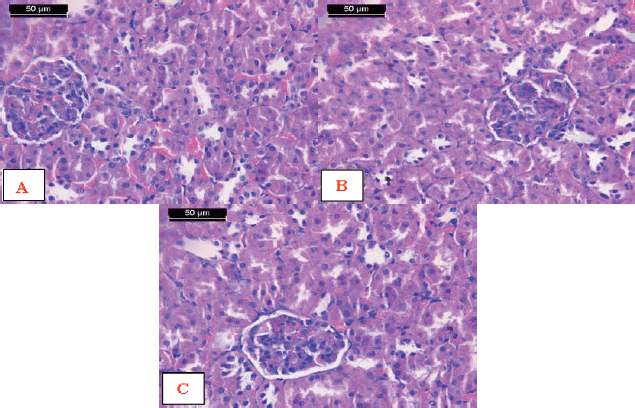 | Figure 9. Photomicrographs of sections of kidey cortex from rats exposed to AFM1 and treated with A) Encapsulated Zizyphusz, B) Encapsulated probiotic, C) Mixture of encapsulated Zizyphusz, encapsulated probiotics, and CM showing the normal structure of the cortex. Notice that distal convoluted tubules (arrows) could be differentiated from the proximal convoluted tubules (arrowheads) as having a larger and well-defined lumen, with less affinity to staining. The renal corpuscles are formed of the glomerulus (asterisk) and urinary space (red arrows) (H&E stain, scale bar 50 μm). [Click here to view] |
The Pro strains were effective in the detoxification and control of the biotransformation of aflatoxins, as well as the prevention of tumor formation (Khorshidian et al., 2016). Several studies have established the feasibility of removing aflatoxin M1 from dairy products via many different bacteria strains (Khadivi et al., 2020; Var and Kabak, 2004). However, there has been little research on the possibility of shielding the organism’s body from AFM1. So, the authors aimed to study the role of encapsulated probiotics or encapsulated Zizyphusz, as well their combination with CM, for occurring protection of the liver against AFM1 toxicity.
Individual treatment of rats with encapsulated probiotics, encapsulated Zizyphusz, or their mix with CM, without being exposed to an AFM1 dose, did not result in biochemical changes, changes in the DNA damage rate, or histological changes compared to the control rats. Our findings showed that encapsulated probiotics or encapsulated Zizyphusz improved liver functions, decreased CAT and lipid peroxidation in the liver, decreased inflammatory markers (TNF-α and NO) in the liver, reduced the rate of liver DNA damage, and decreased kidney functions in rats intoxicated with AFM1. Also, rats administrated with encapsulated Pro or encapsulated Zizyphusz fruit extract attenuated the histological changes in the livers and kidneys in rats intoxicated with AFM1. For the treatment in Group 8, where a lower quantity of probiotics (0.4 ml) with equal quantities (0.3 ml) of CM and Zizyphusz was utilized, the result differs from the individual application. The result for a combination of encapsulated materials with CM was the least promising in terms of liver protection from AFM1 maybe due to the low dose of encapsulated Pro and encapsulated Zizyphusz. This result could be explained as the binding process of lactic acid bacteria strains to mycotoxins is dosage dependent. Suppression of mycotoxins was found when 1 × 107 cells of lactic acid bacteria per animal were used (Mahmood Fashandi et al., 2018). Lactic acid bacteria could bind and eliminate AFM1 through physical absorption between lactic acid bacteria and mycotoxins, or by degradation, the chemicals possessed a high carcinogenic risk into by-products with little or low carcinogenic risk (Mahmood Fashandi et al., 2018).
Although the human body has built-in antioxidant defense systems to avoid the interaction of excess free radicals with biological components, exogenous antioxidants play an important role in controlling this mechanism as well. Natural antioxidants are a logical solution offered by nature to avoid ROS and RNS generation via oxidative stress-induced aflatoxins connected to DNA, protein synthesis, and mitochondria harmful effects and to restore the oxidative balance that mycotoxins have disrupted. One of the most frequent exogenous antioxidant molecules is the probiotics bacteria and their cellular metabolites (Afshar et al., 2020).
Similarly, the antioxidant power of the Zizyphusz may contribute to its role in the protection of the liver from oxidative damage induced by AFM1. The presence of phytochemicals such as cardiac glycosides, polyphenols, saponins, and tannins in Zizyphusz contributes to its antioxidant action (El-Hefny et al., 2018). Pectin and polyphenols content in Zizyphusz may have a positive role in promoting the growth of beneficial bacteria in the gut (Kao and Chen, 2015). Concerning the bioactivity of plant extracts, Loi et al. (2020) reported that bioactive substances derived from plants are valued for their pharmacological and nutraceutical properties, as well as their antifungal and antiaflatoxin properties. Also, Badr et al. (2021b) discussed the antioxidant function of plant phytochemicals against aflatoxins toxicity. Because of the hydroxyl and carboxyl moieties in polyphenols, their presence may contribute to the antioxidant activity of Zizyphusz. They regulate the cellular redox state by directly quenching free radicals and chelating metal ions (promotors of oxidative reactions). They also activate the redox-sensitive transcription factors of antioxidative enzymes. Protein binding and inhibition are mediated via hydrogen interactions between the hydroxyl moiety of phenols and the carboxyl and thiol groups of proteins. The aromatic ring, on the other hand, can make van der Waals (hydrophobic) contact with proteins (Loi et al., 2020).
Contrary to the norm, the combination of probiotics and Zizyphusz in ’CM, at a low bacterial concentration, did not show the best efficacy compared to the application of each capsule separately in AFM1 toxicity reduction in rats (for histopathology of tissues or biochemical parameters). The authors expected that the presence of Zizyphusz (with a bacterial concentration lower than 107) would support the probiotics efficiency to reduce AFM1 toxicity. Despite the efficiency of Zizyphusz in reducing AFM1 toxicity, the effective mechanism differs from that by bacterial strains. Therefore, the authors suggest more trials in the next investigation that will explain the suitable ratio of mix process between probiotics strains and Zizyphuszizyphus, which achieve the efficacy for AFM1 toxicity reduction.
CONCLUSION
Aflatoxin M1 is a hazardous contaminant found in dairy products and causes several health issues. In rats exposed to AFM1, encapsulated materials (probiotics or Zizyphusz) suppressed the elevation of liver functions, oxidative marker (MDA), inflammatory marker (TNF-α), and DNA damage. The combination of encapsulated materials within CM was the least promising in terms of liver protection from AFM1 exposure. This result could be explained as a result of the plant extracts’ failure to compensate for the decrease in bacteria concentration of the mixture, where the extract did not support the low bacterial concentration to reduce AFM1 toxicity like that at 107 CFU/ml. In this regard, there is an urgent need for more research with several Zizyphusz doses to determine the suitable mixture ratio. The results recommended encapsulated probiotics and encapsulated Zizyphusz for the promising removal of AFM1 throughout its in vivo contamination.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The Medical Research Ethics Committee of the National Research Centre in Cairo, Egypt, as well as the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdel-Fattah SM, Badr AN, Seif FAHA, Ali SM, Hassan RA. Antifungal and anti-mycotoxigenic impact of eco-friendly extracts of wild stevia. J Biol Sci, 2018; 18(8):488–99; http://doi.org/10.3923/jbs.2018.488.499
Abdel-Salam AM, Badr AN, Zaghloul AH, Farrag ARH. Functional yogurt aims to protect against the aflatoxin B1 toxicity in rats. Toxicol Rep, 2020; 7:1412–20; http://doi.org/10.1016/j.toxrep.2020.10.012
Afshar P, Shokrzadeh M, Raeisi SN, Ghorbani-HasanSaraei A, Nasiraii LR. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon, 2020; 178:50–8; http://doi.org/10.1016/j.toxicon.2020.02.007
Badr AN, Gromadzka K, Shehata MG, Stuper-Szablewska K, Drzewiecka K, Abdel-Razek AG, Youssef MM. Encapsulated Bioactive Ingredients of grape by-products applicate in fresh-cut fruit and juices diminished the ochratoxins. J Food Process Preserv, 2021a; 45(2):e15112; http://doi.org/10.1111/jfpp.15112
Badr AN, Youssef M, Abdel-Razek AG, Shehata MG, Hassanien MM, Amra H. Natural antioxidants: preservation roles and mycotoxicological safety of food. Egypt J Chem, 2021b; 64(1):285–98; http://doi.org/10.21608/EJCHEM.2020.51183.3048
Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biolo Chem, 1952; 195(1):133–40.
Ben Salah-Abbès J, Abbès S, Jebali R, Haous Z, Oueslati R. Potential preventive role of lactic acid bacteria against aflatoxin M- immunotoxicity and genotoxicity in mice. J Immunotoxicol, 2015; 12(2):107–14; http://doi.org/10.3109/1547691x.2014.904025
Ben Salah-Abbès J, Belgacem H, Ezdini K, Mannai M, Oueslati R, Abbès S. Immunological effects of AFM1 in experimental subchronic dosing in mice prevented by lactic acid bacteria. Immunopharmacol Immunotoxicol, 2020; 42(6):572–81; http://doi.org/10.1080/08923973.2020.1824237
Benkerroum N. Chronic and acute toxicities of aflatoxins: mechanisms of action. Int J Environ Res Public Health, 2020; 17(2):423; http://doi.org/10.3390/ijerph17020423
Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J Biol Chem, 1946; 164:321–9.
Betts CA, Stuart B. Determination of serum total iron-binding capacity. J Clin Pathol, 1973; 26(6):457; http://doi.org/10.1136/jcp.26.6.457-a
Blasiak J, Arabski M, Krupa R, Wozniak K, Zadrozny M, Kasznicki J, Zurawska M, Drzewoski J. DNA damage and repair in type 2 diabetes mellitus. Mutat Res, 2004; 554(1):297–304; http://doi.org/10.1016/j.mrfmmm.2004.05.011
Cavalcante Caetano M, Ortiz Ortiz TT, Lopes Da Silva SG, Suano De Souza FI, Saccardo Sarni ROJR. Complementary feeding: inappropriate practices in infants. Rev Chil Pediatr, 2012; 83(5):503; http://doi.org/10.4067/s0370-41062012000500014
Cencic A, Chingwaru W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients, 2010; 2(6):611–25; http://doi.org/10.3390/nu2060611
Collins A, Dušinská M, Franklin M, Somorovská M, Petrovská H, Duthie S, Fillion L, Panayiotidis M, Rašlová K, Vaughan N. Comet assay in human biomonitoring studies: Reliability, validation, and applications. Environ Mol Mutagen, 1997; 30:139–46.
Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clinica Chimica Acta, 1997; 258(1):21–30; http://doi.org/10.1016/s0009-8981(96)06447-9
Drabkin DL. The standardization of hemoglobin measurement. Am J Med Sci, 1949; 292:386–99; http://doi.org/10.1097/00000441-194801000-00017
El-Hefny M, Mohamed AA, Salem MZM, Abd El-Kareem MSM, Ali HM. Chemical composition, antioxidant capacity and antibacterial activity against some potato bacterial pathogens of fruit extracts from Phytolacca dioica and Ziziphus spina-christi grown in Egypt. Sci Hortic, 2018; 233:225–32; http://doi.org/10.1016/j.scienta.2018.01.046
Fawcett JK, Scott JE. A rapid and precise method for the determination of Urea. J Clin Pathol, 1960; 13(2):156–9; http://doi.org/10.1136/jcp.13.2.156
Kamyar S, Movassaghghazani M. Reduction of aflatoxin m1 in milk using kefir starter. Iran J Toxicol, 2017; 11(6):27–31; http://doi.org/10.29252/arakmu.11.6.27
Kao TH, Chen BH. Functional components in zizyphus with emphasis on polysaccharides, in polysaccharides: bioactivity and biotechnology. In: Ramawat KG, Mérillon JM (eds.). Springer International Publishing Cham, Switzerland, pp 795–827, 2015; http://doi.org/10.1007/978-3-319-03751-6_15-1
Khadivi R, Razavilar V, Anvar A, Akbari-adergani B. Aflatoxin M1-binding ability of selected lactic acid bacteria strains and Saccharomyces boulardii in the experimentally contaminated milk treated with some biophysical factors. Arch Razi Inst, 2020; 75(1):63–73; http://doi.org/10.22092/ari.2019.123985.1265
Khorshidian N, Yousefi Asli M, Hosseini H, Shadnoush M, Mortazavian AM. Potential anticarcinogenic effects of lactic acid bacteria and probiotics in detoxification of process-induced food toxicants. Iran J Cancer Prev, 2016; 9(5):1–13; http://doi.org/10.17795/ijcp-7920
Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG. Aflatoxins: a global concern for food safety, human health and their management. Front Microbiol, 2017; 7:2170; http://doi.org/10.3389/fmicb.2016.02170
Larsen K. Creatinine assay by a reaction-kinetic principle. Clinica Chimica Acta, 1972; 41:209–17; http://doi.org/10.1016/0009-8981(72)90513-X
Li H, Xing L, Zhang M, Wang J, Zheng N. The toxic effects of aflatoxin B1 and aflatoxin M1 on kidney through regulating l-proline and downstream apoptosis. BioMed Res Int, 2018; 2018:9074861; http://doi.org/10.1155/2018/9074861
Loi M, Paciolla C, Logrieco AF, Mulè G. Plant bioactive compounds in pre- and postharvest management for aflatoxins reduction. Front Microbiol, 2020; 11:243; http://doi.org/10.3389/fmicb.2020.00243
Mahmood Fashandi H, Abbasi R, Mousavi Khaneghah A. The detoxification of aflatoxin M1 by Lactobacillus acidophilus and Bifidobacterium spp.: a review. J Food Process Preserv, 2018; 42(9):e13704; http://doi.org/10.1111/jfpp.13704
Marchese S, Polo A, Ariano A, Velotto S, Costantini S, Severino L. Aflatoxin B1 and M1: biological properties and their involvement in cancer development. Toxins, 2018; 10(6):214; http://doi.org/10.3390/toxins10060214
Messina A, Luce E, Hussein M, Dubart-Kupperschmitt A. Pluripotent-stem-cell-derived hepatic cells: hepatocytes and organoids for liver therapy and regeneration. Cells, 2020; 9(2):420; http://doi.org/10.3390/cells9020420
Montgomery HAC, Dymock JF. The determination of nitrite in water. Analyst, 1961; 86:414–6.
Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972; 46(2):849–54.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 1979; 95(2):351–8; http://doi.org/10.1016/0003-2697(79)90738-3
Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med, 1967; 70(1):158–69.
Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol, 2015; 52(12):7577–87; http://doi.org/10.1007/s13197-015-1921-1
Rasheed Z. Medicinal values of bioactive constituents of camel milk: a concise report. Int J Health Sci, 2017; 11(5):1–2.
Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76a rodent diet. J Nutr, 1993; 123(11):1939–51; http://doi.org/10.1093/jn/123.11.1939
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol, 1957; 28(1):56–63.
Rheinhold J, Seligron D. Total protein, albumin and globulin in standard methods of clinical chemistry. J Academic press, Inc., New York, NY, 88 p, 1953.
Saha Turna N, Wu F. Aflatoxin M1 in milk: a global occurrence, intake, & exposure assessment. Trends Food Sci Technol, 2021; 110:183–92; http://doi.org/10.1016/j.tifs.2021.01.093
Samedi L, Charles AL. Viability of 4 probiotic bacteria microencapsulated with arrowroot starch in the simulated gastrointestinal tract (GIT) and yoghurt. Foods, 2019; 8(5):175; http://doi.org/10.3390/foods8050175
Shirani K, Riahi Zanjani B, Mehri S, Razavi-Azarkhiavi K, Badiee A, Hayes AW, Giesy JP, Karimi G. miR-155 influences cell-mediated immunity in Balb/c mice treated with aflatoxin M1. Drug Chem Toxicol, 2021; 44(1):39–46; http://doi.org/10.1080/01480545.2018.1556682
Shirani K, Zanjani BR, Mahmoudi M, Jafarian AH, Hassani FV, Giesy JP, Karimi G. Immunotoxicity of aflatoxin M1: as a potent suppressor of innate and acquired immune systems in a subacute study. J Sci Food Agric, 2018; 98(15):5884–92; http://doi.org/10.1002/jsfa.9240
Stookey LL. Ferrozine--a new spectrophotometric reagent for iron. Anal Chem, 1970; 42(7):779–81; http://doi.org/10.1021/ac60289a016
Szasz G. A kinetic photometric method for serum γ-glutamyl transpeptidase. Clin Chem, 1969; 15(2):124–36.
Var I, Kabak B. Removal of aflatoxins by viable and heat-killed lactic acid bacteria and bifidobacteria. Archiv Für Lebensmittelhygiene, 2004; 55(5):106–9.
Vaz A, Cabral Silva AC, Rodrigues P, Venâncio A. Detection methods for Aflatoxin M1 in dairy products. Microorganisms, 2020; 8(2):246; http://doi.org/10.3390/microorganisms8020246
Yousof SSM, El Zubeir IEM. Chemical composition and detection of Aflatoxin M1 in camels and cow’s milk in Sudan. Food Addit Contam: Part B, 2020; 13(4):298-304. http://doi.org/10.1080/19393210.2020.1796826