INTRODUCTION
Opioids belong to a class of drugs that produce beneficial effects, such as gastric slowing, cough suppression, and analgesia (Schaefer et al., 2007). However, opioid addiction has become a major health problem all over the world. Tramadol is a synthetic analgesic opioid that acts as a weak agonist for μ-opioid receptors (Gillen et al., 2000). Several lines of evidence highlighted that the tramadol exhibits antidepressant effects (Rojas-Corrales et al., 1998). Also, tramadol can produce adverse effects that vary based on several factors. In a case study reported by Osman and Mustafa (2018), it was noted that the prescription of 50 mg tramadol to a female with no psychiatric history was correlated with an elevation in the mood accompanied with acceleration in the flow of speech when prescribed, albeit such a case is rare. Furthermore, it was noted that tramadol overdose causes serotonin syndrome due to its ability to block the reuptake of serotonin (Tashakori and Afshari, 2010).
Despite the fact that the tramadol has low potential to cause addiction compared to other opioids, it is considered as the third abused opioid and results in the symptoms of opioid withdrawal in several cases, even in patients with no history of addiction (De Conno et al., 2005; Pollice et al., 2008). A 61-year-old patient described that she was very agitated if she skipped or delayed tramadol and that the nervousness disappeared after tramadol intake (Pollice et al., 2008). Additionally, several reports showed that the intravenous administration of tramadol causes seizures (Raiger et al., 2012). It is widely accepted that addiction affects the metabolism of biochemical parameters that take part in energy production and fatigue. Thus, the effect of the chronic administration of tramadol on several biochemical parameters was explored in this study.
Importantly, the ability of tramadol (similar to other opioids) to cross the blood-brain barrier (BBB) contributes to its benefits and risks (Schaefer et al., 2017). Several researches reviewed by Tardy et al. (2020) addressed the role of many vitamins and minerals in energy-yielding processes, oxygen (O2) transport, and brain and muscle functions. Thus, these vitamins and minerals are considered significant contributors to fatigue (a sense of energy depletion) in its physical and mental forms (Ryan and Frederick, 1997). Accumulating lines of evidence demonstrated that the brain consumes 20% of the energy produced from glucose (Herculano-Houzel, 2011). Cellular energy production has many steps starting from the oxidation of macronutrients into acetyl coenzyme A, leading to the generation of pyruvate from glucose followed by the citric acid cycle (CAC) (Alberts et al., 2017). The CAC produces flavin adenine dinucleotide (FADH2) and dihydronicotinamide adenine dinucleotide (NADH) via oxidative processes that require several vitamins and minerals, including vitamin B12, Fe, and magnesium (Mg+2) (Alberts et al., 2017). The final process includes the transfer of electrons from FADH2 and NADH electron carriers to the electron transport chain to generate adenosine triphosphate (ATP), a process that also needs some vitamins and minerals such as ferrous (Fe) (Alberts et al., 2017). Notably, triglycerides (TG) contribute to the transfer of blood glucose from the liver (Alves-Bezerra and Cohen, 2017). Besides, Mg+2 has a major role in energy production and utilization by forming a complex with ATP (Yamanaka et al., 2016). Mg+2 is also needed as a cofactor for kinases in the glycolysis process, in CAC and ATP export from mitochondria to the cytosol (Yamanaka et al., 2016). Mg+2 affects muscle strength, cardiorespiratory functions, and neuromuscular performance (Zhang et al., 2017). Another important parameter that contributes to energy production or depletion (fatigue) is the availability of O2 for the brain and muscles as well as the availability of adequate amounts of vitamins and minerals needed for its availability (Tardy et al., 2020). It is worth mentioning that iron in the Fe+2 state can bind to the porphyrin ring of heme in cytochromes (the electron carriers needed for energy production), allowing the reversible binding of O2 (Aggett, 2012). In addition, Fe is crucial for the O2 transport in the myoglobin heme protein (Aggett, 2012). Thus, the deficiency of Fe reduces O2 transport and supply to blood and muscles, leading to impairment in the energetic efficiency (Aggett, 2012). In the context of the effect of Fe deficiency on neuronal fatigue, Munoz and Humeres (2012) provided a solid basis for the effect of Fe deficiency on the neurotransmitter system. Also, Fe deficiency can lead to a decrease in the activity of Fe-containing enzymes and O2 delivery to different tissues and thus to fatigue (Yokoi and Konomi, 2017). Additionally, it was noted that vitamin B12 is involved in DNA synthesis, activation of folate, the production of red blood cells, and O2 delivery (Hoffbrand and Jackson, 1993). Moreover, several lines of evidence revealed the antioxidative properties of zinc (Zn), which is a cofactor for several enzymes that take part in energy production (Holt et al., 2012; Mezzaroba et al., 2019). Another aspect that can be considered in energy production is the protein levels. Proteins are fundamental for structural integrity and important biochemical reactions (Tóthová et al., 2018). Proteins have roles in homeostasis, coagulation, energy production, enzymes activity, platelet adhesion, and aggregation (Tóthová et al., 2018). Despite that, the increase in serum protein concentration indicates diseases and health disorders in the body (Anderson and Anderson, 2002).
Aim of the study
In this study, we tested the effect of the oral administration of tramadol hydrochloride (HCl) on serum levels of some biochemical parameters that play key roles in several processes, including energy production and O2 transport. In more detail, we measured the levels of Fe+2, glucose, vitamin B12 (cobalamin), total protein (TP), TG, Zn, and Mg+2 in rats.
Ethical approval
All experiments were approved by the Animal Ethics Committee at the Applied Science Private University, Amman, Jordan (Ethical approval no. 2021-PHA-11).
MATERIALS AND METHODS
Materials
Capsules of tramadol HCl were purchased from Standardarzneimittel Deutscher Apotheker (Italy), while diethyl ether was from Scharlau, Barcelona, Spain. Kits for measuring the levels of Fe+2, Mg+2, glucose, TP, TG, and Zn were brought from Abbott Diagnostics (Milano, Italy). The vitamin B12 kit was provided by DiaSorin (Via Crescentino, Italy). Syringes and needles were from Thermo Fisher Scientific, Waltham.
Methods
Animals
Male Wistar rats (150–200 g) were used in this study. The animals were kept at the Animal House of the Applied Science Private University, Amman, Jordan, in a temperature-controlled environment (at 22°C ± 1°C) with a 12/12 hours light-dark cycle. Food pellets and water were provided ad libitum. All experiments were approved by the Animal Eethics Ccommittee at the Applied Science Private University, Amman, Jordan (Ethical Aapproval number no. 2021-PHA-11).
Treatment of animals
Before starting the experiment, the animals were allowed to acclimatize to the conditions of the experimental room. The animals were divided into control and experimental groups (n = 8 in each group). All animals were weighed to ensure the accuracy of the administered doses. Tramadol HCl capsules (100 mg each) were finely ground into a powder using a mortar and pestle and then were dissolved in normal saline to make a stock solution. Tramadol HCl was freshly prepared on the day of treatment. The control group received oral gavage of normal saline, while the experimental groups received 50 or 100 mg/kg tramadol HCl orally. The oral treatments were administered to the animals every day for a total period of 30 days pursuant to El-Gaafarawi (2006). No anesthesia was used during the treatment.
Blood collection and serum preparation
The animals were sacrificed under ether anesthesia after 30 days of receiving the oral gavage treatments to collect blood from the eyes. The skin around the eye of the rat was pulled with fingers. After that, a 25-gauge needle was fixed to a syringe and inserted at a 30° angle into the medial canthus of the eye until the needed volume of blood was withdrawn. The blood was kept to settle for 30 minutes at room temperature to allow coagulation and separate the serum. Samples were then centrifuged at 1,300 g for 15 minutes. The supernatant was preserved at −80°C.
Determining the levels of serum parameters
On the day of conducting the biochemical analysis, all serum samples were removed from the deep freeze and were allowed to thaw. The levels of Fe+2, Mg+2, glucose, TP, TG, and Zn were assayed using the Architect i2000SR analyzer and kits from Abbott Diagnostics (Milano, Italy). Vitamin B12 was determined using the LIAISON vitamin B12 automated assay and kits from DiaSorin (Via Crescentino, Italy). All samples were analyzed according to the kit manufacturer’s instructions.
Statistical analysis
Data were presented as the mean ± standard error of the mean (SEM). The normality test was conducted for all groups using the Shapiro–Wilk test. The statistical significance of the difference between groups was assessed by one-way analysis of variance (ANOVA) followed by Dunnett’s test using GraphPad Prism version 7. P < 0.05 was considered to be significant. Graphs were created using GraphPad Prism version 7.
RESULTS AND DISCUSSION
It is widely accepted that addiction affects the metabolism of biochemical parameters that take part in energy production and fatigue (Karam et al., 2004). The effects of addiction vary based on the abused substance, duration of addiction, dose of substance, and type of species used in the experiments. In this study, we investigated the effect of the oral administration of tramadol HCl on different serum parameters in male rats. The results showed that daily oral gavage of 50 or 100 mg/kg tramadol HCl for a month caused noticeable and significant reductions in the levels of Fe+2 and glucose as presented in Figure 1a and b. In more detail, the oral treatment of 50 and 100 mg/kg tramadol HCl decreased the levels of Fe+2 in the serum by 73.31% and 65.54%, respectively. We chose to test Fe+2 as it is the form that binds to heme and ferritin (Lane et al., 2015). Additionally, the decrease in glucose levels reached 52.60% in the group that received 50 mg/kg tramadol HCl and 54.57% in the animals treated with 100 mg/kg tramadol HCl. Notably, only the dose of 50 mg/kg tramadol HCl increased the levels of vitamin B12 in the serum (887 ± 51.88 pg/ml) compared to the control group (699.1 ± 41.03 pg/ml), Figure 2a. On the other hand, the low and high doses of tramadol HCl augmented TP, TG, and Mg+2 serum levels significantly compared to the control group (Figure 2b–d). The dramatic and highest rise was found in the TG levels, whereby 115.1% and 96.99% increases were revealed in the animals treated with 50 and 100 mg/kg tramadol HCl, respectively. Notably, the administration of tramadol HCl had no effect on the serum levels of Zn compared to the control group nor on body weight difference (Figure 3a and b). It is noteworthy that the treated animals showed aggressive behavior, which was eliminated upon receiving the daily dose of tramadol.
As in other opioids, the main explanation for the results is that the effects produced by tramadol HCl were due to its ability to cross the BBB and to produce effects on the central nervous system (CNS), thus affecting the metabolic response of various vitamins and minerals in the body (Molina et al., 1994). In this regard, earlier reports indicated that normal Zn levels are important for the suppression of β-amyloid-induced neurotoxicity (Mezzaroba et al., 2019). Moreover, Fe is considered a crucial element for the anabolic processes in mitochondria and for binding to ferritin in heme (Lane et al., 2015). Previous studies reported that massive Fe depletion causes cell death, indicating the importance of Fe for cell survival (Lawen and Lane, 2013). Besides, Fe-starved cells tend to stop other biochemical pathways that rely on Fe as well as storage pathways (Duke University Medical Center, 2008). Importantly, the cells keep tight control for Fe levels, whereby an increase or decrease in its levels is detrimental (Lawen and Lane, 2013). Accordingly, the reduction of serum Fe+2 levels after tramadol HCl administration presented in this study suggests that several vital processes (e.g., energy-yielding processes) in the body were affected, leading to detrimental effects. Moreover, the decrease in glucose levels observed in this study can be due to a reshuffling mechanism from the use of Fe to glucose for energy production, a mechanism suggested to be activated during Fe starvation in the cells (Duke University Medical Center, 2008). In more detail, mitochondria that need Fe for its processes shut down energy production during Fe deficiency leading to the utilization of glucose outside mitochondria (Duke University Medical Center, 2008). Despite the fact that the reshuffling mechanism was identified in yeasts, the researchers stressed the fact that these results are predicted to be involved in humans due to conserved mechanisms (Duke University Medical Center, 2008). All of these factors can explain the feeling of tiredness and lethargy during Fe deficiency due to the correlation between Fe, glucose, and energy (Duke University Medical Center, 2008).
In addition, the hypoglycemic effect of tramadol HCl shown in the current study can be related to the decrease in the production of hepatic glucose that is correlated with the rate of glycogen breakdown and/or inhibition of glucagon release from α cells (Molina et al., 1994). Also, it can be attributed to a suppressive effect of CNS on beta- (β-) cell function, a direct effect of tramadol HCl on β-cells, or to the rise in catecholamines levels that inhibit insulin release (Molina et al., 1994). Additionally, there could be an inhibitory effect for tramadol HCl on somatostatin release (Molina et al., 1994). Further research can provide answers for the tramadol HCl mechanism of action. Of note, earlier work showed that the effect of morphine on glucose differs according to the route of administration, whereby the intrathecal administration caused a hypoglycemic effect in contrast to the intracerebroventricular administration of morphine (Molina et al., 1994). Similar variations are expected upon using other substances such as tramadol HCl.
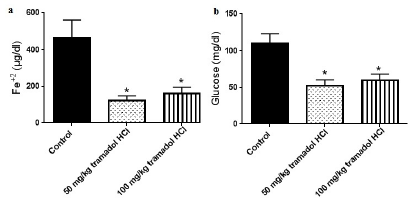 | Figure 1. Tramadol HC1 decreases Fe+2 (a) and glucose (b) serum levels. Data presented as mean ± SEM, n = 8. *Significant compared to control group, p < 0.05. One way ANOVA followed by Dunnett’s test post-hoc test. [Click here to view] |
In contrast to the suppression of Fe+2 and glucose levels demonstrated in this study, the daily oral gavage of tramadol HCl augmented the levels of TP, TG, vitamin B12, and Mg+2. By looking at the literature, it was reported that vitamin B12 deficiencies could lead to fatigue, diminished energy, and shortness of breath (Stabler, 2013). Based on the findings in this work, the increase in serum vitamin B12 concentration can be justified as a defense mechanism in the body to avoid energy depletion caused by imbalances of multiple parameters. Further, several studies revealed the link between vitamin B12 and cognition, indicating that the level of vitamin B12 is important for mental fatigue (Kennedy, 2016). Regarding Mg+2, Zhang et al. (2017) reported that the increase in Mg+2 supply enhanced the availability of glucose in the brain, muscles, and blood and decreased the requirements for O2. Thus, the huge decrease in glucose levels exhibited in this study can be a contributing factor to the increase in Mg+2 that can enhance glucose availability and decrease O2 requirements. The role of Mg+2 is emphasized by the translocation of Mg+2 to the locations of energy production (Zhang et al., 2017). Also, it was noted that the levels of plasma Mg+2 decreased in alcohol abusers compared to healthy people (Nechifor, 2011). Additionally, the findings of this study proved that tramadol HCl treatment caused an increase in the levels of TG. It is well established that the brain uses glucose as a major energy fuel compared to muscles that utilize glycogen (Bordone et al., 2019). However, when glucose levels reduce, the brain can use alternative substrates for energy, such as medium-chain TG and other substrates (Bordone et al., 2019). Accordingly, the increase in the levels of TG after tramadol HCl treatment can be a compensatory mechanism for the decrease in glucose that is needed for energy production.
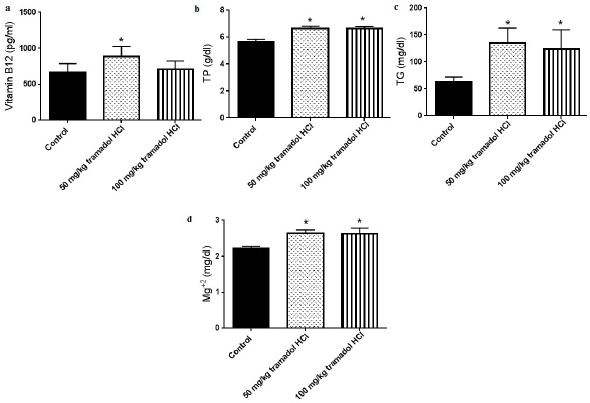 | Figure 2. Tramadol HC1 increases vitamin B12 (a) TP (b) TG (c) and Mg+2 (d) serum levels. Data presented as mean ± SEM, n = 8. *Significant compared to control group, p < 0.05. One way ANOVA followed by Dunnett’s test post-hoc test. [Click here to view] |
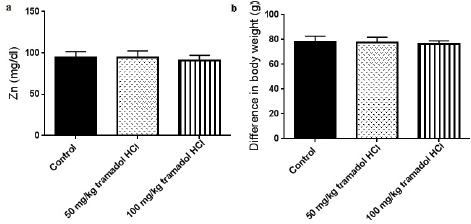 | Figure 3. Tramadol HC1 had no effects on Zn serum levels (a) or body weight differences of animals (b). Data presented as mean ± SEM, n = 8. *Significant compared to control group, p < 0.05. One way ANOVA followed by Dunnett’s test post-hoc test. [Click here to view] |
With respect to TP, it is noteworthy to mention that the serum TP measures the amount of albumin and globulin proteins. High levels of serum protein may indicate cancer, inflammation, chronic kidney disease, liver disease, or dehydration (Tóthová et al., 2018; Wu et al., 2019;). As serum proteins include albumin and globulin, there are two possibilities for explaining the increase in their levels. The first possibility is the increase in albumin-to-globulin ratio, a matter that can refer to the occurrence of leukemia or underproduction of antibodies (Busher, 1990). The other possibility includes high globulin levels compared to albumin, which may indicate the existence of an autoimmune disease, liver cirrhosis, nephrotic syndrome, multiple myeloma, gastrointestinal diseases, or internal parasitism (Diogenes et al., 2010; Tóthová et al., 2018; Wu et al., 2019).
Notably, the dose of 50 mg/kg tramadol HCl produced an increase in vitamin B12 levels that were not exhibited in the group treated with 100 mg/kg. In fact, by considering the Food and Drug Administration guidelines (2005) on converting the doses used in humans and animals, the dose of 50 mg/kg tramadol HCl used in rats is equivalent to the ones consumed by humans. On the other hand, although calcium (Ca+2) and phosphorus (P) levels were not measured in this study, a change in their levels is expected as Mg+2 is involved in the active transport of Ca+2 and potassium across cell membranes (Kirkland et al., 2018). Also, phosphorus is involved in the phosphorylation of many sugars and proteins, activity of enzymes, intracellular storage of energy, and maintenance of pH (Heaney, 2012). Further investigation into the effect of tramadol HCl on other biochemical parameters is recommended in order to apply strategies that can help in decreasing fatigue among addicted people.
CONCLUSION
Taken together, the results of this study showed that oral gavage of tramadol HCl caused a reduction in the levels of Fe+2 and glucose accompanied by a noticeable enhancement effect for vitamin B12, TP, TG, and Mg+2 concentrations. Tramadol HCl treatment had no change in Zn levels. We strongly recommend a periodic nutritional follow-up for addicted people to avoid fatigue symptoms due to malnutrition. Based on the outcome of biochemical analysis, vitamin and mineral supplementations can contribute to a better health status for addicted people.
ACKNOWLEDGEMENTS
The authors acknowledge the Applied Science Private University, Amman, Jordan, for funding this research.
CONFLICT OF INTEREST
The authors had no conflicts to declare.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aggett PJ. Iron in present knowledge in nutrition. In: Erdman JW, Macdonald IA, Zeisel SH (eds.). Present knowledge in nutrition. 10th edition, Wiley-Blackwell, Washington, DC, pp 506–20, 2012. CrossRef
Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P. Cell chemistry and bioenergetics. In Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P, Wilson J, Hunt T: Molecular biology of the cell, Garland-Sciences-CRCPress, New York, NY, 43–108, 2017. CrossRef
Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol, 2017; 8:1–22. CrossRef
Anderson NL, Anderson NG. The human plasma proteome. Mol Cell Proteom, 2002; 1:845–67. CrossRef
Bordone MP, Salman MM, Titus HE, Amini E, Andersen JV, Chakraborti B, Diuba AV, Dubouskaya TG, Ehrke E, Espindola de Freitas A, Braga de Freitas G, Gonçalves RA, Gupta D, Gupta R, Ha SR, Hemming IA, Jaggar M, Jakobsen E, Kumari P, Lakkappa N, Marsh APL, Mitlöhner J, Ogawa Y, Paidi RK, Ribeiro FC, Salamian A, Saleem S, Sharma S, Silva JM, Singh S, Sulakhiya K, Tefera TW, Vafadari B, Yadav A Yamazaki R, Seidenbecher CI. The energetic brain—a review from students to students. J Neurochem, 2019; 151(2):139–65. CrossRef
Busher JT. Serum albumin and globulin. In: Walker HK, Hall WD, Hurst JW (eds.). Clinical methods: the history, physical and laboratory examinations, 3rd edition, Butterworths, Boston, MA, 1990.
De Conno F, Ripamonti C, Brunelli C. Opioid purchases and expenditure in nine western European countries: are we killing off morphine? Palliat Med, 2005; 19(3):179–84. CrossRef
Duke University Medical Center. Why cells starved of iron burn more glucose. In: ScienceDaily, Duke University Medical Center, 2008. Available via www sciencedaily com/releases/2008/06/080609090709 htm (Accessed 2 June 2021).
Diogenes PVA, Suassuna ACD, Ahid SMM, Soto-Blanco B. Serum protein electrophoretic profile of goats infected with Haemonchus contortus. J Anim Vet Adv, 2010; 9:1603–6. CrossRef
El-Gaafarawi II. Biochemical toxicity induced by tramadol administration in male rats. Egypt J Hosp Med, 2006; 23(1):353–62. CrossRef
Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity potency and efficacy of tramadol and its metabolites at the cloned human μ-opioid receptor. Naunyn-Schmiedeberg’s Arch Pharmacol, 2000; 362:116–21. CrossRef
Food and drug administration. Guidance for industry estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Food and drug administration, Rockville, US, pp 1–27, 2005.
Heaney RP, Phosphorus. In: Erdman JW, Macdonald IA, Zeisel SH (eds.). Present knowledge in nutrition, 10th edition, Wiley-Blackwell, Washington, DC, 447–58, 2012.
Herculano-Houzel S. Scaling of brain metabolism with a fixed energy budget per neuron: Implications for neuronal activity, plasticity and evolution. PLoS One, 2011; 6:e17514. CrossRef
Hoffbrand AV, Jackson BF. Correction of the DNA synthesis defect in vitamin B12 deficiency by tetrahydrofolate: evidence in favour of the methyl-folate trap hypothesis as the cause of megaloblastic anemia in vitamin B12 deficiency. Br J Haematol, 1993; 83:643–7. CrossRef
Holt RR, Uriu-Adams JY, Keen CL. Zinc in present knowledge in nutrition. In: Erdman JW, Macdonald IA, Zeisel SH (eds.). Wiley-Blackwell, Washington, DC, pp 521–39, 2012. CrossRef
Karam GA, Reisi M, Kaseb AA, Khaksari M, Mohammadi A, Mahmoodi M. Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol, 2004; 9(1):53–8. CrossRef
Kennedy DOB. Vitamins and the brain: mechanisms dose and efficacy- a review. Nutrients, 2016; 8:68. CrossRef
Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients, 2018; 10:730. CrossRef
Lane D, Merlot A, Huang M, Bae D, Jansson P, Sahni S, Kalinowski D, Richardson D. Cellular iron uptake trafficking and metabolism: key molecules and mechanisms and their roles in disease. Biochim Biophys Acta Mol Cell Res, 2015; 1853(5):1130–44. CrossRef
Lawen A, Lane DJR. Mammalian iron homeostasis in health and disease: uptake storage transport and molecular mechanisms of action. Antioxid Redox Signal, 2013; 18:2473–507. CrossRef
Mezzaroba L, Alfieri DF, Colado Simao AN, Vissoci Reiche EM. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology, 2019; 74:230–41. CrossRef
Molina PE, Hashiguchi Y, Ajmal M, Mazza M, Abumrad NN. Differential hemodynamic metabolic and hormonal effects of morphine and morphine-6-glucuronide. Brain Res, 1994; 664(1-2):126–32. CrossRef
Munoz P, Humeres A. Iron deficiency on neuronal function. Biometals, 2012; 25:825–35. CrossRef
Nechifor M. Magnesium in drug abuse and addiction. In: Vink R, Nechifor M (eds.). Magnesium in the Central Nervous System, University of Adelaide Press, Adelaide, Australia, 331–42, 2011.
Osman M, Mustafa M. Tramadol-induced mood elevation in a patient with no previous psychiatric history. Case Rep Psychiatry, 2018; 2018:1–3. CrossRef
Pollice R, Casacchia M, Bianchini V, Mazza M, Conti CM, Roncone R. Severe Tramadol addiction in a 61 year-old woman without a history of substance abuse. Int J Immunopathol Pharmacol, 2008; 21(2):475–6. CrossRef
Raiger LK, Naithani U, Bhatia S, Chauhan SS. Seizures after intravenous tramadol given as premedication. Indian J Anaesth, 2012; 56(1):55. CrossRef
Rojas-Corrales M, Gibert-Rahola J, Micó J. Tramadol induces antidepressant-type effects in mice. Life Sci, 1998; 63(12):PL175–80. CrossRef
Ryan RM, Frederick C. On energy personality and health: subjective vitality as a dynamic reflection of well-being. J Peers, 1997; 65:529–65. CrossRef
Schaefer CP, Tome ME, Davis TP. The opioid epidemic: a central role for the blood brain barrier in opioid analgesia and abuse. FBCNS, 2017; 14(1):32. CrossRef
Stabler SP. Clinical practice, vitamin B12 deficiency. N Engl J Med, 2013; 368:149–60. CrossRef
Tardy A, Pouteau E, Marquez D, Yilmaz C, Scholey A. Vitamins and minerals for energy fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients, 2020; 12(1):228. CrossRef
Tashakori A, Afshari R. Tramadol overdose as a cause of serotonin syndrome: a case series. Clin Toxicol, 2010; 48(4):337–41. CrossRef
Tóthová C, Mihajlovi?ová X, Nagy O. The use of serum proteins in the laboratory diagnosis of health disorders in ruminants. In Muhammad Abubakar, Ruminants - the husbandry economic and health aspects, IntechOpen, 105–46, 2018. CrossRef
Wu P, Hsieh Y, Kor C, Chiu P. Association between albumin–globulin ratio and mortality in patients with chronic kidney disease. J Clin Med, 2019; 8(11):1991. CrossRef
Yamanaka R, Tabata S, Shindo Y, Hotta K, Suzuki K, Soga T, Oka K. Mitochondrial Mg(2+) homeostasis decides cellular energy metabolism and vulnerability to stress. Sci Rep, 2016; 6:30027. CrossRef
Yokoi K, Konomi A. Iron deficiency without anemia is a potential cause of fatigue: meta-analyses of randomised controlled trials and cross-sectional studies. Br J Nutr, 2017; 117:1422–31. CrossRef
Zhang Y, Xun P, Wang R, Mao L, He K. Can magnesium enhance exercise performance? Nutrients, 2017; 9:946. CrossRef