INTRODUCTION
Lead (Pb) is a toxic heavy metal in the environment, and it represents a great concern to human health worldwide (Abdelhamid et al., 2020). It presents in multiple forms and sources, such as food and air pollution (Kosnett et al., 2007). It causes multiorgan toxicities and damages almost all vital organs such as the testes, brain, kidney, liver, and hematopoietic tissues (Manoj Kumar et al., 2017). Lead-contaminated food and water are absorbed through the duodenum via divalent metal transporter 1 and leached into the circulation (Kosnett et al., 2007). More than 95% of the lead in the circulation binds to proteins in the erythrocytes (Gonick, 2011); thus, it is stored in internal organs such as the liver and the kidney (Nakhaee et al., 2019). One of the key mechanisms implicated in lead-induced toxicity is oxidative stress, which is an imbalance between oxidant and antioxidant systems due to an excess of reactive oxygen species (ROS). This excessive formation of ROS by lead consequently results in mitochondrial impairment and cell damage (Zolkipli-Cunningham and Falk, 2017). Several heavy metal chelating agents have been applied as therapeutic drugs for lead-exposed patients, such as succimer, dimercaprol, dimercaptosuccinic acid, and CaNa2-ethylenediaminetetraacetic acid (EDTA). However, these chelators have multiple side effects (Kosnett, 2010). Therefore, great attention has been given to natural compounds that could attenuate the deleterious effects of lead poisoning and protect the cell from lead-induced damage. Such natural compounds include vitamin C (Haridy et al., 2014) and vitamin E (Khodamoradi et al., 2015) and flavonoids from natural plants (Adhikari et al., 2018).
Flavonoid and other polyphenol mixtures can be found in several plants, and they are widely known for their antioxidant activity (Pandey and Rizvi, 2009). In a previous experimental animal study, the effects of lead were neutralized by a mixture of flavonoids and polyphenols via heavy-metal chelation and antioxidant activity (Adhikari et al., 2018). Moreover, several flavonoid-containing medicinal plants, such as Thunbergia laurifolia Linn. (Tangpong and Satarug, 2010), green tea extract (Mehana et al., 2012), Fumaria parviflora L (Dorostghoal et al., 2014), Ginkgo biloba extract (Yallapragada and Velaga, 2015), and Tinospora cordifolia (Sharma and Pandey, 2010), also exhibited protective effects against lead-induced toxicity. However, Paederia foetida Linn., a flavonoid-enriched plant from the southern region of China, Vietnam, India, Japan, and Thailand (Ahmed et al., 2014) that is used as a folk medicine to treat rheumatoid arthritis (Kumar et al., 2015; Soni et al., 2013), diarrhea (Afroz et al., 2006), asthma (Khushbu et al., 2010), and diabetes (Kumar et al., 2014), has not been investigated for its protective effects on lead-induced toxicity yet. Therefore, this study sought to investigate the protective effects of a methanolic extract of P. foetida against low-dose, lead-induced toxicity, particularly in hematologic, renal, and liver organs of rats.
MATERIALS AND METHODS
Ethical approval
The study protocol was approved by the Animal Ethics Committee, Walailak University, under Approval No. 008/2019. All animal procedures were carried out in accordance with the Guide for the Care and Use of Animals for Scientific Purposes, National Committee for Research Animal Development, National Research Council of Thailand.
Paederia foetida Linn. extraction
Paederia foetida leaves were obtained from Khlong Krabue, Pak Phanang District, Nakhon Si Thammarat Province, Thailand (8°17?54.5?N, 100°09?13.1?E); and the leaves were identified by Assistant Professor Dr. Kingkan Bunluepuech, Applied Thai Traditional Medicine Department, School of Medicine, Walailak University, Thailand. The voucher sample (Ref. HA1601061505) was deposited at the School of Medicine, Walailak University. Healthy leaves of P. foetida Linn. were collected between March and May 2019. The collected leaves of P. foetida were gently washed with tap water. The leaves were dried at 45°C for 48 hours in a hot air oven and then ground into a powder. 50 g of ground leaves was then extracted with 500 ml of methanol for 7 days at room temperature. The plant remains were removed by filtering with Whatman filter paper (No. 1). The filtrated extraction was then concentrated using a rotary vacuum evaporator under reduced pressure at 45°C (Heidolph Hei-VAP Advantage Rotary Evaporator). The concentrated were dried at 45°C in a water bath, resulting in a crude semisolid residue (percentage yield, 20%). The final yield was stored at 4°C for further investigation. In animal studies, it was suspended in a 0.25% solution of carboxymethyl cellulose in distilled water. Doses of the methanolic PFE were modified from Kumar et al. (2014, 2018).
Phenolic content of PFE
The amount of phenolic content of PFE was determined using Folin–Ciocalteu’s reagent. Briefly, the crude methanolic extract of PFE (1 mg/ml) was mixed with Folin–Ciocalteu’s phenol reagent (250: 250 μl) for 5 minutes. Then, 500 μl of a sodium carbonate (Na2CO3) solution (7% w/v) was added to the mixture, and the volume was adjusted to 1.5 ml with distilled water. The reactions were allowed to occur for 90 minutes at room temperature (in the dark). Next, 200 μl of reaction samples was transferred to 96-well microplates, and its absorbance was measured at 765 nm (JASCO FP-8200, JASCO FP-8200 microreader). The amount of phenolic concentration (mg in equivalent/g dry weight) in the plant extract was calculated by using a standard curve that was generated from gallic acid (0–500 μg/l). All determinations were conducted in triplicate experiments.
Flavonoid content of PFE
To determine the amount of flavonoid content of PFE, 50 μl of P. foetida crude extract (1 mg/ml) was incubated with 200 μl of distilled water. Then, 5% NaNO2 (300 μl) and 10% AlCl3 (300 μl) solutions were added, and the final volume was brought to 1 ml. After 5 minutes of incubation, the absorbance was measured at 510 nm and the amount of flavonoid concentration was calculated by using a standard curve that was generated from quercetin (0–500 μg/l). All determinations were conducted in triplicate experiments.
Lead acetate preparation
Lead (II) acetate trihydrate was obtained from Merck Company, USA. In previous studies, ingestion of low levels of lead at doses of 1, 7.5, 20, 50, and 60 mg/kg bodyweight (BW) caused ROS generation and tissue damage (Agodi et al., 1990; El-Masry et al., 2011; Haridy et al., 2014; Kumar Singh et al., 2018; Offor et al., 2017; Shukla et al., 2003; Tham et al., 2013). Therefore, this study focused on lead acetate at 50 mg/kg BW for lead toxicity induction in rats. A solution was prepared by suspending 5 g of lead acetate in 20 ml of sterile water. A fresh lead acetate solution was prepared daily.
Animals
All animals were obtained from Nomura Siam International, Thailand. They were provided with commercial feed and distilled water. They were maintained in well-ventilated cages, which were kept under a regular light/dark cycle (12/12 hours), constant room temperature (23°C ± 2°C), and relative humidity (50%–60%). The rats were allowed free access to a standard diet and water throughout the experimental period. The animals were acclimatized for 7 days before the experiments. Bodyweight was recorded weekly for each animal throughout the experiment.
Experimental design
Thirty-six healthy male Wistar rats weighing about 180–200 g at 6 weeks of age were randomly divided into six groups: i) animal control group, ii) lead-induced group (50 mg/kg BW of lead acetate), iii) cotreatment of 50 mg/kg BW PFE with lead acetate group, iv) cotreatment of 100 mg/kg BW PFE with lead acetate group, v) cotreatment of 500 mg/kg BW PFE with lead acetate group, and vi) cotreatment of 1,000 mg/kg BW PFE with lead acetate group.
All rat groups were orally administered selected PFE doses based on previous studies (Arunkumar et al., 2016; Kumar et al., 2014). Treatments lasted for 8 weeks. Eight weeks of lead acetate administration was carried out to mimic subchronic lead exposure and sufficiency for lead toxicity induction (Obafemi et al., 2019). This also is an appropriate time for investigating the protective effects of PFE (Kumar et al., 2014). The bodyweight of each animal was recorded weekly for 8 weeks.
Dose fixation of the plant drug
The lethal dose or LD50 of the PFE, > 2,000 mg/kg BW, was reported previously (Das et al., 2013; Kumar, 2014). In their study, animals received graded doses of PFE raised from 100 to 2,000 mg/kg BW for 24 hours. They found that no animals showed any change in behavior, anatomy, or neurological patterns from any of the doses. The results from this group suggest that doses up to 2,000 mg/kg of PFE are safe for use. Moreover, several studies used concentrations of PFE between 100 and 500 mg/kg BW for 28 days, and they showed remarkable protective effects, such as antihyperglycemic, antihyperlipidemic, and antioxidant activities, in Wistar rats (Kumar et al., 2014). In addition, at concentrations between 100 and 400 mg/kg BW of PFE, hepatoprotective effects against carbon tetrachloride were seen as well in rats (Arunkumar et al., 2016). Therefore, we chose the dosages of PFE based on these publications and added two more dosages [one lower (50 mg/kg) and one higher (1,000 mg/kg)] to cover the range of promising doses that could protect against lead-induced toxicity.
Blood collection for hematological and biochemical studies
After 8 weeks of treatment, the rats were deeply anesthetized by using a mix of zoletil100 and xylazine at a ratio of 25:5 mg/kg BW, intraperitoneal injection. The combination of zoletil100 and xylazine is appropriate to use as an anesthetic since it prevents serum hemolysis and hepatic metabolism in rats (Machado et al., 2009). Blood samples were collected from the heart. Whole blood was collected in EDTA tubes for analyzing complete blood count, including i) red blood cell (RBC) count, ii) white blood cell (WBC) count, iii) hemoglobin concentration (Hb), and iv) % hematocrit (Hct), as well as red blood cell indices including v) mean corpuscular volume (MCV), vi) mean corpuscular hemoglobin (MCH), and vii) mean corpuscular hemoglobin concentration (MCHC). These parameters were analyzed by a Mindray Broucher BC-5180 Auto Hematology Analyzer (Aspen Diagnostics, India).
The blood samples were also utilized for biochemical studies. Serum was collected by centrifugation at 3,000 rpm for 30 minutes and then kept in a freezer at −70°C until used. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine were evaluated. These parameters were measured by an automated analyzer (Mindray BS-400 Chemistry Analyzer, Aspen Diagnostics, India).
Histopathological study
Histopathological changes in the liver and kidney were determined in this study. The liver and kidney tissues were immediately removed from the rats after blood collection. The tissues were then weighed, fixed in 10% neutral buffered formalin, and embedded in paraffin. After that, sections (5 µm thickness) of paraffin-embedded tissues were stained with hematoxylin and eosin. The histological slides and representative photomicrographs were examined and captured using a light microscope, respectively. The relative weight of the organs was calculated by using the following formula:
Statistical analysis
Data are presented as means ± SEM. Prism 9 software (GraphPad, La Jolla, CA) was used for data analysis. Statistical analyses were carried out using two-tailed unpaired Student’s t-test when comparing two groups or with a one-way analysis of variance followed by Dunnett’s multiple comparisons test when three or more groups were compared. A p-value less than 0.05 (p < 0.05) was considered statistically significant.
RESULTS
Phenolic and flavonoid content of PFE
According to a previous report, P. foetida contains a large amount of flavonoid constituents (Ojha et al., 2018). Therefore, we first determined the phenolic and flavonoid contents in P. foetida extracts using garlic and quercetin as the equivalent standard. The phenolic and flavonoid contents of the P. foetida extract were 26.47% (3.97 ± 0.17 mg gallic acid equivalents (GAE)/g) and 42.86% (21.43 ± 1.77 mg quercetin equivalents (QE)/g), respectively, indicating that P. foetida contained a good source of flavonoid content.
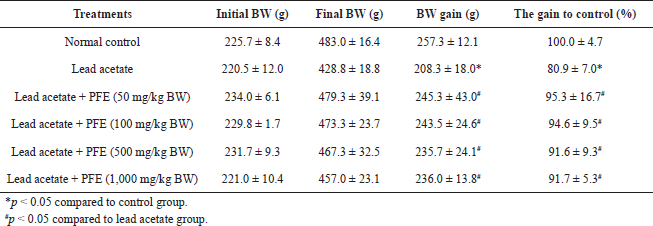 | Table 1. Effects of PFE on bodyweight gain in rats with lead acetate toxicity. [Click here to view] |
Effects of lead and PFE on body and organ weight
To determine the toxic effects of PFE on organs and bodyweight, Wistar rats were given lead (50 mg/kg BW) and various concentrations of PFE ranging from 0 to 1,000 mg/kg BW. After 8 weeks of intervention, the result showed that the bodyweight of rats receiving the lead acetate alone exhibited less increase in comparison with rats receiving normal saline (control group) (p < 0.05). Moreover, there was no difference in the weight of rats that received lead acetate with any concentration of PFE (50, 100, 500, and 1,000 mg/kg BW) compared to control animals (p > 0.05) (Table 1).
The livers and kidneys of the animals taken from the sacrificed animals were weighed and averaged. For the weight of the liver, the results showed no difference in its weight or its weight ratio (weight of liver divided by the bodyweight of rat) in rats exposed to lead acetate compared to controls. Also, there was no difference in the weight or weight ratio of the liver in rats exposed to lead acetate + PFE at any concentration compared to the control group or rats exposed to lead acetate alone. For the weight of the kidney, the results showed no difference in the weight or weight ratio of the kidney in lead-exposed rats compared to controls. However, in Table 2, there was a difference in the weight ratio of the kidney in rats exposed to lead acetate with PFE at 1,000 mg/kg BW compared to controls (p < 0.05).
Effects of lead and PFE on hematological parameters
RBC count
The results showed a significant decrease in the RBC count among rats exposed to lead acetate alone compared to controls ( p < 0.05). Rats exposed to both lead acetate and PFE (at 500 and 1,000 mg/kg BW) exhibited a significant increase in RBC count compared to rats exposed to lead acetate alone (p < 0.05).
Hb concentration
The results showed a significant decrease in Hb concentration among rats exposed to lead acetate alone compared to controls (p < 0.05). Rats exposed to both lead acetate and PFE (at 50, 100, 500, and 1,000 mg/kg BW) exhibited a significant increase in Hb concentration compared to rats exposed to lead acetate alone (p < 0.05) (Fig. 1B).
Hematocrit
The results showed a significant decrease in Hct among rats exposed to lead acetate alone compared to controls (p < 0.05). Rats exposed to both lead acetate and PFE (at 50, 100, 500, and 1,000 mg/kg BW) exhibited a significant increase in Hct compared to rats exposed to lead acetate alone (p < 0.05) (Fig. 1C).
Mean corpuscular volume
The results showed a significant decrease in MCV among rats exposed to lead acetate alone compared to those in the control (p < 0.05). Rats exposed to both lead acetate and PFE (at 500 and 1,000 mg/kg BW) exhibited a significant increase in MCV compared to rats exposed to lead acetate alone (p < 0.05) (Fig. 1D).
Mean corpuscular hemoglobin
The results showed a significant decrease in MCH among rats exposed to lead acetate alone compared to controls (p < 0.05). There was no difference in MCH between rats exposed to both lead acetate and PFE (at 50, 100, 500, and 1,000 mg/kg BW) and rats exposed to lead acetate alone (p > 0.05) (Fig. 1E).
Mean corpuscular hemoglobin concentration
The results showed a significant decrease in MCHC among rats exposed to lead acetate alone compared to those in the control (p < 0.05). Rats exposed to both lead acetate and PFE (at 100, 500, and 1,000 mg/kg BW) exhibited a significant increase in MCHC compared to rats exposed to lead acetate alone (p < 0.05) (Fig. 1F).
Red cell distribution width (RDW)
The results showed no difference in RDW between rats exposed to lead acetate alone and rats in the control (p > 0.05). There was also no difference in MCH between rats exposed to both lead acetate and PFE (at 50, 100, 500, and 1,000 mg/kg BW) and rats exposed to lead acetate alone (p > 0.05) (Fig. 1G).
WBC Count
The results showed a significant increase in WBC count among rats exposed to lead acetate alone compared to controls (p < 0.05). However, rats exposed to both lead acetate and PFE (at 50, 100, 500, and 1,000 mg/kg BW) exhibited a significant decrease in WBC count compared to rats exposed to lead acetate alone (p < 0.05) (Fig. 1H).
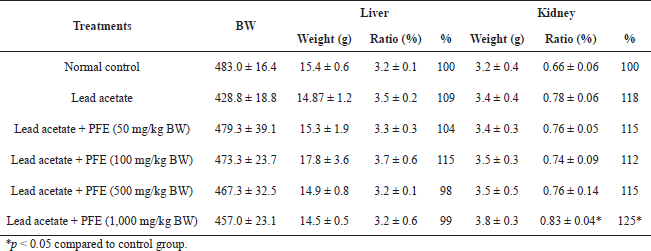 | Table 2. Effects of PFE on organ weight ratio in rats induced with lead acetate. [Click here to view] |
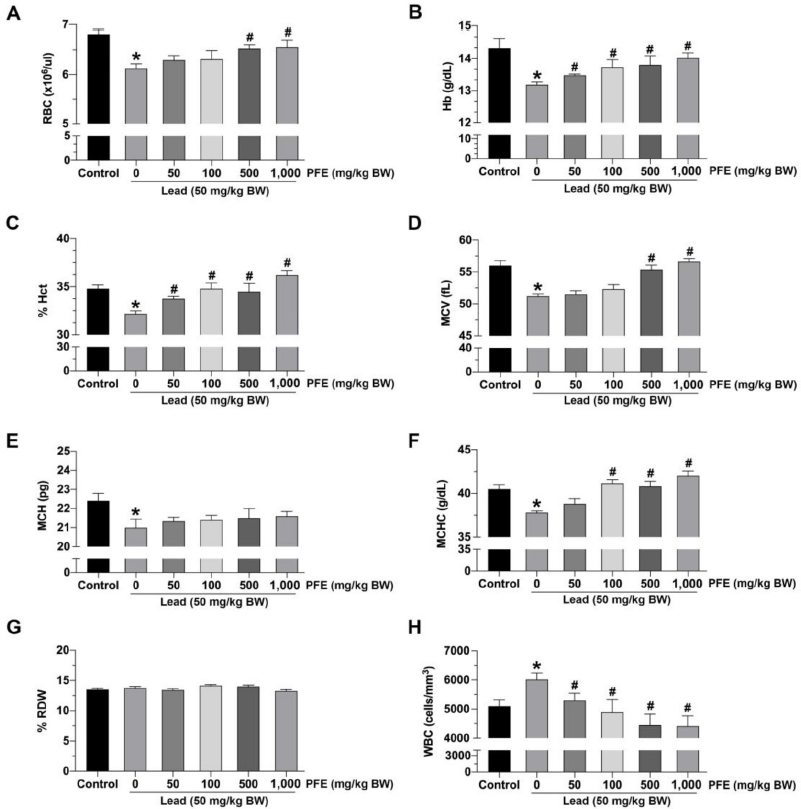 | Figure 1. Effects of lead acetate and the PFE on hematological parameters. (A) Red blood cell (RBC) count; (B) Hemoglobin (Hb) concentration; (C) % hematocrit (Hct); (D) mean corpuscular volume (MCV); (E) mean corpuscular hemoglobin (MCH); (F) mean corpuscular hemoglobin concentration (MCHC); (G) % red cell distribution width (RDW); (H) white blood cell (WBC) count; PFE: methanol extract of Paederia foetida leaves (mg/kg BW); lead (50 mg/kg BW). *p < 0.05 compared to control, #p < 0.05 compared between the lead acetate and PFE cotreatment groups. [Click here to view] |
Effects of PFE on liver function and histopathology
Alanine aminotransferase
The results showed that rats exposed to lead acetate alone exhibited significantly increased ALT compared to controls (p < 0.05) (Fig. 2A). However, rats exposed to lead acetate with PFE (at 50, 100, 500, and 1,000 mg/kg BW) exhibited significantly decreased ALT compared to rats exposed to lead acetate alone (p < 0.05) (Fig. 2A).
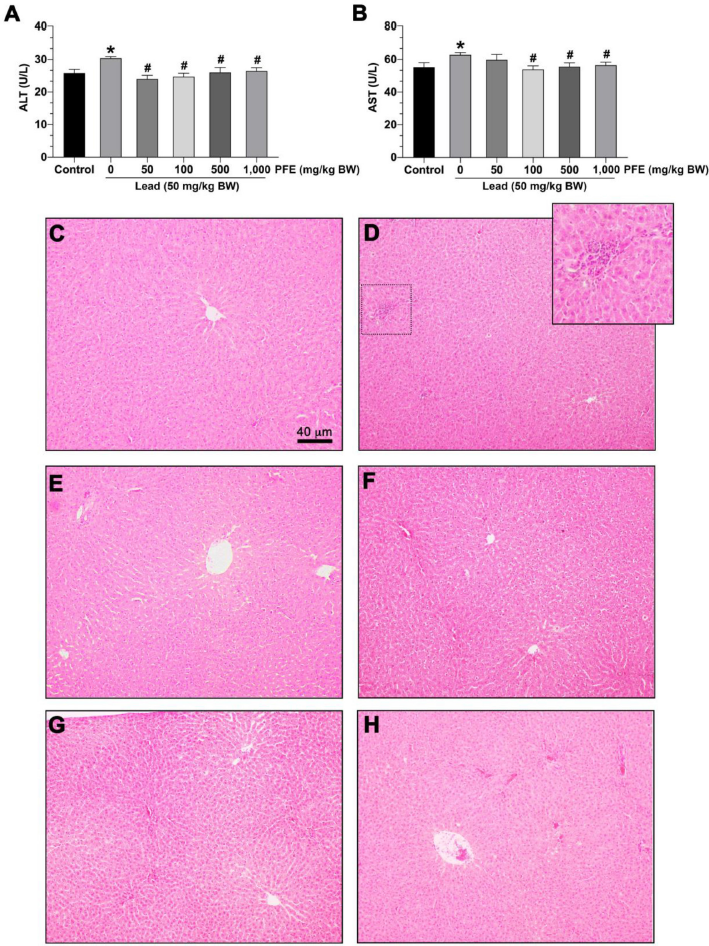 | Figure 2. Effects of the PFE on liver function and histopathology. (A) Serum aspartate aminotransferase (AST) level; (B) serum alanine aminotransferase (ALT) level. *p < 0.05 compared to the control, #p < 0.05 compared between the lead acetate and the PFE cotreatment groups. Photographs show representatie hepatocytes stained with hematoxylin and eosin. (C) Hepatocytes in the control group; (D) hepatocytes in the lead acetate group; (E) hepatocytes in lead + PFE 50 mg/kg BW group; (F) hepatocytes in lead + PFE 100 mg/kg BW group; (G) hepatocytes in lead + PFE 500 mg/kg BW group; and (E) hepatocytes in lead + PFE 1,000 mg/kg BW group. Scale bar = 40 µm (magnification = 400×). [Click here to view] |
Aspartate aminotransferase
The results showed that rats exposed to lead acetate alone exhibited significantly increased AST compared to controls (p < 0.05) (Fig. 2B). Rats exposed to lead acetate with PFE (at 100, 500, and 1,000 mg/kg BW) exhibited significantly decreased AST compared to rats exposed to lead acetate alone (p < 0.05) (Fig. 2B).
Histopathology
No histopathological abnormalities were observed in the hepatocytes of rats in the control group (Fig. 2C). Rats exposed to lead acetate alone showed some neutrophil infiltration around the periportal areas (Fig. 2D). However, no histopathological abnormalities were observed in the hepatocytes of rats exposed to lead acetate and PFE at 50 mg/kg BW (Fig. 2E), 100 mg/kg BW (Fig. 2F), 500 mg/kg BW (Fig. 2G), and 1,000 mg/kg BW (Fig. 2H).
Effects of PFE on kidney function and histopathology
Blood urea nitrogen
The results showed that rats exposed to lead acetate alone exhibited significantly increased BUN compared to the control group (p < 0.05). Rats exposed to lead acetate with PFE (at 100, 500, and 1,000 mg/kg BW) exhibited significantly decreased BUN compared to rats exposed to lead acetate alone (p < 0.05) (Fig. 3A).
Creatinine
The results showed no difference in creatinine levels between rats exposed to lead acetate alone and the control group (p > 0.05). Also, there was no difference in creatinine levels between rats exposed to both lead acetate and PFE (at 50, 100, 500, and 1,000 mg/kg BW) and rats exposed to lead acetate alone (p > 0.05) (Fig. 3B).
Kidney histopathology
No histopathological abnormalities could be observed in the kidneys of rats in the control group (Fig. 3C). However, there was mild parenchymal cell degeneration among rats exposed to lead acetate alone (Fig. 3D) and rats exposed to both lead acetate and PFE at 50 mg/kg BW (Fig. 3E), 100 mg/kg BW (Fig. 3F), 500 mg/kg BW (Fig. 3G), and 1,000 mg/kg BW (Fig. 3H).
DISCUSSION
Lead is a highly toxic heavy metal that has a variety of pathological and physiological effects in both animals and humans (El-Boshy et al., 2019; El-Tantawy, 2016). In this work, lead acetate-induced hepato-, renal- and hematotoxicities in rats. However, the toxic and harmful effects of lead acetate were alleviated by the P. foetida extract (PFE) starting at the dose of 100 mg/kg BW, which may have a higher potency and similar efficacy when compared with higher doses. PFE at a dose of 100 mg/kg BW also showed several other effective characteristics; for example, i) administration of PFE for 5 days significantly prevented hepatotoxicity after injection of CCl4 for 48 hours in rats (Arunkumar et al., 2016) and ii) PFE at a daily dose of 100 mg/kg for 28 days significantly decreased the level of fasting blood glucose, total cholesterol, and triglyceride, and it modulated the pathology of the kidney and liver in streptozotocin-induced diabetic rats (Kumar et al., 2014). Moreover, the maximum effective dose observed in diabetic rats was 500 mg/kg BW, which was slightly aligned with our hematology results. It has also been reported that PFE can protect against colitis in rats at the higher dose of 500 mg/kg BW (Das et al., 2013). It seems that the higher concentration of PFE was also safe and effective and showed no signs of toxicity as observed in our studies and previous reports, which found that PFE doses up to 2,000 mg/kg BW (p.o.) are safe with an LD50 of more than 2,000 mg/kg BW (Das et al., 2013; Kumar et al., 2014).
The present study proposed that PFE could attenuate the lead-induced reduction in bodyweight and restore hematologic alteration, renal function, and liver function in lead acetate-induced rats. For lead-induced reductions in bodyweight, the data from National Health and Nutrition Examination Survey (NHANES) 1999–2006 revealed that blood lead levels were associated with lower bodyweights in adults (Scinicariello et al., 2013), which can potentially be caused by lead-induced metabolic changes. Moreover, previous studies indicated subchronic exposure to heavy metals induced weight loss (Nwokocha et al., 2011, 2012). The mechanism of lead-induced weight loss is not well understood. A previous study suggested that the loss of bodyweight might be caused by the lead-induced oxidative stress in the muscular system through ROS generation, leading to muscular atrophy (Buck and Chojkier, 1996). Following PFE treatment in lead-exposed rats, the present study showed that PFE in any concentration could improve the bodyweight of lead-exposed rats when compared to lead-exposed rats without PFE. The antioxidant properties of PFE could potentially explain its weight-restorative effect among lead-exposed rats.
The present study also showed no difference in the weight or weight ratio of the internal organs (liver and kidney) between rats exposed to lead acetate alone, the control group, or rats exposed to lead acetate + PFE. These results are partially consistent with the study of Amjad et al., (2013), who reported that the weight of the kidney was significantly increased at 4 weeks, following daily induction (i.p.) of lead acetate at 8 mg/kg BW, and it did not change significantly at 6 weeks. Therefore, the alteration of the internal organs among lead-exposed rats is dependent on the duration of exposure. The mechanism underlying the increased internal organ weight could be explained by lipid accumulation in the liver and kidney, resulting in an increased weight of those organs (Alwaleedi, 2016).
For liver function and histopathology, the present study showed that AST and ALT levels were significantly increased in lead-exposed rats compared to control, suggesting lead-mediated hepatic damage. Moreover, neutrophil infiltration could be observed around the periportal areas of liver tissue in lead-exposed rats. Interestingly, PFE administration at 100, 500, and 1,000 mg/kg BW decreased ALT and AST and reversed or prevented the histopathology of the liver tissue. These results suggest that PFE can protect against hepatic injuries and prevent the hepatic alterations that are caused by the toxic effects of lead acetate exposure. These results are consistent with the findings of previous studies that demonstrated the hepatoprotective activity of PFE in hepatotoxin-induced rats (Arunkumar et al., 2016; Sumithra et al., 2014; Uddin et al., 2011). The mechanism of lead-induced hepatic injuries is not well understood. Previous studies showed that lead acetate could induce the production of ROS-related oxidative damage to the cell membrane of hepatocytes, which leads to hepatic enzyme leakage (Poli et al., 1987; Wang et al., 2013).
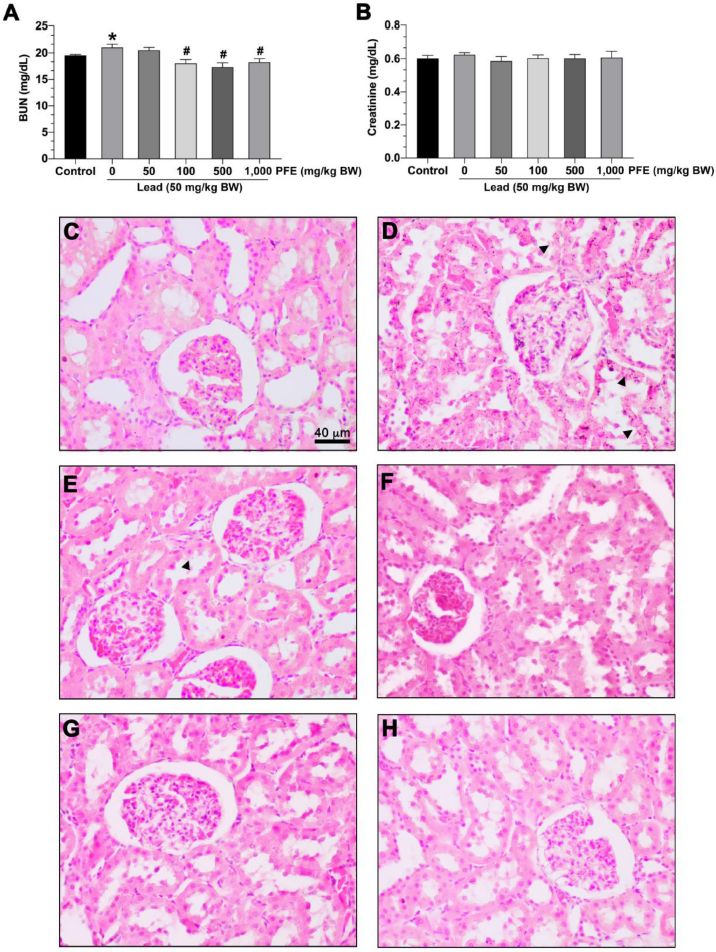 | Figure 3. Effects of PFE on kidney function and histopathology. (A) Blood urea nitrogen (BUN) level; (B) serum creatinine level. *p < 0.05 compared to the control, #p < 0.05 compared between the lead acetate and the PFE cotreatment groups. Photographs show representative renal cells stained with hematoxylin and eosin. (C) Renal cells control group; (D) renal cells in the lead acetate group; (E) renal cells in lead + PFE 50 mg/kg BW group; (F) renal cells in lead + PFE 100 mg/kg BW group; (G) renal cells in lead + PFE 500 mg/kg BW group; (E) renal cells in lead + PFE 1,000 mg/kg BW group. Scale bar = 40 µm (magnification = 400×). ? = parenchymal cell degeneration. [Click here to view] |
For the kidney function and histopathology, the present study showed an increased BUN but not creatinine levels among the rats exposed to lead acetate alone. These results indicate that the alteration in BUN was more sensitive than those of creatinine for the early stages of the lead-induced renal impairment. This was corroborated by another study that showed that increased creatinine was frequent in moderate to severe renal dysfunction (Wong Vega et al., 2020). In addition, mild parenchymal cell degeneration was observed among rats exposed to lead acetate alone (Fig. 3D). Following the administration of lead + PFE at any dose, BUN was significantly decreased (Fig. 3A). Interestingly, mild parenchymal cell degeneration was also observed among rats exposed to lead acetate alone or both lead acetate and PFE. As BUN and creatinine are commonly used as renal impairment biomarkers, the increased BUN level might be an early sign of renal dysfunction. Decreased BUN after lead acetate administration suggests the possibility of the protective effect of PFE.
To determine the long-term effects of lead plus P. foetida intake in humans, low-dose lead acetate with P. foetida was administered for 8 weeks rats. In this study, lead acetate at a dose of 50 mg/kg BW caused mild liver injury, which is slightly different from other groups that showed more liver damage in rats (Abdelhamid et al., 2020; Adhikari et al., 2018; Offor et al., 2017; Sumithra et al., 2014). This discrepancy could be due to the shorter time point at which the other groups observed the effect of lead exposure (4 weeks). In addition, another report found that 2% lead acetate, which is a subtoxic concentration, causes changes in hepatocytes, portal triads, and sinusoids with an aggregation of inflammatory cells in the portal triad one month after lead exposure (Jarrar and Taib, 2012). However, the liver normally provides a great ability to tolerate several kinds of injuries and toxicants. After chemically induced liver damage, the process of inflammation can neutralize injurious agents and restore liver function. Therefore, the liver might have been repaired and recovered when exposed to lead for a long time, as seen by our liver histology slides that showed only mild liver injury (Fig. 2). This result aligns with another report of normal portal hepatic tissue with a mild aggregation of inflammatory cells in the liver in rats seven months after lead exposure (Jarrar and Taib, 2012).
In the hematological study, the results showed a significant decrease in the hematological parameters, including the RBC count, Hb concentration, % hematocrit, MCV, and MCHC in the lead-exposed rats compared to controls. The results of this study were consistent with the findings of previous studies (Haridy et al., 2014; Obafemi et al., 2019). However, PFE cotreatment with lead acetate showed effective improvement in these hematological parameters. The mechanisms of lead-induced hematological alterations in rats are well described, including i) lead-related RBC membrane fluidity that leads to increased erythrocyte hemolysis rates (Mannem, 2014; Ray, 2016), ii) lead interference with the heme synthesis pathway, such as the downregulation of δ-aminolevulinic acid dehydratase, resulting in low Hb concentration (Obafemi et al., 2019), or iii) the interference of lead with iron and copper metabolism in the RBCs (Klauder and Petering, 1977). While most of the RBC parameters were altered, the present study showed that the leukocyte count was also increased in lead-exposed rats. This result is consistent with another study, which also showed a lead-induced increase in leukocyte count (Shah and Altindog, 2005). Following PFE administration at any concentration, our results showed restoration of leukocyte counts back to the physiological levels. This result suggests that PFE can reduce the inflammatory responses that are induced by lead toxicity.
To the best of our knowledge, this study is the first report of PFE effectiveness against lead-induced toxicity. A possible mechanism could be due to the effect of various phytochemicals found within PFE (Ojha et al., 2018; Wang et al., 2014). For example, flavonoids such as kaempferol (Satapathy and Pattnaik, 2019) found in PFE could retain their heavy-metal binding activity and act as chelating agents that neutralize the Pb2+ charge (Adhikari et al., 2018; Bagchi et al., 2015; Cornard and Merlin, 2003). This fact could reduce ROS and other free-radical-induced oxidative stress and inflammation during Pb2+ exposure in the cells. Flavonoids also retain their antioxidant and anti-inflammation properties after Pb2+ chelation resulting in even more health benefits. Since significant oxidative stress is generated due to cellular Pb2+ exposure, an exogenously supplied chelator with sustainable antioxidant activity and a low cost, like PFE, could be a promising agent for treating lead-induced diseases. Moreover, oxidative stress markers, such as protein carbonylation and nonprotein thiols, and antioxidant enzymes, such as catalase and superoxide dismutase, need to be further evaluated in P. foetida. Finally, future studies should include clinical trials to assess the protective effects of P. foetida against high blood lead levels in lead-exposed workers.
CONCLUSION
Our investigation clearly indicated that methanolic P. foetida leaf extracts, at doses of 100, 500, and 1,000 mg/kg BW, improve bodyweight and the function of the liver, renal, and hematologic systems, following low-level exposure to lead. This study is the first to demonstrate the effects of methanolic extracts from P. foetida leaves on the hematologic system and the function of the liver and kidney in lead acetate-induced rats. PFE starting at the doses of 100 mg/kg BW might be beneficial against low doses of lead exposure in rats. However, the potential protective effects of dietary supplements containing PFE following lead poisoning in humans need to be investigated further.
ACKNOWLEDGMENTS
The authors would like to express their sincere gratitude to Associate Professor Dr. Jitbanjong Tangpong and Associate Professor Dr. Wiyada Kwanhian Klangbud for providing some laboratory equipment.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest related to this work.
FUNDING
This work was supported by the Research Institute for Health Sciences, Walailak University under Grant [number WU-IRG-62-011].
AUTHORS’ CONTRIBUTIONS
T.K., P.P., S.Y., and S.K. carried out the experiments; T.K., P.P., and S.K. analyzed the data; T.K., P.P., S.Y., M.K., and S.K. interpreted the results of the experiments. T.K. and S.K. prepared the figures; T.K., P.P., S.Y., M.K., and S.K. drafted, edited, revised, and approved the final version of the manuscript; T.K., P.P., S.Y., and S.K. conceived and designed the research.
ETHICAL APPROVAL
The study protocol was approved by the Animal Ethics Committee, Walailak University (Protocol Number: 008/2019).
REFERENCES
Abdelhamid FM, Mahgoub HA, Ateya AI. Ameliorative effect of curcumin against lead acetate–induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ Sci Pollut Res, 2020; 27:10950–65. CrossRef
Adhikari A, Darbar S, Chatterjee T, Das M, Polley N, Bhattacharyya M, Bhattacharya S, Pal D, Pal SK. Spectroscopic studies on dual role of natural flavonoids in detoxification of lead poisoning: bench-to-bedside preclinical trial. ACS Omega, 2018; 3(11):15975–87. CrossRef
Afroz S, Alamgir M, Khan MT, Jabbar S, Nahar N, Choudhuri MS. Antidiarrhoeal activity of the ethanol extract of Paederia foetida Linn. (Rubiaceae). J Ethnopharmacol, 2006; 105(1–2):125–30. CrossRef
Agodi A, Viola M, Alberghina M, Giaffri da Stella AM. Effect of low-dose lead acetate exposure on the metabolism of nucleic acids and lipids in cerebellum and hippocampus of rat during postnatal development. J Neurosci Res, 1990; 25(1):131–8. CrossRef
Ahmed A, Islam M, Rahman A, Hossain A. Thrombolytic, cytotoxic and antidiabetic effects of Paederia foetida L. leaf extract. J Adv Med Med Res, 2014; 4(5):1244–56. CrossRef
Alwaleedi S. Hematobiochemical changes induced by lead intoxication in male and female albino mice. Natl J Physiol Pharm Pharmacol, 2016; 6(1):46–51. CrossRef
Amjad Z, Iqbal M, Shoro A. Lead-induced reduction in body and kidney weight of Wistar albinorats ameliorated by Ginkgo biloba extract (EGb 761). Biochem Physiol, 2013; 2(2):1–4. CrossRef
Arunkumar R, Chowdhury A, Chowdhury B. Hepatoprotective activity of Paederia foetida in vitro and in vivo studies. J Evid Based Med Healthc, 2016; 3(18):733–7. CrossRef
Bagchi D, Chaudhuri S, Sardar S, Choudhury S, Polley N, Lemmens P, Pal SK. Modulation of stability and functionality of a phyto-antioxidant by weakly interacting metal ions: curcumin in aqueous solution. RSC Adv, 2015; 5(124):102516–24. CrossRef
Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J, 1996; 15(8):1753–65. CrossRef
Cornard JP, Merlin JC. Comparison of the chelating power of hydroxyflavones. J Mol Struct, 2003; 651:381–7. CrossRef
Das S, Kanodia L, Mukherjee A, Hakim A. Effect of ethanolic extract of leaves of Paederia foetida Linn. on acetic acid induced colitis in albino rats. Indian J Pharmacol, 2013; 45(5):453–7. CrossRef
Dorostghoal M, Seyyednejad SM, Jabari A. Protective effects of Fumaria parviflora L. on lead-induced testicular toxicity in male rats. Andrologia, 2014; 46(4):437–46. CrossRef
El-Boshy ME, Refaat B, Qasem AH, Khan A, Ghaith M, Almasmoum H, Mahbub A, Almaimani RA. The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ Sci Pollut Res Int, 2019; 26(22):22736–46. CrossRef
El-Masry TA, Emara AM, El-Shitany NA. Possible protective effect of propolis against lead-induced neurotoxicity in animal model. J Evol Biol Res, 2011; 3(1): 4–11.
El-Tantawy WH. Antioxidant effects of Spirulina supplement against lead acetate-induced hepatic injury in rats. J Tradit Complement Med, 2016; 6(4):327–31. CrossRef
Gonick HC. Lead-binding proteins: a review. J Toxicol, 2011; 2011:686050. CrossRef
Haridy M, Al-Amgad Z, Sakai H, Mohi-Eldin M. Ameliorating effects of garlic, calcium, and vitamin C on chronic lead toxicity in albino rats. Comp Clin Pathol, 2014; 23(5):1215–23. CrossRef
Jarrar BM, Taib NT. Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi J Biol Sci, 2012; 19(2):203–10. CrossRef
Khodamoradi N, Komaki A, Salehi I, Shahidi S, Sarihi A. Effect of vitamin E on lead exposure-induced learning and memory impairment in rats. Physiol Behav, 2015; 144:90–4. CrossRef
Khushbu C, Anar P, Mayuree P, Carol M, Roshni S, Subodh A. Paederia foetida Linn. as a potential medicinal plant: a review. J Pharm Res, 2010; 3(12):3135–7.
Klauder DS, Petering HG. Anemia of lead intoxication: a role for copper. J Nutr, 1977; 107(10):1779–85. CrossRef
Kosnett MJ. Chelation for heavy metals (arsenic, lead, and mercury): protective or perilous? Clin Pharmacol Ther, 2010; 88(3):412–5. CrossRef
Kosnett MJ, Wedeen RP, Rothenberg SJ, Hipkins KL, Materna BL, Schwartz BS, Hu H, Woolf A. Recommendations for medical management of adult lead exposure. Environ Health Perspect, 2007; 115(3):463–71. CrossRef
Kumar Singh P, Kumar Singh M, Singh Yadav R, Kumar Dixit R, Mehrotra A, Nath R. Attenuation of lead-induced neurotoxicity by Omega-3 fatty acid in rats. Ann Neurosci, 2018; 24(4):221–32. CrossRef
Kumar V, Al-Abbasi FA, Ahmed D, Verma A, Mujeeb M, Anwar F. Paederia foetida Linn. inhibits adjuvant induced arthritis by suppression of PGE(2) and COX-2 expression via nuclear factor-kappaB. Food Funct, 2015; 6(5):1652–66. CrossRef
Kumar V, Anwar F, Ahmed D, Verma A, Ahmed A, Damanhouri ZA, Mishra V, Ramteke PW, Bhatt PC, Mujeeb M. Paederia foetida Linn. leaf extract: an antihyperlipidemic, antihyperglycaemic and antioxidant activity. BMC Complement Altern Med, 2014; 14:76. CrossRef
Kumar V, Kaithwas G, Anwar F, Rahman M, Patel DK, Singh Y, Verma A. Effect of variable doses of Paederia foetida L. combat against experimentally-induced systemic and topical inflammation in Wistar rats. Curr Bioact Compd, 2018; 14(1):70–9. CrossRef
Machado EF, Normand AC, Nunes LA, Brenzikofer R, Macedo DV. Effects of different general anesthetics on serum hemolysis and hepatic and muscular glycogenolysis in rats. Braz J Med Biol Res, 2009; 42(11):1035–8. CrossRef
Mannem P. Lead toxicity on hematological changes and amelioration with ginger (Zingiber officinale) extract in male albino rats. Int J Adv Res, 2014; 2(4):23–8.
Manoj Kumar V, Henley AK, Nelson CJ, Indumati O, Prabhakara Rao Y, Rajanna S, Rajanna B. Protective effect of Allium sativum (garlic) aqueous extract against lead-induced oxidative stress in the rat brain, liver, and kidney. Environ Sci Pollut Res, 2017; 24(2):1544–52. CrossRef
Mehana EE, Meki AR, Fazili KM. Ameliorated effects of green tea extract on lead induced liver toxicity in rats. Exp Toxicol Pathol, 2012; 64(4):291–5. CrossRef
Nakhaee S, Amirabadizadeh A, Brent J, Mehrpour O. Impact of chronic lead exposure on liver and kidney function and haematologic parameters. Basic Clin Pharmacol Toxicol, 2019; 124(5):621–8. CrossRef
Nwokocha CR, Nwokocha MI, Owu DU, Obi J, Olatunde B, Ebe C, Nwangwu O, Iwuala MO. Comparative analysis on the effect of palm oil (Elaeis guineensis) in reducing cadmium and lead accumulation in liver of Wistar rats. Pharmacognosy Res, 2012; 4(4):214–8. CrossRef
Nwokocha CR, Owu DU, Ufearo CS, Iwuala MOE. Comparative study on the efficacy of Garcinia kola in reducing some heavy metal accumulation in liver of Wistar rats. J Ethnopharmacol, 2011; 135(2):488–91. CrossRef
Obafemi TO, Onasanya A, Adeoye A, Falode JA, Daniel DJ, Irefo EF, Ojo AO, Fadaka A, Afolabi OB, Awe JO, Omiyale BO. Protective effect of methanolic and flavonoid-rich leaf extracts of Synsepalum dulcificum (Danielli) on lead-acetate-induced toxicity in Wistar albino rats. J Appl Pharm Sci, 2019; 9(5):65–72. CrossRef
Offor SJ, Mbagwu HO, Orisakwe OE. Lead induced hepato-renal damage in male albino rats and effects of activated charcoal. Front Pharmacol, 2017; 8:107. CrossRef
Ojha S, Raj A, Roy A, Roy S. Extraction of total phenolics, flavonoids and tannins from Paederia foetida L. leaves and their relation with antioxidant activity. Pharmacogn J, 2018; 10(3):541–7. CrossRef
Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev, 2009; 2(5):270–8. CrossRef
Poli G, Albano E, Dianzani MU. The role of lipid peroxidation in liver damage. Chem Phys Lipids, 1987; 45(2–4):117–42. CrossRef
Ray RR. Haemotoxic effect of lead: a review. Proc Zoo Soc, 2016; 69(2):161–72. CrossRef
Satapathy S, Pattnaik G. Antioxidant and HPLC analysis of an Indian medicinal herb: Paederia foetida L. (Prasarini). Int J Life Sci, 2019; 7(2):249–55.
Scinicariello F, Buser MC, Mevissen M, Portier CJ. Blood lead level association with lower body weight in NHANES 1999-2006. Toxicol Appl Pharmacol, 2013; 273(3):516–23. CrossRef
Shah SL, Altindog A. Alteration in the immunological parameters Tench (Tinca tinca L. 1758) after acute and chronic exposure to lethal and sublethal treatments with mercury, cadmium and lead. Turk J Vet Anim Sci, 2005; 29:1163–8.
Sharma V, Pandey D. Protective role of Tinospora cordifolia against lead-induced hepatotoxicity. Toxicol Int, 2010; 17(1):12–7. CrossRef
Shukla PK, Khanna VK, Khan MY, Srimal RC. Protective effect of curcumin against lead neurotoxicity in rat. Hum Exp Toxicol, 2003; 22(12):653–8. CrossRef
Soni RK, Irchhaiya R, Dixit V, Alok S. Paederia foetida Linn: phytochemistry, pharmacological and traditional uses. Int J Pharm Sci Res, 2013; 4(12):4525.
Sumithra M, Chitra V, Anbu J, Suman R, Shubaranshu, Nithya S. Hepatoprotective activity of leaf extract of Paederia foetida in experimental liver cirrhosis. Invent Rapid Ethnopharmacol, 2014; (2):1–6.
Tangpong J, Satarug S. Alleviation of lead poisoning in the brain with aqueous leaf extract of the Thunbergia laurifolia (Linn.). Toxicol Lett, 2010; 198(1):83–8. CrossRef
Tham CS, Chakravarthi S, Haleagrahara N, De Alwis R. Morphological study of bone marrow to assess the effects of lead acetate on haemopoiesis and aplasia and the ameliorating role of Carica papaya extract. Exp Ther Med, 2013; 5(2):648–52. CrossRef
Uddin B, Nahar T, Basunia MA, Hossain S. Paederia Foetida protects liver against hepatotoxin-induced oxidative damage. Adv Biol Res, 2011; 5(5):267–72.
Wang J, Zhu H, Yang Z, Liu Z. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J Pharmacol, 2013; 45(4):395–8. CrossRef
Wang L, Jiang Y, Han T, Zheng C, Qin L. A phytochemical, pharmacological and clinical profile of Paederia foetida and P. scandens. Nat Prod Commun, 2014; 9(6):879–86. CrossRef
Wong Vega M, Swartz SJ, Devaraj S, Poyyapakkam S. Elevated serum creatinine: but is it renal failure? Pediatrics, 2020; 146(1):e20192828. CrossRef
Yallapragada PR, Velaga MK. Effect of Ginkgo biloba Extract on lead-induced oxidative stress in different regions of rat brain. J Environ Pathol Toxicol Oncol, 2015; 34(2):161–73. CrossRef
Zolkipli-Cunningham Z, Falk MJ. Clinical effects of chemical exposures on mitochondrial function. Toxicology, 2017; 391:90–9. CrossRef