INTRODUCTION
Urolithiasis also termed kidney stone or nephrolithiasis, is a common worldwide disease with a high prevalence and recurrence rate. Chronic diseases, including diabetes mellitus, obesity, kidney disease, metabolic disorder, and hypertension, have a high tendency to form stones compared with healthy people (Besiroglu and Ozbek, 2019). It causes pyelonephritis or acute renal failure and severe acute back pain (Namburu et al., 2017; Yasui et al., 2017). Urolithiasis typically occurs between the age of 20 and 60 years and is more occurrences in hot climates. It affects nearly 10% of men and 6% of women over their lifetime. The recurrence rate is 50% within 5–10 years and 75% within 20 years of initial treatment (Dawson and Tomson, 2012; Yasui et al., 2017). The overall recurrence rate is high in male (70%–80%) as compared with female (47%–60%) (Ahmed et al., 2018; Wang et al., 2019). Approximately, 80% of stones are calcium-containing stones which are found as pure calcium oxalate (CaOx) (50%), pure calcium phosphate (1%), or a mixture of both (45%), and other stones are struvite (10%), uric acid (9%), and cystine (1%) (Bashir and Gilani, 2009; Namburu et al., 2017). CaOx (CaC2O4) stones are found as CaOx monohydrate or CaOx dehydrate form. Struvite stones are also called triple phosphate or infection stones or magnesium ammonium phosphate and develop when the urease-producing bacteria such as proteus cause the urine pH higher than 7.2 by ammonia (urea convert in ammonia in presence of urease enzyme) (Ahmed et al., 2018).
Caesalpinia bonducella Roxb. (Caesalpiniaceae) is extensively used in the traditional medicine (Bawari et al., 2020; Bashir and Gilani, 2009; Datte et al., 1998; Iheagwam et al., 2019; Liu et al., 2020). The various parts of C. bonducella have been reported for anti-cancer and cytotoxic activities (Iheagwam et al., 2019); contractile activity of uterine smooth muscle (Datte et al., 1998); inhibit nuclear factor-kappa B and type-4 phosphodiesterase (Liu et al., 2020); antimicrobial activities (Arif et al., 2009). The objective of this study is to perform toxicity study (acute and subacute oral toxicity) and to evaluate antiurolithiatic activity of ECB in experimentally induced CaOx stone model.
MATERIALS AND METHODS
Material
Chemicals of analytical grade were utilized in the studies. Cystone was procured from Varanasi’s local market. Albumin, total protein, calcium, phosphorous, magnesium, uric acid, urea, creatinine, uric acid estimation kits were procured from Coral Clinical Systems, a division of Tulip Diagnostics (P) Ltd., Uttarakhand, India. Alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT) were procured from Autospan Liquid Gold, Arkray Healthcare Pvt. Ltd., Maharastra, India. UV-Visible spectrophotometer (Systronics Double Beam UV-VIS Spectrophotometer: 2202) was used for analysis.
Plant material and extraction
The plant seeds samples were collected from the local market in Varanasi, India. A voucher specimen was deposited (number 201901) and authenticated by Dr. Ashwani K. Kushwaha, Department of Dravyguna, Faculty of Ayurveda, Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi. The coarsely powdered seeds of C. bonducella (100 g) were soaked in 1 l of 95% ethanol for a week with occasional shaking. The crude extract was obtained by filtration through filter paper. The filtrate was evaporated by rotatory evaporator to give a viscous brownish dark mass and the extract was preserved in a refrigerator at 2°C–8°C for use in the experiments (Bashir and Gilani, 2009). The yield value of the extract was 6% (w/w). The extract was suspended in 0.5% carboxy methyl cellulose (CMC) for pharmacological screening.
Animals
Adult albino Wistar rats, 12 weeks old, 130–150 g bodyweight, were used for the study. They were placed under standard conditions (22°C ± 3°C) and allowed free access to standard food pellets and water. After 1 week of acclimatization, rats were used for the experiment. Rats were handled according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines and procedures were approved by the ethical committee of the Institute of Medical Sciences, BHU, Varanasi, India (2211, 07.11.2020) for the care and use of Laboratory Animals.
Toxicity study
Acute oral toxicity
The acute toxicity was performed according to the Organisation for Economic Co-operation and Development (OECD) (423, December 17, 2001) test guidelines. Rats were divided into two groups (n = 3). Group I (control) received p.o. vehicle (0.5% CMC), while Group II was treated with ECB at a single dose of 2,000 mg/kg p.o.
Subacute oral toxicity
The subacute toxicity was performed according to the OECD (407, October 3, 2008) guidelines with slight modification. Rats were divided into four groups (n = 5). Group I (control) received vehicle orally (0.5% CMC), while Group II, III, and IV received orally 250, 500, and 1,000 mg/kg per day dose of ECB, respectively, for 28 consecutive days (Ekanayake et al., 2019; Hasan et al., 2020; Kharchoufa et al., 2020; Loha et al., 2019; Meguellati et al., 2019; Porwal et al., 2017; Silva et al., 2011).
Behavioral changes and mortality
Animals were observed for food intake, water intake, bodyweight, behavioral changes, seizure, hair loss, allergic reaction, hazardous symptoms, and number of deaths during the entire experiment.
Hematological and biochemical analysis
Animals were anesthetized with chloroform once the toxicity investigation was completed, and blood was taken via retro-orbital puncture with Ethylenediamine tetraacetic acid (EDTA) coated capillary tube. Hematological parameters were determined by Auto-Haematology analyser (Arkray Autocell Plus). The serum parameters were determined by using standard diagnostic kits.
Histopathological studies
After completion of toxicity study duration, all animals were anesthetized with chloroform and sacrificed. The organs, like liver, heart, lungs, and kidney, were collected and preserved in 10% formalin solution. The preserved organs were fixed in melted paraffin wax, then allowed to solidify. The tissue sections were prepared using a microtome (thickness of 4 μm). After staining with hematoxylin and eosin, the pathological changes of the tissue sections were examined and identified under microscope [Olympus Magnus Inverted (INVI) inverted microscope] in comparison with the control group (Ekanayake et al., 2016).
In vivo urolithiasis
Ethylene glycol (EG) induced urolithiasis
The antiurolithiasis activity of ECB was evaluated by EG induced urolithiasis model (Bawari et al., 2020; Lonkala, 2020; Moriyama et al., 2009; Patel and Acharya, 2020; Tzou et al., 2016) with slight modification. Animals were divided into five groups (n = 5). Group I (normal control) received p.o. vehicle (0.5% CMC) for 28 days. Group II, III, IV, and V received p.o. 0.75% v/v EG and 1% w/v ammonium chloride for 28 days. Group II (induced control) received vehicle 0.5% CMC p.o. once daily from 15th day to 28th day. Group III, IV, and V received p.o. 200, 400 mg/kg dose of ECB and 750 mg/kg dose of cystone tablet (a standard polyherbal formulation), respectively, once daily from 15th day to 28th day.
Sodium oxalate (SO) induced urolithiasis
The antiurolithiasis activity of ECB was evaluated by SO induced urolithiasis model (Araujo et al., 2020; Elhan et al., 2014; Tzou et al., 2016) with slight modification. Rats were divided into five groups (n = 5). Group I (normal control) received vehicle 0.5% CMC p.o. once daily for 21 days. Group II, III, IV, and V received SO (70 mg/kg per day, i.p.) for 7 days. Group II (induced control group) received vehicle 0.5% CMC p.o. once daily for next 14 days. Group III, IV, and V received p.o. 200, 400 mg/kg dose of ECB and 750 mg/kg dose of cystone, respectively, once daily for next 14 days.
Glycolic acid (GA) induced urolithiasis
The antiurolithiasis activity of ECB was evaluated by GA induced urolithiasis model (Mitra et al., 1998; Sathya and Kokilavani, 2012; Shehzad et al., 2021; Tzou et al., 2016) with slight modification. The rats were divided into five groups (n = 5). Group I (normal control) received vehicle 0.5% CMC p.o. once daily for 21 days. Group II, III, IV, and V received 3% v/v GA per day, p.o. for 7 days. Group II (induced control) received vehicle 0.5% CMC p.o. once daily from for next 14 days. Group III, IV, and V received p.o. 200, 400 mg/kg dose of ECB and 750 mg/kg dose of cystone, respectively, once daily for next 14 days.
Body weight
In EG induced urolithiatic model, changes in bodyweight of individual rats were measured on the 1st, 14th, and 28th days while, in the GA and SO induced urolithiatic model, the body weight was measured on 1st, 7th and 21st days.
Urine analysis
At the end of experiment, all animals were kept in metabolic case for 24 hours urine collection. A drop of conc. Hydrochloric acid (HCl) was mixed in urine to prevent microbial growth (Patel and Acharya, 2020). Urine samples were used to measurement of urine volume and then quantitative estimation of calcium, phosphorus, and magnesium. Urine pH was analyzed by pH meter (Eutech Instruments CyberScan pH 510)
Biochemical analysis
Animals were anesthetized with chloroform once the toxicity investigation was completed, and blood was taken via retro-orbital puncture with capillary. After centrifugation at 5,000 g for 15 minutes, serum was collected and stored in a deep freezer (Patel and Acharya, 2020). The serum parameters were determined by using diagnostic kits.
Histopathological studies
At the end of experiment, all rats were anesthetized with chloroform and sacrificed and the kidneys were collected and preserved in 10% formalin. Kidney sections were prepared as the above-described procedure. The pathological changes in kidney sections were identified by comparison with control group and photographs were taken by microscope (Olympus Magnus INVI inverted microscope).
Statistical analysis
The data were expressed as mean ± SEM and analyzed by using software GraphPad Prism 8.0.2. ordinary one-way and two-way analysis of variance with Dunnett’s multiple comparisons test was applied.
RESULTS AND DISCUSSION
In order to investigate the effect of medicinal plants on urolithiasis, we chosen C. bonducella that have been claimed to have diverse therapeutic characteristics. Extraction of C. bunducella seed were performed in ethanol (95%) based on the literature studies stating that ethanol (95%) can offer superior extraction results for phenolic like chemicals because it dissolves the most polar and non-polar compounds. Extraction was done using the cold percolation process, and the operation was repeated three times with new solvents for a greater yield. Semisolid extracts were obtained and kept in the freezer for scientific investigations. In this investigation, ethanolic solvent yielded higher extract yields for C. bonducella (6%).
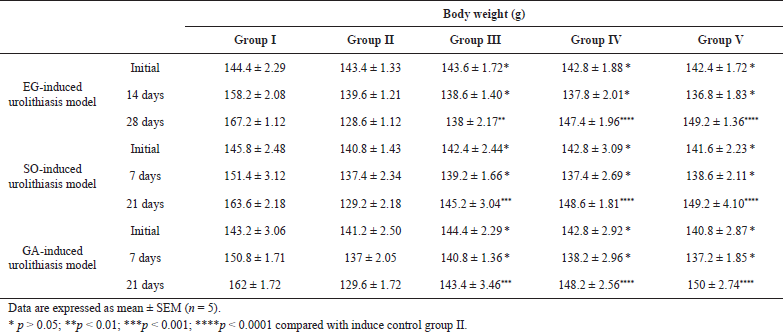
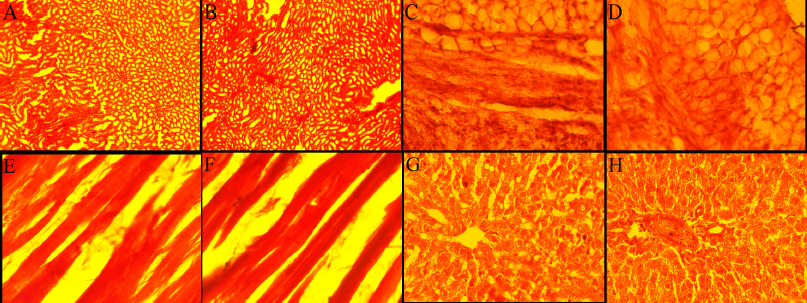
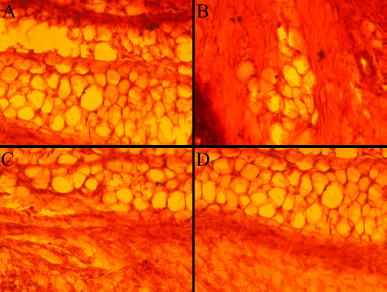
The signs and symptoms of toxicity, including morbidity or mortality, were not observed in both acute and subacute oral toxicity. LD50 of ECB could be greater than 2,000 mg/kg because there was no death recorded in rats during treatment. In acute and subacute toxicity study, the bodyweight of both the treated and normal control group were gradually increased and no significant change in mean body weight gain (Table 2 and 4). The results of hematological and biochemical parameters of treated groups were showed non-significant (p > 0.05), similar and within range as compared with normal control group in both acute (Table 1 and 2) and subacute oral toxicity study (Table 3 and 4). The results of hematological and biochemical parameters of all groups were found to be normal and nearly similar to previously reported studies (Han et al., 2015; Porwal et al., 2017; Silva et al., 2011). The histopathology of the liver showed no inflammation, normal hepatocytes cords, no acute or chronic damage in both acute (Fig. 1D), and subacute oral toxicity (Fig. 4) study. The histopathology of the kidney showed normal glomeruli, tubules, vessels with no features of damage or inflammation in both acute (Fig. 1A), and subacute oral toxicity (Fig. 2). The section of lungs showed normal patent alveoli lined by pneumocytes and there is no collapse, exudate, inflammatory infiltrate, or necrosis in the lung parenchyma in both acute (Fig. 1B) and subacute oral toxicity (Fig. 3). The section of the heart showed cardiomyocytes arranged in pseudosyncytium with normal thickness and there is no myonecrosis or inflammation in both acute (Fig. 1C) and subacute oral toxicity (Fig. 5).
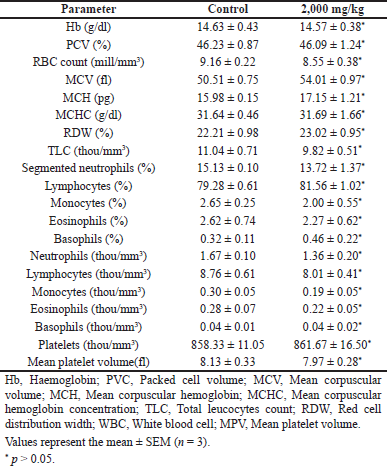 | Table 1. Effects of C. bonducella extract on haematological parameters in acute oral toxicity study. [Click here to view] |
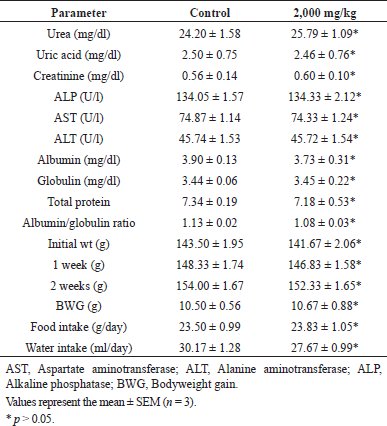 | Table 2. Effects of C. bonducella extract on biochemical parameters in acute oral toxicity study. [Click here to view] |
In EG, GA, and SO-induced urolithiasis model, the bodyweight of both C. bonducella and cystone-treated groups was increased significantly (p < 0.01 to p< 0.0001) as compared with the induced control group (Table 5). Administration of EG, GA, and SO caused induction of renal stone formation and led to a significant decrease in body weight in the induced control group when compared with the normal control group. EG, GA, and SO caused body weight loss. After treatment with the 200 mg/kg of C. bonducella, slight body weight gain was observed; and treatment with 400 mg/kg of C. bonducella, maximum body weight gain was observed which was similar to cystone-treated group. Ahmed et al. (2013); Bawari et al. (2020); Bayir et al. (2011), Shehzad et al. (2021); Kaushik et al., (2019) reported that lithogenic treatment causes a decrease in body weight.
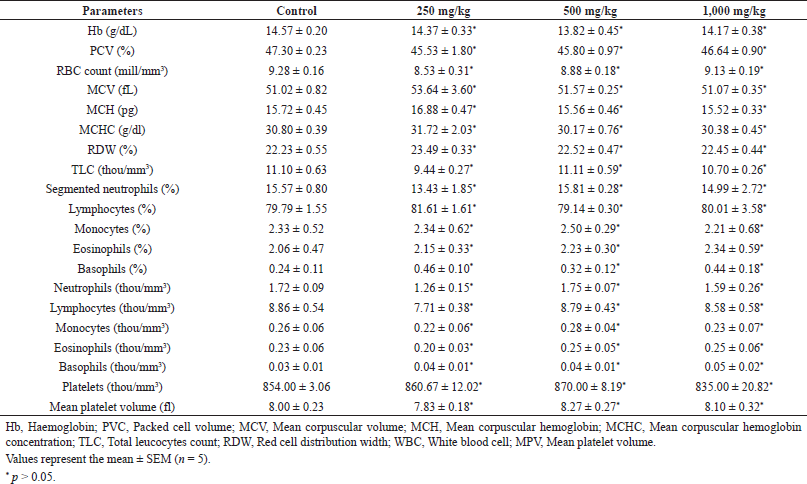 | Table 3. Effects of C. bonducella extract on haematological parameters in subacute oral toxicity study. [Click here to view] |
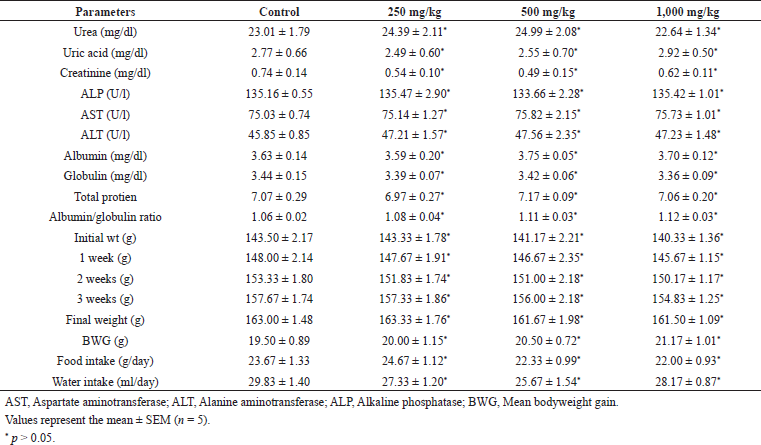 | Table 4. Effects of C. bonducella extract on biochemical parameters in subacute oral toxicity study. [Click here to view] |
 | Table 5. Body weight change during treatment. [Click here to view] |
 | Figure 1. Histology of albino Wistar rat’s kidney (A), lung (C), heart (E), liver (G), of the control group and kidney (B), lung (D), heart (F), liver (H) of the group treated with 2,000 mg/kg dose in acute oral toxicity. [Click here to view] |
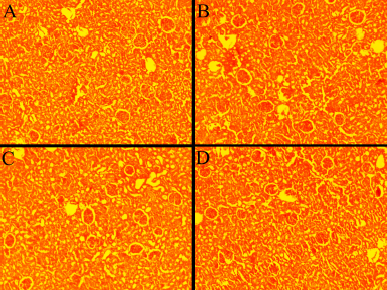 | Figure 2. Histology of albino Wistar rat’s kidney (A) control group (A) 250 mg/kg (B), 500 mg/kg (C), and 1,000 mg/kg (D) treated group in subacute oral toxicity study. [Click here to view] |
 | Figure 3. Histology of albino Wistar rat’s lung (A) control group (A) 250 mg/kg (B), 500 mg/kg (C), and 1,000 mg/kg (D) treated group in subacute oral toxicity study. [Click here to view] |
EG, GA, and SO administration cause significant (p < 0.05 to p < 0.0001) increase in the urinary calcium, phosphorus level and significant (p <0.05 to p < 0.0001) decrease in the urine pH, urine volume, urinary magnesium level in the induced control group as compared with the normal control group. Treatment with 200 and 400 mg/kg of C. bonducella extract causes a significant (p < 0.05 to p < 0.0001) decrease in the levels of urinary calcium and phosphorus along with an increase in magnesium urinary level as compared with the induced control group (Table 6). The lithogenic substance decreases the urinary pH by its metabolism in the acidic compound which causes urinary acidosis. The decrease in urinary pH favors the CaOx precipitation by decreasing CaOx solubility and saturation level in urine which promotes stone formation. Low urinary output also plays a major role by decreasing the volume of urine which got easily supersaturated with oxalates and calcium ions (Patel and Acharya, 2020). The urinary pH and volume of the induced control group were decreased significantly and urine gets supersaturated with CaOxs, initiating stone formation. Volume and pH of urine in C. bonducella extract (200 and 400 mg/kg) and cystone-treated group was found significantly increased, which indicates that CaOx solubility and saturation level were increased in urine. Maximum recovery in urinary parameters were seen with highest dose (i.e., 400 mg/kg) which is similar with cystone-treated group.
Urinary magnesium works as an inhibitor in CaOx stone formation. Magnesium preferentially binds with oxalate and forms a stable complex that is highly water-soluble (Bano et al., 2018; Kaushik et al., 2019). A low level of magnesium is seen in the induced control group and after treatment with the C. bonducella extract, a nearly normal Mg level was resumed. Increased levels of calcium and phosphorous in the urine favor the growth of CaOx crystals. Phosphorous as phosphates was deposited on CaOx stone (Kaushik et al., 2019). The level of calcium and phosphorous was decreased in the C. bonducella extract-treated group.
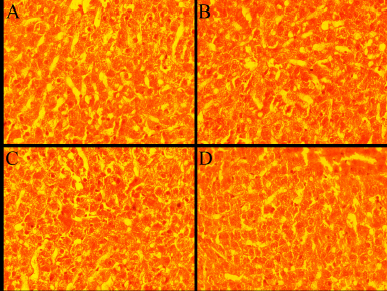 | Figure 4. Histology of albino Wistar rat’s liver (A) control group (A) 250 mg/kg (B), 500 mg/kg (C), and 1,000 mg/kg (D) treated group in subacute oral toxicity study. [Click here to view] |
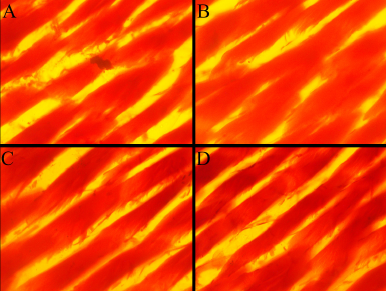 | Figure 5. Histology of albino Wistar rat’s heart (A) control group (A) 250 mg/kg (B), 500 mg/kg (C), and 1,000 mg/kg (D) treated group in subacute oral toxicity study. [Click here to view] |
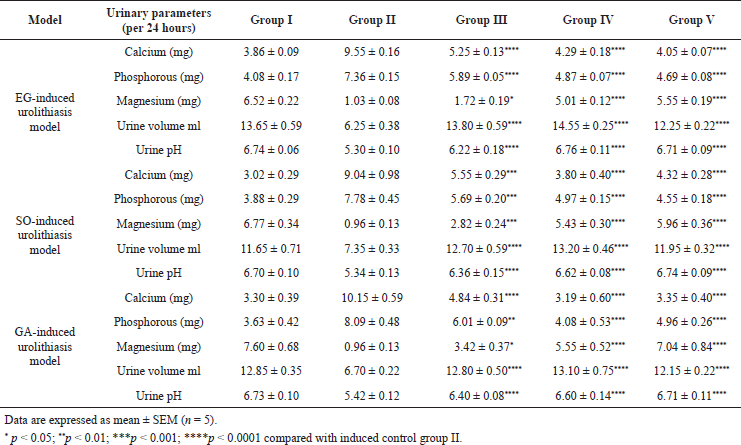 | Table 6. Effect of C. bonducella on urine parameter in urolithiasis. [Click here to view] |
The serum biochemical parameters like uric acid, urea, creatinine, BUN, ALP level were significantly (p < 0.05 to <0.0001) increased in the induced control group as compared to the normal group. However, these biochemical parameters were found to be significantly (p < 0.05 to <0.0001) decreased in both C. bonducella extract and cystone-treated groups as compared to the induced control group (Table 7). Maximum recovery in biochemical parameters were seen with highest dose (i.e., 400 mg/kg) which is similar with cystone-treated group. Stone deposition in tubules and glomerulus obstruct the outflow of urine and decreases the glomerular filtration rate which leads to the accumulation of nitrogenous waste products (urea, BUN, creatinine, and uric acid) in the blood (Bano et al., 2018; Bayir et al., 2011; Kaushik et al., 2019). However, the accumulation of nitrogenous waste in blood was reduced on treatment with C. bonducella extract. ALP level is a marker for kidney damage. In urolithiasis, apical membranes of renal tubular epithelial are damaged because of CaOx crystals deposition. This membrane damage leads to a rise in ALP (Kaushik et al., 2019).
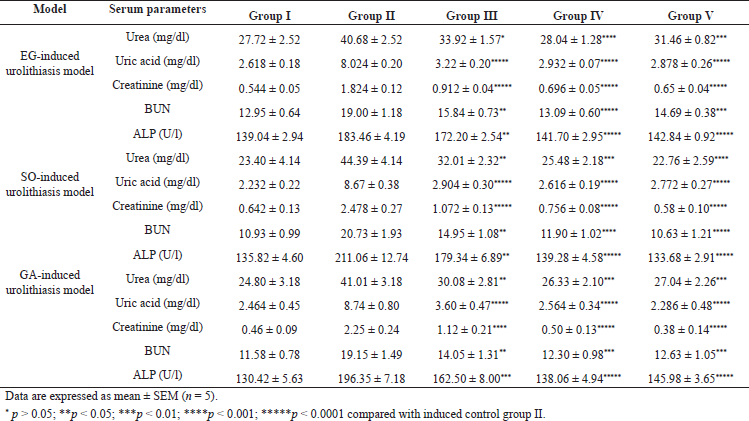 | Table 7. Effect of C. bonducella on serum biochemical parameters in urolithiasis [Click here to view] |
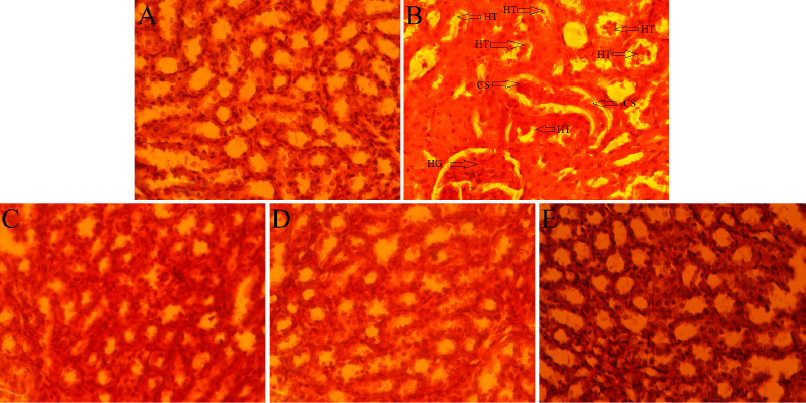 | Figure 6. Histology of albino Wistar rat’s kidney of the normal control group (A), induced control group (B), 200 mg/kg (C), 400 mg/kg (D), and cystone (E) treated group in EG-induced urolithiasis model. [Click here to view] |
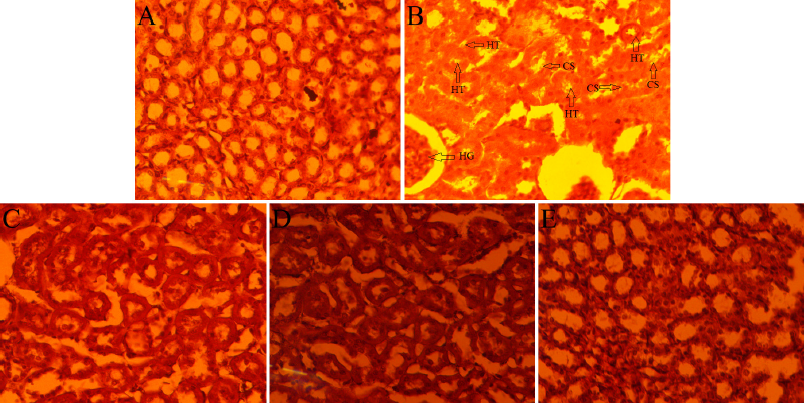 | Figure 7. Histology of albino Wistar rat’s kidney of the normal control group (A), induced control group (B), 200 mg/kg (C), 400 mg/kg (D), and cystone (E) treated group in GA-induced urolithiasis model. [Click here to view] |
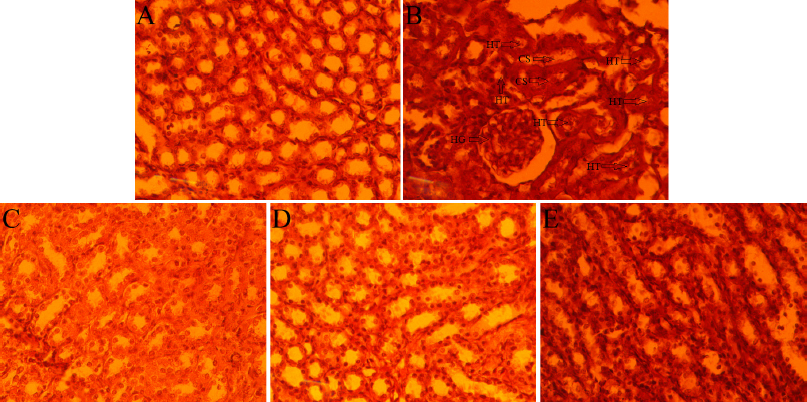 | Figure 8. Histology of albino Wistar rat’s kidney of the normal control group (A), induced control group (B), 200 mg/kg (C), 400 mg/kg (D), and cystone (E) treated group in SO-induced urolithiasis model. [Click here to view] |
The histopathological section of the kidney showed a mild increase in glomerular hypercellularity with severe tubular hydropic degeneration and casts in EG induced control group (Fig. 6B); moderate increased glomerular hypercellularity with moderate tubular hydropic degeneration and casts in GA induced control group (Fig. 7B); moderate increased glomerular hypercellularity, mild tubular hydropic degeneration and casts in SO-induced control group (Fig. 8B) as compared with the respective normal control group. In C. bonducella extract (200 and 400 mg/kg) treated rat’s kidney section showed that glomerular hypercellularity, tubular hydropic degeneration, and casts were recovered in a concentration-dependent manner (Figs. 6C and D, 7C and D, 8C and D); and rats in the cystone-treated group were also recovered (Figs. 6E, 7E and 8E) as compared with the respective induced control group.
CONCLUSION
From the above results, it can be concluded that the ethanolic extract of C. bonducella has no toxic effect on albino Wistar rats in an acute and subacute oral study. 400 mg/kg of C. bonducella seed extract showed preventive effect in experimentally EG, GA, and SO-induced urolithiasis by normalizing imbalanced electrolytes in urine, nitrogenous substances in serum, and decreasing supersaturation of CaOx. Therefore, it is concluded that 400 mg/kg of C. bonducella seed extract could be used as a therapeutic agent to prevent urolithiasis and attenuate the problems associated with it.
ACKNOWLEDGMENTS
The authors would like to acknowledge the facility provided by the Department of Pharmacology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
All the experimental procedures were approved by the ethical committee of the Institute of Medical Sciences, BHU, Varanasi, India (2211, 07.11.2020) for the care and use of Laboratory Animals.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmed A, Wadud A, Jahan N, Bilal A, Hajera S. Efficacy of Adiantum capillus veneris Linn in chemically induced urolithiasis in rats. J Ethnopharmacol, 2013; 146(1):411–6. CrossRef
Ahmed S, Hasan MM, Khan H, Mahmood ZA, Patel S. The mechanistic insight of polyphenols in calcium oxalate urolithiasis Mitigation. Biomed Pharmacother, 2018; 106:1292–9. CrossRef
Araújo DL, Costa-Pessoa JM, de Ponte MC, Oliveira-Souza M. Sodium oxalate-induced acute kidney injury associated with glomerular and tubulointerstitial damage in rats. Front Physiol, 2020; 11:1076. CrossRef
Arif T, Mandal TK, Kumar N, Bhosale JD, Hole A, Sharma GL, Padhib MM, Lavekarb GS, Dabur R. In vitro and in vivo antimicrobial activities of seeds of Caesalpinia bonduc (Lin.) Roxb. J Ethnopharmacol, 2009; 123(1):177–80. CrossRef
Bano H, Jahan N, Makbul SAA, Kumar BN, Husain S, Sayed A. Effect of Piper cubeba L. fruit on ethylene glycol and ammonium chloride induced urolithiasis in male Sprague Dawley rats. Integr Med Res, 2018; 7(4):358–65. CrossRef
Bashir S, Gilani AH. Antiurolithic effect of Bergenia ligulata rhizome: an explanation of the underlying mechanisms. J Ethnopharmacol, 2009; 122(1):106–16. CrossRef
Bawari S, Sah AN, Tewari D. Anticalcifying effect of Daucus carota in experimental urolithiasis in Wistar rats. J Ayurveda Integr Med, 2020; 11(3):308–15. CrossRef
Bayir Y, Halici Z, Keles MS, Colak S, Cakir A, Kaya Y, Akcay F. Helichrysum plicatum DC. subsp. plicatum extract as a preventive agent in experimentally induced urolithiasis model. J Ethnopharmacol, 2011; 138(2):408–14. CrossRef
Besiroglu H, Ozbek E. Association between blood lipid profile and urolithiasis: a systematic review and meta-analysis of observational studies. Int J Uro, 2019; 26(1):7–17. CrossRef
Datte JY, Traore A, Offoumou AM, Ziegler A. Effects of leaf extract of Caesalpinia bonduc (Caesalpiniaceae) on the contractile activity of uterine smooth muscle of pregnant rats. J Ethnopharmacol, 1998; 60(2):149–55. CrossRef
Dawson CH, Tomson CRV. Kidney stone disease: pathophysiology, investigation and medical treatment. Clin Med, 2012; 12(5):467–71. CrossRef
Ekanayake CP, Thammitiyagodage MG, Padumadasa S, Seneviratne B, Padumadasa C, Abeysekera AM. Acute and subacute toxicity studies of the ethyl acetate soluble proanthocyanidins of the immature inflorescence of Cocos nucifera L. in female Wistar rats. BioMed Res Int, 2019; 2019:8428304; doi:10.1155/2019/8428304 CrossRef
Elhan M, Ergene B, Süntar I, Özbilgin S, ÇitoLlu GS, Demirel MA, Keles H, Altun L, Akkol EK. Preclinical evaluation of antiurolithiatic activity of Viburnum opulus L. on sodium oxalate-induced urolithiasis rat model. Evid Based Complement Alternat Med, 2014; 2014:578103; doi:10.1155/2014/578103 CrossRef
Han C, Kim M, Moon S, Jeon Y, Hwang J, Nam C, Park C, Lee S, Na J, Park C, Park H, Lee J, Jang H, Park S, Han K, Choi YW, Lee HY, Kang J. Acute and 28-day subacute toxicity studies of hexane extracts of the roots of Lithospermum erythrorhizon in Sprague-Dawley rats. Toxicol Res, 2015; 31(4):403–14. CrossRef
Hasan M, Mahmud AA, Alam MJ, Siddiqui SA, Arman MSI, Mahmud MH, Amin MN, Imtiaz O, Shahriar M, Jakaria M. Subacute oral toxicity of ayurvedic anti-diabetic preparation Jambadyarista in Sprague-Dawley rats. Toxicol Rep, 2020; 7:1616–21. CrossRef
Iheagwam FN, Ogunlana OO, Ogunlana OE, Isewon I, Oyelade J. Potential anti-cancer flavonoids isolated from Caesalpinia bonduc young twigs and leaves: molecular docking and in silico studies. Bioinfo Bio Insights, 2019; 13:1177932218821371; doi:10.1177/1177932218821371 CrossRef
Kaushik J, Tandon S, Bhardwaj R, Kaur T, Singla SK, Kumar J, Tandon C. Delving into the antiurolithiatic potential of Tribulus terrestris extract through in-vivo efficacy and preclinical safety investigations in Wistar rats. Sci Rep, 2019; 9:15969; doi:10.1038/s41598-019-52398-w CrossRef
Kharchoufa L, Bouhrim M, Bencheikh N, Assri SE, Amirou A, Yamani A, Choukri M, Mekhfi H, Elachouri M. Acute and subacute toxicity studies of the aqueous extract from Haloxylon scoparium Pomel (Hammada scoparia (Pomel)) by oral administration in rodents. BioMed Res Int, 2020; 2020:4020647. CrossRef
Liu T, Wang M, Qi S, Shen X, Wang Y, Jing W, Yang Y, Li X, Gao H. New cassane-type diterpenoids from kernels of Caesalpinia bonduc (Linn.) Roxb. and their inhibitory activities on phosphodiesterase (PDE) and nuclear factor-kappa B (NF-κB) expression. Bioorg Chem, 2020; 96:103573; doi:10.1016/j.bioorg.2020.103573 CrossRef
Loha M, Mulu A, Abay SM, Ergete W, Geleta B. Acute and subacute toxicity of methanol extract of Syzygium guineense leaves on the histology of the liver and kidney and biochemical compositions of blood in rats. Evid Based Complement Alternat Med, 2019; 10:5702159. CrossRef
Lonkala S. In-vitro anti-urolithiatic evaluation of tamsulosin against urolithiasis induced in Wistar albino rats. J Forensic Sci Toxicol, 2020; 3(1):1011.
Meguellati H, Ouafi S, Saad S, Djemouai N. Evaluation of acute, subacute oral toxicity and wound healing activity of mother plant and callus of Teucrium polium L. subsp. geyrii Maire from Algeria. South Afri J Bot, 2019; 127:25–34. CrossRef
Mitra SK, Gopumadhavan S, Venkataranganna MV, Sundaram R. Effect of cystone, a herbal formulation, on glycolic acid-induced urolithiasis in rats. Phytother Res, 1998; 12(5):372–4. CrossRef
Moriyama MT, Suga K, Miyazawa K, Tanaka T, Higashioka M, Noda K, Oka M, Tanaka M, Suzuki K. Inhibitions of urinary oxidative stress and renal calcium level by an extract of Quercus salicina Blume/Quercus stenophylla Makino in a rat calcium oxalate urolithiasis model. Int J Uro, 2009; 16(4):397–401. CrossRef
Namburu SL, Dodoala S, Koganti B, Prasad KVSRG. Antiurolithiatic activity of Phaseolus vulgaris seeds against ethylene glycol-induced renal calculi in Wistar rats. Int J Gre Pharm, 2017; 11(4):281–9.
Patel VB, Acharya N. Effect of Macrotyloma uniflorum in ethylene glycol induced urolithiasis in rats. Heliyon, 2020; 6(6):e04253. CrossRef
Porwal M, Khan NA, Maheshwari KK. Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Sci Pharm, 2017; 85(3):29. CrossRef
Sathya M, Kokilavani R. Antiurolithiatic activity of ethanolic root extract of Saccharum spontaneum on glycolic acid induced urolithiasis in rats. J Drug Del Therap, 2012; 2(5):86–9. CrossRef
Shehzad A, Saleem U, Shah MA, Cruz CVL, Khan AH, Ahmad B. Antiurolithic evaluation of Cucurbita pepo seeds extract against sodium oxalate?induced renal calculi. Pharmacog Mag, 2021; 16(68):174–80. CrossRef
Silva MGB, Aragão TP, Vasconcelos CFB, Ferreira PA, Andrade BA, Costa IMA, Costa-Silva JH, Wanderley AG, Lafayette SSL. Acute and subacute toxicity of Cassia occidentalis L. stem and leaf in Wistar rats. J Ethnopharmacol, 2011; 136(2):341–6. CrossRef
Tzou DT, Taguchi K, Chi T, Stoller ML. Animal models of urinary stone disease. Int J Surg, 2016; 36(Pt D):596–06. CrossRef
Wang X, Wang M, Ruan J, Zhao S, Xiao J, Tian Y. Identification of urine biomarkers for calcium-oxalate urolithiasis in adults based on UPLC-Q-TOF/MS. J Chromat B. 2019; 1124:290–7. CrossRef
Yasui T, Okada A, Hamamoto S, Ando R, Taguchi K, Tozawa K, Kohri K. Pathophysiology-based treatment of urolithiasis. Int J Uro, 2017; 24(1):32–8. CrossRef