INTRODUCTION
Human breast cancer is the most prevalent cancer in the world with the death rate 17.71 per 100,000 women (Bray et al., 2018). The current treatment to control the mortality rate of breast cancer has as yet not been optimal, due to the emergence of apoptotic resistance in cancer stem cells (CSCs) to several chemotherapeutic agents (Hermansyah et al., 2021). This is supposed to be due to the presence of CSCs within a tumor which have the ability to protect themselves from the apoptotic program by releasing the anti-apoptotic protein (He et al., 2014). On the other hand, tumor-associated macrophages (TAMs) as a CSCs niche play a significant role in promoting cancer cell proliferation by constructing immunotolerance and actively enhancing the CSC apoptotic resistance (Ge et al., 2020). TAMs enhance the apoptotic resistance of CSCs by extremely decreasing the low levels of inducible nitric oxide and a reactive oxygen intermediate (Perrotta et al., 2018). The lower level of reactive oxygen species (ROS) also inhibits the production of extracellular hydrogen peroxide on M2 macrophages leading to decreased signal transducer and activator of transcription 3 activation that inhibits apoptosis in cancer cells (Griess et al., 2020). On the other hand, heat shock protein-70 (Hsp-70) expression may modulate TAMs to upregulate those molecules (Gabai et al., 2016; Komarova et al., 2020). iNO stimulates the expression of Hsp-70, by means of ROI-mediated modification in intracellular antioxidant levels leading to apoptosis inhibition (Manucha and Valles, 2008). Currently, several drugs have been developed that target the tumor microenvironment (Bissell et al., 2011; Wartha et al., 2014), but our options to target TAMs are still limited. Therefore, the target Hsp-70 TAMs leading to induction of the apoptotic program are a promising strategy for the treatment of cancer.
Typhonium flagelliforme (TF) is a herbal medicinal plant widely used in various anticancer studies (Chodidjah et al., 2017; Lai et al., 2008; Purwaningsih et al., 2014). A previous study reported that TF contains saturated hydrocarbons, aliphatic acids, fatty acids, and linoleate acid (LA) (Lai et al., 2010; Mankaran et al., 2013). A previous study also reported that TF extract has the capability to inhibit cell growth of P388 lymphocytic leukemia cells (Choo et al., 2001). TF also induces apoptosis and G0/G1 cycle arrest in cellosaurus cells by upregulating caspase-9 and release of cytochrome c (Mohan et al., 2010). Apoptotic inducted LA is thought to be by binding on CSCs-peroxisome proliferator-activated receptors (PPARγ) (Arnett et al., 2018). The activated complexes of LA-CSCs-PPARγ initiate the activation of several proapoptotic proteins (Hsu and Ip, 2006). Our previous study also found that TF triggered apoptosis in CSCs by decreasing survivin expression and elevating caspase-3 and caspase-9 levels (Putra et al., 2018). However, the effect of TF in increasing Hsp-70 expression of TAMs leading to apoptosis improvement remains unclear. The current study is a continuation of our previous study, which has been previously reported (Putra et al., 2018). In the present study, we aimed to identify a possible pathway of Hsp-70-TAM-targeted apoptosis induction in CSCs by TF.
MATERIALS AND METHODS
Extraction of TF
TF (Araceae) herbs were collected from Semarang, Central Java, Indonesia (latitude 7.0051°S and longitude 110.4381°E). Authentication was conducted at the Plant Systematic Laboratory, Faculty of Biology, Universitas Gadjah Mada. 360.4 g of the fresh plant was harvested and washed thoroughly with circulating distilled water, followed by tubers being chopped into small pieces and dried (Memmert, Models 100–800, Schwabach, Germany). The dried tubers were powdered and macerated with hexane for 3 days to remove the nonpolar fractions before being treated with dichloromethanes for 7 days with intermittent shaking (Edmund Buchler Shaker, Model KS-15, Hechingen, Germany) and then filtered and vacuum-evaporated using a rotary evaporator (Buchi, R-210, Postfach, Switzerland) (Suzery et al., 2020).
Breast CSCs sorting
CSCs were isolated using magnetic-activated cell sorting (MACS) (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, total populations of MCF-7 breast cancer cells were harvested into a single cell suspension and counted to validate the quantity of the total cells. 300 g of 1 × 107 MCF-7 breast cancer cells was centrifuged for 10 minutes to obtain the pellet. The pellet was incubated with 10 μl microbeads directly conjugated to human monoclonal anti-human CD24-biotin antibody at 2°C–8°C for 15 minutes. Subsequently, the suspended cells were washed by a 1 ml buffer and added to the MACS column that was positioned in a MACS separator’s magnetic field. The unlabeled CD24− cells were eluted from the column by a 500 μl buffer and collected as positively selected cells for further investigation. CD24− cell fractions were centrifugated at 300 g for 10 minutes, and then the cell pellet was resuspended in an 80 μl buffer. Finally, 20 μl CD44 microbeads were added to cell suspensions, and these were mixed and then incubated at 2°C–8°C for 15 minutes and then resuspended in a 500 μl buffer. The separation of CD44+ was performed by 500 μl flowed down the column MACS separator, followed by cell suspensions. The labeled cell fractions were flushed out by pushing the plunger into the column rapidly. The CD24−/CD44+ cell fractions were collected into a 15 ml tube.
CSCs validation
To induce mammosphere formation, several isolated CSCs (CD24−/CD44+) were plated in Dulbecco’s modified Eagle medium F-12 (DMEM F-12), enriched with 1% bovine serum albumin, 1 μM insulin, 10 ng/ml basic fibroblast growth factor, 20 ng/ml epidermal growth factor, and B-27 supplement, in a low-cell-binding dish. Mammospheres emerged as tight, spherical structures floating in the medium.
Macrophage-polarized TAMs sorting
For the isolation of TAMs using MACS, the peripheral blood mononuclear cells (PBMCs) derived from human breast cancer patients were cocultured with MCF-7 in Roswell Park Memorial Institute media on 72 hours incubation. Human TAMs were purified by MACS with anti-CD163 followed by antiphycoerythrin microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer’s protocol.
Hsp-70 analysis
Briefly, 5 × 104 CSCs and TAM ml−1 were seeded on adhesive slides-coated slides (Matsunami, Japan) and treated by TF concentration at 50.89 µg/ml as IC50 according to our previous study (Putra et al., 2018). The cells were incubated at atmospheric pressure of 5% CO2 and temperature 37°C for 24 hours and observed under an inverted microscope. After incubation, cells were fixed with 4% paraformaldehyde for 10 minutes, rinsed with phosphate buffer saline, and permeabilized for 10 minutes with 0.1% Triton-X100. Hsp-70 antigen retrieval was performed in a microwave for 20 minutes with 10 mM citrate buffer (pH 6.0). Endogenous peroxidase was then inactivated for 10 minutes in methanol with 3% H2O2. Ten percent of normal goat serum (Nichirei, Tokyo, Japan) was administered to the cells for 10 minutes and incubated with primary antibodies for Hsp-70 (rabbit polyclonal, Abcam, 1:100) at 4°C overnight. The secondary antibody, biotin-conjugated anti-rabbit IgG (Nichirei, Tokyo, Japan), was used to visualize antibody binding using peroxidase-conjugated streptavidin (Nichirei, Tokyo, Japan), followed by 3,3′-diaminobenzidine (DAB) solution (Metal-Enhanced DAB Substrate Kit, Thermo Scientific, USA). The cells were dehydrated and mounted after being counterstained with new hematoxylin (Muto Pure Chemicals Co., Ltd., Tokyo, Japan). The presence of Hsp-70 staining in TAMs was investigated.
The UALCAN database and the Kaplan-Meier Plotter (KM Plotter)
The UALCAN (http://ualcan.path.uab.edu/) database serves as a portal for tumor gene expression and survival studies (Wu et al., 2020). This study’s screening conditions are as follows: Gene: HSPA1A; HSP1A1 is Hsp-70 kDa protein 1A that molecular chaperone implicated in a wide variety of cellular processes. The analysis type of this study used was Breast Cancer versus. Normal Analysis; Cancer Type: Breast Invasive Carcinoma; and Data Type: the Cancer Genome Atlas Project (TCGA) dataset. We also evaluated the survival analysis using the breast cancer dataset of the KM Plotter. The screening conditions are as follows: “Cancer: Breast Cancer”; “Gene symbol: HSPA1A”; “Affy id: 203326_at”; “Survival: Relapse free survival” (Amalina et al., 2021; Cahyono et al., 2021; Mursiti et al., 2021).
Statistical analysis
All studies were performed at least three times, and 200 cells were evaluated for each plot in the immunocytochemical staining analysis. Results are expressed as mean ± SD and analyzed using one-way analysis of variance. Differences were analyzed by the Duncan post hoc test for all groups. p < 0.05 was considered to be significant. The statistical analysis was performed with Statistical Package for the Social Sciences version 22.0 (SPSS Inc., Chicago, IL).
RESULTS AND DISCUSSION
Cancer cells have high expression of chaperones, particularly Hsp-70, which promote proliferation and escape from programmed cell death (Albakova et al., 2020; Komarova et al., 2020). Naturally, Hsp-70 can be released into the extracellular milieu by cancer cells without an additional trigger (Seclì et al., 2021). In addition, the higher level of Hsp-70 in cancer cells is associated with more chaperone molecules in the microenvironment of CSCs (Kabakov et al., 2020; Vega et al., 2008). Furthermore, TAMs constitute the tumor microenvironment cellular population which strongly influence cancer cell tumorigenicity including promoting cancer progression, development, metastasis, cancer stemness, and escape immune surveillance (Ge et al., 2020). A previous study reported that a pancreas cancer patient with a high TAM density had a lower survival rate (Jung et al., 2015). In fact, TAM depletion slows the progression of lung cancer (Fritz et al., 2014). In addition, the presence of tumors influences the maintenance of TAM polarization, indicating that tumor cells can maintain TAM polarization. The maintenance of TAM polarization is dependent on the presence of tumors, which suggests that tumor cells can maintain TAM polarization. A previous study also reported that TAMs can promote tumor progression and immune protection through inducing secretion of transforming growth factor-β, vascular endothelial growth factor, fibroblast growth factor, and several matrix metalloproteinases (Ge et al., 2020). Therefore, to establish a possible effect of Hsp-70-TAM-targeted apoptosis induction in CSCs by TF tuber extract as a natural chemotherapeutic agent is needed.
CSCs isolation and characterization
MCF-7 cells were used to isolate the CSC population based on the expression of CD44+/CD24− by MACS. The isolated CSC population expressed the highest CD44+/CD24− surface marker up to 93.80% ± 0.50% compared to CD44−/CD24− (3.10%), CD44−/CD24+ (0.10%), and CD44+/CD24+
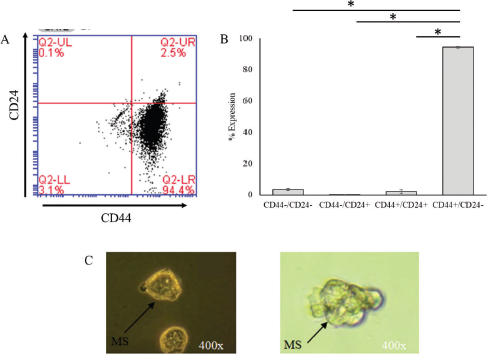 | Figure 1. Characterization and validation of clone CSCs. (A) Flow cytometry detection of CD44 and CD24 markers on the surface of CSCs. (B) Percentage of surface marker CSCs, positive CD44, and CD24 negative cells. (C) The formation of mammosphere clones of CSCs. Black arrows indicate the morphology of mammospheres with a size in the range of 80–230 µm (*p < 0.05). [Click here to view] |
MCF-7-induced TAMs
PBMC was cocultured with the MCF-7 cell line to trigger the polarization of macrophages into TAMs. This study showed that the morphological change of macrophages occurred along with the increase of the MCF-7 population (Fig. 2A–F). This finding indicated that the M1 macrophage successfully polarized into the M2 macrophage leading to TAM formation.
TAM isolation and characterization
In order to investigate the effect of TF administration on coculture of a TAM and CSCs, first, we isolated a TAM from the tumor microenvironment using MACS using the CD163 surface marker. The isolated cell expressed a high level of CD163 up to 99.90% on flow cytometry analysis (Fig. 3A) and showed different morphological features compared to the negatively expressed CD163 cell colony (Fig. 3B). TAM colonies were used to analyze the effect of TF on Hsp-70 expression in coculture with a TAM and CSCs.
TF induces the expression of Hsp-70 in TAMs
Our previous study found that 50.89 µg/ml TF was shown to reduce 50% cell viability in CSCs (Putra et al., 2018). In order to further evaluate the activity of Hsp-70 on coculture CSCs with a TAM, we performed an immunocytochemistry assay. This study found that TF administration highly induces Hsp-70 expression in TAMs up to 67.67% (Fig. 4). Hsp-70 was localized on the cell membrane surface of cancer cells. Expression of HSPA1A (Hsp-70) across TCGA tumors indicated that the expression level of Hsp-70 was higher in all TCGA tumors than its matched normal tissue in most of the tumors (Fig. 5A), including in breast cancer invasive tumor. We also gathered clinical data for 1,097 cancer patients from the TCGA. Patients with high expression of Hsp-70 had significantly less likelihood of survival than patients with low medium expression of Hsp-70 (Fig. 5B). The results suggest that the presence of Hsp-70 in breast cancer cell predictors can be an indicator of likelihood of survival in breast invasive carcinoma patients.
The positive expression of Hsp-70 expression in TAMs in this study indicated that TF might induce the expression of Hsp-70 TAMs and then is secreted into an extracellular niche which bind to the transmembrane receptor of the TAM itself. This may lead to polarization of the TAM (type 2 macrophage) into a type 1 activated macrophage (M1) (Orecchioni et al., 2019). The active macrophages release the TNF-α ligand molecules (proinflammatory cytokines) and may bind to tumor necrosis factor receptor-1 of CSCs, leading to apoptosis of CSCs via an extrinsic apoptotic pathway (Ge et al., 2020; Holdbrooks et al., 2018). This is supported by a previous study where the increase of intracellular Hsp-70 expression would be secreted in part extracellularly and interacts with the surrounding microenvironment including macrophages that may bind to PPARγ (Wahli and Michalik, 2012). This suggested that TAM polarization to type 1 may strengthen apoptotic induction to CSCs. The limitation of this study is that we did not analyze the polarization of TAMs into type 1 activated macrophages as well as the soluble molecule released. Taken together, our data suggest that TF may enhance the CSCs cell death associated with positive expression of Hsp-70 in TAMs.
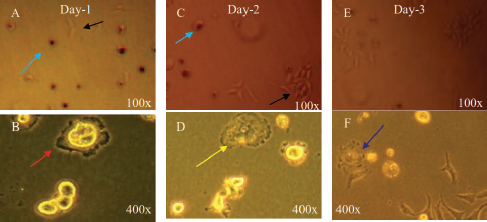 | Figure 2. Morphological change in the PBMC cocultured with the MCF-7 cell line. (A) The PBMC on day 1 after coculture (blue arrow) with the MCF-7 cell line (black arrow). (B) Pseudophili formation (red arrow) indicates the polarization of the hematopoietic progenitor into a macrophage. (C–D) The increased number of the MCF-7 population triggers macrophages to undergo the inactive phase (yellow arrow). (E) The number of the MCF-7 population continues to increase leading to macrophage polarization into TAMs (F) (blue arrow). [Click here to view] |
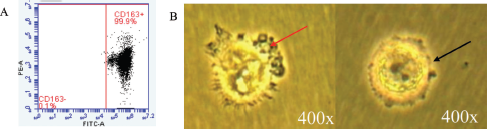 | Figure 3. CD163+ on coculture of TAM and CSCs. (A) Flow cytometry detection of CD163+ and (B) morphological features of the coculture population. Pseudophili formation (red arrow) and macrophage polarization into TAMs (black arrow). [Click here to view] |
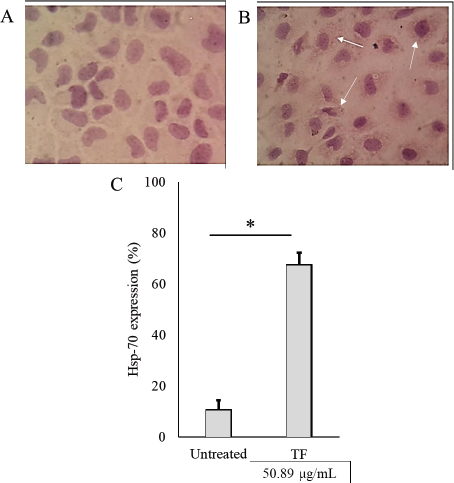 | Figure 4. Figure 4. Hsp-70 immunocytochemistry staining, the expression of Hsp-70 determined by brown color on the surface of the cell. (A) Control cells negatively express Hsp-70, (B) highly express Hsp-70 (white arrow), and (C) quantification of Hsp-70 expression. Values in the bar charts are presented as means ± standard deviation. *p < 0.05 versus untreated cells. [Click here to view] |
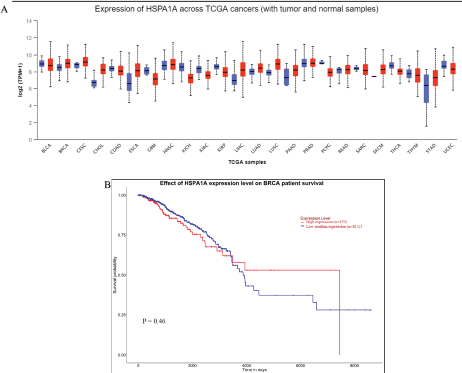 | Figure 5. (A) Expression of HSPA1A (Hsp-70) across TCGA cancers versus normal. (B) Survival curve analysis of TCGA breast cancer patients based on HSPA1A (Hsp-70) expression. The patients were divided into two groups. One group had high expression of Hsp-70, and the other group had low medium expression of Hsp-70. [Click here to view] |
CONCLUSION
Based on our findings, we conclude that the TF as a natural chemotherapeutic agent may decrease the CSC population by increasing Hsp-70 expression in TAMs. These findings also indicate that the potential anticancer activities of TF associated with TAM polarization should be further developed for cancer treatment in the future.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
The authors have declared that no conflicts of interest exist.
FUNDING
There is no funding to report.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Albakova Z, Armeev GA, Kanevskiy LM, Kovalenko EI, Sapozhnikov AM. HSP70 multi-functionality in cancer. Cells, 2020; 9(3):1–26. CrossRef
Amalina ND, Wahyuni S, Harjito. Cytotoxic effects of the synthesized Citrus aurantium peels extract nanoparticles against MDA-MB-231 breast cancer cells. J Phys Conf Ser, 2021; 1918(3):032006. CrossRef
Arnett E, Weaver AM, Woodyard KC, Montoya MJ, Li M, Hoang KV, Hayhurst A, Azad AK, Schlesinger LS. PPARγ is critical for Mycobacterium tuberculosis induction of Mcl-1 and limitation of human macrophage apoptosis. PLoS Pathog, 2018; 14(6):1–24. CrossRef
Bissell MJ, Hines WC, Berenblum I. Why don’t we get more cancer?? A proposed role of the microenvironment in restraining cancer progression. Nat Med, 2011; 17(3):320–9. CrossRef
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018; 68(6):394–424. CrossRef
Cahyono B, Amalina ND, Suzery M, Bima DN. Exploring the capability of Indonesia natural medicine secondary metabolite as potential inhibitors of SARS-CoV-2 proteins to prevent virulence of COVID-19?: in silico and bioinformatic approach. Open Access Maced J Med Sci, 2021; 9:336–42. CrossRef
Chodidjah C, Widayati E, Nasihun T. Treatment of thyponium flageliforme in combination with Curcuma zedoaria, and Phyllanthus niruri synergistically enhances apoptotic and anti-proliferative effect on breast cancer. J Nat Remedies, 2017; 17(1):1–8. CrossRef
Choo CY, Chan KL, Sam TW, Hitotsuyanagi Y, Takeya K. The cytotoxicity and chemical constituents of the hexane fraction of Typhonium flagelliforme (Araceace). J Ethnopharmacol, 2001; 77(1):129–31. CrossRef
Crabtree JS, Miele L. Breast cancer stem cells. Biomedicines, 2018; 6(77):133–51. CrossRef
Fritz JM, Tennis MA, Orlicky DJ, Lin H, Ju C, Redente EF, Choo KS, Staab TA, Bouchard RJ, Merrick DT, Malkinson AM. Depletion of tumor-associated macrophages slows the growth of chemically induced mouse lung adenocarcinomas. Front Immunol, 2014; 5:1–11. CrossRef
Gabai VL, Yaglom JA, Wang Y, Meng L, Shao H, Kim G, Colvin T, Gestwicki J, Sherman MY. Anticancer effects of targeting Hsp70 in tumor stromal cells. Cancer Res, 2016; 76(20):5926–32. CrossRef
Ge Z, Ding S. The crosstalk between tumor-associated macrophages (TAMs) and tumor cells and the corresponding targeted therapy. Front Oncol, 2020; 10:1–23. CrossRef
Griess B, Mir S, Datta K, Teoh-Fitzgerald M. Free radical biology and medicine scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic Biol Med, 2020; 147:48–60. CrossRef
He YC, Zhou FL, Shen Y, Liao DF, Cao D. Apoptotic death of cancer stem cells for cancer therapy. Int J Mol Sci, 2014; 15(5):8335–51. CrossRef
Hermansyah D, Putra A, Munir D, Lelo A, Amalina ND, Alif I. Synergistic effect of Curcuma longa extract in combination with Phyllanthus niruri extract in regulating Annexin A2, epidermal growth factor receptor, matrix metalloproteinases, and pyruvate kinase M1/2 signaling pathway on breast cancer stem cell. Open Access Maced J Med Sci, 2021; 9:271–85. CrossRef
Holdbrooks AT, Britain CM, Bellis SL. ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. J Biol Chem, 2018; 293(5):1610–22. CrossRef
Hsu YC, Ip MM. Conjugated linoleic acid-induced apoptosis in mouse mammary tumor cells is mediated by both G protein coupled receptor- dependent activation of the AMP-activated protein kinase pathway and by oxidative stress. Bone, 2006; 23(1):1–7.
Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, Park YJ. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med, 2015; 49(4):318–24. CrossRef
Kabakov A, Yakimova A, Matchuk O. Molecular chaperones in cancer stem cells: determinants of stemness and potential targets for antitumor therapy. Cells, 2020; 9(4):892. CrossRef
Komarova EY, Marchenko LV, Zhakhov AV, Nikotina AD, Aksenov ND, Suezov RV, Ischenko AM, Margulis BA, Guzhova IV. Extracellular hsp70 reduces the pro-tumor capacity of monocytes/macrophages co-cultivated with cancer cells. Int J Mol Sci, 2020; 21(1):59. CrossRef
Lai C, Mas RHMH, Nair NK, Majid MIA, Mansor SM, Navaratnam V. Typhonium flagelliforme inhibits cancer cell growth in vitro and induces apoptosis?: an evaluation by the bioactivity guided approach. J Ethnopharmacol, 2008; 118:14–20. CrossRef
Lai C, Mas RHMH, Nair NK, Mansor SM, Navaratnam V. Chemical constituents and in vitro anticancer activity of Typhonium flagelliforme (Araceae). J Ethnopharmacol, 2010; 127:486–94. CrossRef
Mankaran S, Kumar D, Sharma D, Singh G. Typhonium flagelliforme: a multipurpose plant. Int Res J Pharm, 2013; 4(3):45–8. CrossRef
Manucha W, G Valles P. Apoptosis modulated by oxidative stress and inflammation during obstructive nephropathy. Inflammation & Allergy-Drug Targets. 2008;11(4):303–12. CrossRef
Mohan S, Bustamam A, Ibrahim S, Al-zubairi AS. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage and cytochrome c release: its activation coupled with G0/G1 phase cell cycle arrest. J Ethnopharmacol, 2010; 131(3):592–600. CrossRef
Mursiti S, Amalina ND, Marianti A. Inhibition of breast cancer cell development using Citrus maxima extract through increasing levels of reactive oxygen species (ROS). J Phys Conf Ser, 2021; 1918(5):052005. CrossRef
Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(Lps+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front Immunol, 2019; 10:1–14. CrossRef
Perrotta C, Cervia D, Di Renzo I, Moscheni C, Bassi MT, Campana L, Martelli C, Catalani E, Giovarelli M, Zecchini S, Coazzoli M. Nitric oxide generated by tumor-associated macrophages is responsible for cancer resistance to cisplatin and correlated with syntaxin 4 and acid sphingomyelinase inhibition. Front Immunol, 2018; 9:1186. CrossRef
Purwaningsih E, Widayanti E, Suciati Y. Cytotoxicity assay of Typhonium flagelliforme Lodd against breast and cervical cells. Universa Med, 2014; 33(2):75–82.
Putra A, Ignatius R, Suharto, Taat P, Indra W. Typhonium flagelliforme extract induce apoptosis in breast cancer stem cells by suppressing survivin. J Cancer Res Ther, 2018; 14(7):1525–34.
Scioli MG, Storti G, D’amico F, Gentile P, Fabbri G, Cervelli V, Orlandi A. The role of breast cancer stem cells as a prognostic marker and a target to improve the efficacy of breast cancer therapy. Cancers (Basel), 2019; 11(7):1021. CrossRef
Seclì L, Fusella F, Avalle L, Brancaccio M. The dark-side of the outside: how extracellular heat shock proteins promote cancer. Cell Mol Life Sci [En ligne], 2021; (0123456789); doi:10.1007/s00018-021-03764-3 CrossRef
Suzery M, Cahyono B, Amalina ND. Antiproliferative and apoptosis effect of hyptolide from Hyptis pectinata (L.) Poit on human breast cancer cells. J Appl Pharm Sci, 2020; 10(02):1–6. CrossRef
Vega VL, Rodríguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol, 2008; 180(6):4299–307. CrossRef
Wu ZH, Zhang YJ, Yue JX, Zhou T. Comprehensive analysis of the expression and prognosis for SFRPs in breast carcinoma. Cell Transplant, 2020; 29:1–11. CrossRef
Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab, 2012; 23(7):351–63. CrossRef