INTRODUCTION
Psyllium is also known as Ispaghula (Masoob and Miraftab, 2010) and is derived from the seed husk of Plantago ovata Forsk which is normally grown in India (Semeco, 2017; Yu et al., 2009). It is a water-soluble supplement (Blackwood et al., 2000). Psyllium husk can be in the form of particles or powder. It is used as a traditional dietary supplement and has been shown to be an effective adjunct to dietary intervention in the control of body weight, body composition and cholesterol, glucose, insulin, and triglycerides levels in both animal (Galisteo et al., 2005; Song et al., 2000) and human studies (Cicero et al., 2007; Feinglos et al., 2013). Not only that, but consumption of psyllium also can cause satiety effects (Brum et al., 2016).
Herbs include many crude plant materials such as leaves, stems, flowers, barks, fruit, seeds, roots, wood, rhizomes, or other plant parts which can be used for medicinal purpose (Ghosh and Mukherjee, 2019; Parkash et al., 2018). The use of herbs in daily life was pioneered in ancient times around the world. The trend of using herbs in supplements also has been increased. The ingredients claimed in the psyllium husk and selected mixed herbal supplement include Plantago ovate, Withania somnifera, Tribulus terrestris, Lepidium meyenii, Serenoa repens, Eurycoma longifolia, Garcinia cambogia, Epimedium, Ptychopetalum olacoides, Tamarindus indica, Cucurbita pepo seeds, marine collagen, and honey. There are some of the mixed herbs in the supplement specified for males, such as E. longifolia, T. terrestris, and P. olacoides. All of the herbs have their own benefits for the body. For example, W. somnifera has antimicrobial (Girish et al., 2006), cardioprotective (Hamza et al., 2008), antioxidant, and anti-inflammatory functions (Mehrotra et al., 2011); S. repens has the anti-inflammatory function (Anastasi et al., 2011); T. indica has the antimicrobial and antiseptic functions (Bhadoriya et al., 2010; Doughari, 2006); L. meyenii has the bioactive effects of antifatigue activity, sexual and fertility enhancement, and memory impairment (Choi et al., 2012; Lembe et al., 2012; Wang et al., 2007). However, herbal supplement overdose may have bad effects. For example, W. somnifera has many issues with toxicity, as its active ingredient called Withaferin A can cause toxicity when overdosed (Mensah, 2016). There are so many supplement products in the market now using mixed herbs. The market value is very high, but few studies have identified the effects of supplements on human health.
According to the National Health and Morbidity Survey in 2015, Malaysians face health problems such as hypertension and obesity at a young age (Yusof, 2018). People may take supplements to prevent disease, as a complement to conventional therapies, and also to promote well-being and health (Anastasi et al., 2011). Health indicators such as lipid profiles, blood pressure, blood glucose, liver function test, and health indicator parameters can be measured to determine the effects of supplementation. Based on the Center for Disease Control and Prevention (2012), health indicators are measurable characteristics that describe the health of the population, such as disease incidence or prevalence and other health consequences.
METHODOLOGY
Research design
An unblinded simple randomized study was carried out in Universiti Malaysia Terengganu. Subject eligibility was screened through Health and Lifestyle Questionnaire. A total of 83 respondents have been screened during one and half month period. For sample size calculation, a formula from Charan and Biswas (2013) was used, where Zα = 1.96 (from Z table) at type I error of 5%, Zpower = 0.842 (from Z table) at 80% power, and SD is the standard deviation of 7.2 mg/dl from Soltanian et al. (2018). From the formula, 13 subjects per group should be recruited, and considering any dropout, the sample size was increased by 105; therefore, a total of 30 respondents were included in the study. The subject’s inclusion criteria included 20–40-year-old healthy male adults (without chronic diseases and other major illnesses). Subjects also had not participated in any clinical trial for at least 3 months and were willing to make a blood donation (if related) and undergo other clinical trials. The present study was approved by the Research Management and Innovation Centre Human Ethics Committee, Universiti Malaysia Terengganu with reference number UMT/JKEPHMK/2020/43. Consent forms were signed and collected from all study participants.
The material used in the present study was a local commercially available product containing psyllium husk and selected mixed herbs in powder form. Apart from psyllium husk, the ingredients claimed for the supplement consist of psyllium husk, E. longifolia Jack, T. terrestris, Epimedium, W. somnifera, L. meyenii, S. repens, G. cambogia, P. olacoides, C. pepo seeds, T. indica, honey, and marine collagen. Most of the herbs used have been explicitly recommended for male use. This supplement was provided in a sealed plastic package (25 grams per packet) and the dose given to the subject was one packet per day for 1 month of intervention. Supplements were acquired as a gift from a particular local business, which has been operating for almost 20 years.
Research instrument and measurements
There were three types of measurements involved in this study: anthropometric, clinical, and biochemical measurements. For the anthropometric measurements, a portable stadiometer (SECA, Germany) and Tanita digital body fat monitor/scale model UM-026 (Tanita, UK) were used for height and body weight, respectively. The body weight and height of subjects were measured to the nearest 0.1 kg and 0.1 cm, respectively. Omron Automatic Blood Pressure Monitor HEM-7080 model was used for the blood pressure measurements. A 5–7 ml blood sample was taken from the subjects after 8–12 hours of fasting by medical personnel at the Universiti Malaysia Terengganu Health Centre. The blood was sent to an accredited lab to determine the results of the liver function test and lipid profile, while a glucose reagent test strip with Accu-Check Advantage meter (Roche Diagnostics, Germany) was used for fasting blood glucose measurements. A questionnaire was also used. There were four sections in the questionnaire and each was prepared in English and Malay. Section A collected the personal information of respondent; section B included the physical measurements of the respondent; section C included clinical and biochemical measurements; and section D consisted of the health indicator parameters of the respondent, and these subjective symptoms, such as constipation, headache, and gastric pain, were evaluated on a scale of 1–5, whereby scale 1 indicates symptoms not present; 2 rarely present; 3 sometimes; 4 often; and 5 always, using the Anti-Aging QOL Common Questionnaire (AAQol) established and validated by Yonei et al. (2004).
Data analysis
The data were analyzed using Statistical Package for the Social Science program (SPSS) version 20.0 with 95% confidence interval (version 20.0, SPSS Inc. Chicago, IL). Kolmogorov–Smirnov normality test was used for the data distribution (Ghasemi and Zahediasl, 2012). Normally distribution data were presented in mean ± standard deviation (SD). A paired t-test was used to analyze the baseline and end-line of each parameter within the group. The nonnormality distribution data were presented as the median and interquartile range (IQR) and tested using the nonparametric test of the Wilcoxon-signed rank test, while the Mann–Whitney test was used to compare between the control group and the supplemental group for each parameter. In all analyses, significance was indicated by a probability level of p < 0.05.
RESULTS
Baseline characteristics of subjects
The eligible respondents (30 respondents) were recruited to participate in the study and randomly assigned into the control and supplemental groups. Randomization was done using MS Excel function RAND. A total of 15 respondents were allocated to the control group, while another 15 respondents were allocated to the supplemental group. All of the respondents were assumed to be healthy and they were not diagnosed with any serious illness. One of the respondents from the supplemental group withdrew due to feeling uncomfortable after consuming 2 weeks’ worth of supplements. Thus, a total of 29 respondents (14 respondents from the control group and 15 from the supplemental group) were included in the final data analysis. The baselines characteristics of the subjects are shown in Table 1.
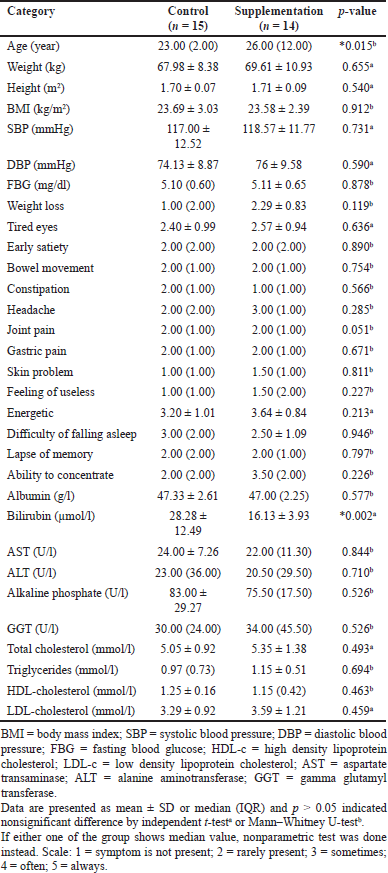 | Table 1. The baseline characteristics of the respondents in both the control and supplemental groups. [Click here to view] |
Effect of psyllium husk and selected mixed herbal supplement on body mass index (BMI), blood pressure, and biochemical parameters
There were significant decreases (p < 0.05) in total bilirubin in both the supplemental group and the control group. In contrast, alanine aminotransferase (ALT) and high density lipoprotein cholesterol (HDL-c) showed significant increases. No other parameters showed any significant changes (p > 0.05) as shown in Table 2.
There were nonsignificant differences (p > 0.05) between the absolute change and percentage change of BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood glucose, liver function test, and lipid profiles. The data are shown in Table 3.
Table 4 shows that only two parameters in the supplemental group on “bowel movement” and “headache” show significant improvement (p < 0.05) after 1-month intervention. However, the other parameters did not show a significant effect (p > 0.05).
DISCUSSION
The present study aims to investigate the effect of psyllium husk and selected mixed herbal supplement on health indicators in healthy male subjects. The parameters used were BMI, blood pressure, biochemical parameters involving lipid profile and liver function test, and subjective symptoms from AAQoL adopted from Yonei et al. (2008). Based on Table 1, there was a significant difference in age between groups, although the subjects were randomly assigned. However, the age difference was acceptable (23 vs. 26 years) and both are classified as young adults (aged 18 to 26 years) (Bonnie et al., 2015). The chance of assuming a false premise as true is high with the small sample size used (Jorge and Lilian, 2014), but the sample size used was calculated and considered adequate to seek a significant difference, if any. The baseline data of bilirubin between the groups also show a significant difference and again the values are still within the normal range. Three of the respondents (10.34%) (one subject from the supplemental group and two from the control group) reported being smokers. Cigarette smoking is one of the factors that may affect the level of total bilirubin. According to Nelofer et al. (2015), bilirubin levels for a current smoker are lower at 6.84–10.84 mmol/l, while those of nonsmokers are 10.84–13.68 mmol/l. Another study conducted by Jo et al. (2012) shows that the total bilirubin for nonsmokers is 16.1 ± 5.6 μmol/l; for ex-smokers is 15.9 ± 5.4 μmol/l; and for current smokers is 14.8 ± 5.3 μmol/l. Thus, it can be concluded that the total bilirubin in the control group should be low. Yet, in the present study, the total bilirubin in the control group was high, as a total of nine of the subjects’ total bilirubin levels from the control group were out of the normal range (3.4–20.5 μmol/l). The reason for being over the normal range of total bilirubin in the control group is unclear, but the findings were certainly reliable as the serums were analyzed by an accredited laboratory. For other parameters, no significant differences were found between groups.
From Table 2, the total bilirubin of subjects in both groups had decreased. However, the effect is not due to the supplement as both groups showed decreased in total bilirubin. Based on Table 3, both aspartate transaminase (AST) and ALT in the control and supplemental groups were increased. The AST shows a slight increase after the intervention period. It can be said that the supplement might have some effects on human liver function tests; however, a longer intervention is necessary. Based on work by Danielsson et al. (2014), ALT has significant interactions between age and BMI. Those younger than 40 years old with a higher BMI will have higher values for ALT. The age range for both the control and supplemental groups was 20–40 years old. Thus, the present study’s results for ALT were in line with those of a previous study, in which subjects younger than 40 years old with the higher BMI had the higher values for ALT.
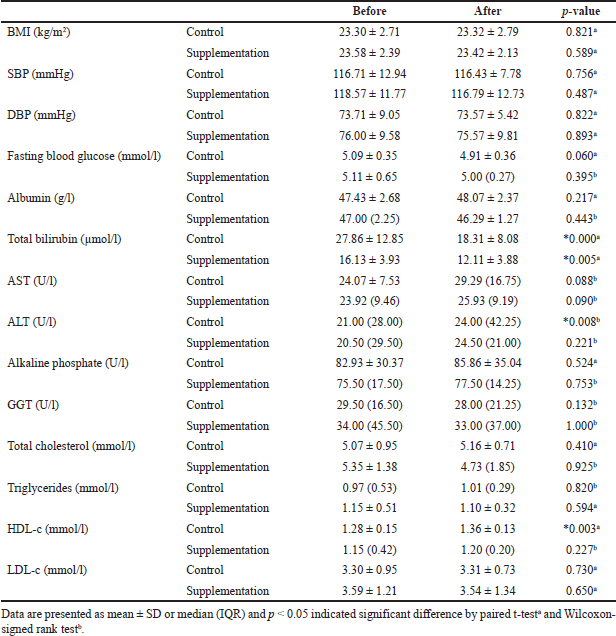 | Table 2. Comparison of BMI, blood pressure, and fasting biochemical parameters in the control and supplemental groups after 1-month intervention period. [Click here to view] |
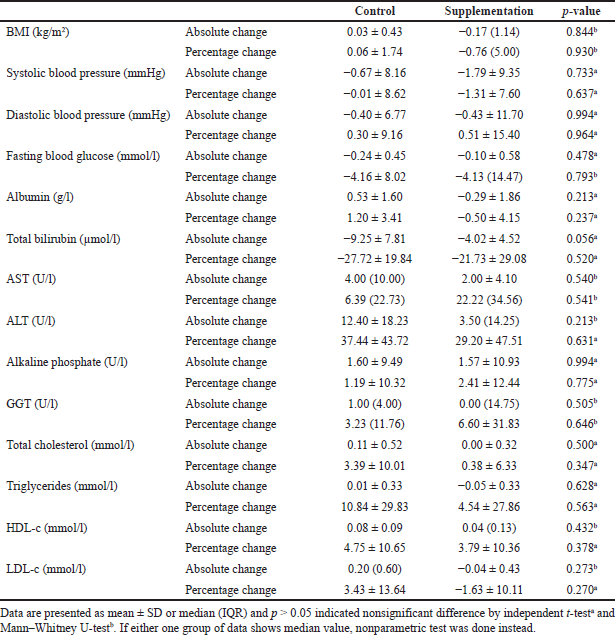 | Table 3. Comparison between absolute change and percentage change of BMI, blood pressure, and fasting biochemical parameters in the control and supplemental groups after 1-month intervention period. [Click here to view] |
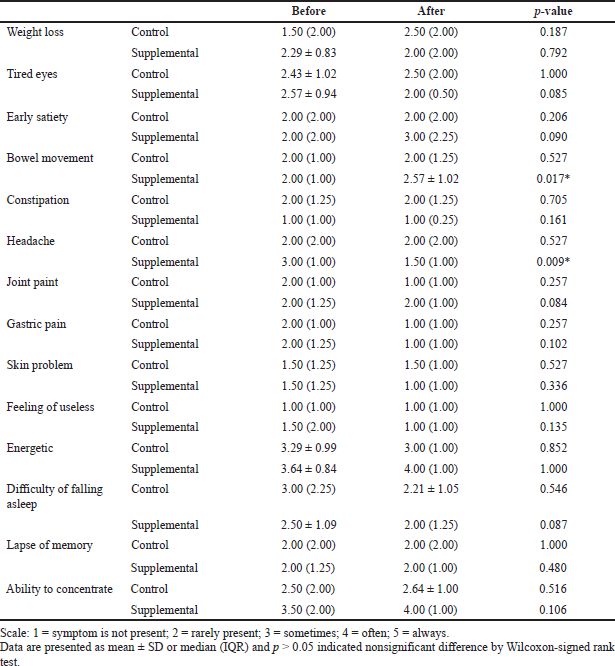 | Table 4. Comparison of health indicator parameters of subjects in the control and supplemental groups. [Click here to view] |
According to Table 2, the significant changes of HDL-c in the control group might be due to the control diet of the subjects. Based on the self-report, two subjects (13.33%) in the control group reported that they controlled their diet during the 1-month intervention although they were advised not to change their usual diet during the intervention period. Additionally, one of the subjects in the control group was undergoing smoking cessation during the intervention month. Forey et al. (2013) reported that smoking cessation for 30 days can increase the HDL-c by +0.035 mmol/l. It can be summarized that the psyllium husk and selected mixed herbal supplement did not show an effect in lowering the lipid profiles as the HDL-c only showed a significant increase in the control group.
According to Erdogan et al. (2016), the frequency of bowel movement for the psyllium intervention group showed a significant increase from 2.76 ± 1.10 to 3.49 ± 1.40 after the 4-week intervention period with the ingestion of 10 g of psyllium per day after meals. However, another study conducted by Soltanian et al. (2018) found that the frequency of bowel movement did not show significant changes in 4 and 12 weeks but only showed significant changes at 8 weeks of intervention with 10 g of psyllium per day. In that study, the frequency of bowel movement at baseline was 1.5 ± 0.7, increasing to 1.7 ± 0.8 after 4 weeks, then continuing to increase to 2.0 ± 0.8 in 8 weeks, and decreasing to 1.8 ± 1.0 after 12 weeks.
The present study also found that the frequency of headaches in the supplemental group was decreased significantly. There is evidence demonstrating that the psyllium husk and selected mixed herbal supplement has a positive effect on headache. Only one of the selected mixed herbs has shown a positive effect on relieving headache, namely, maca, which is most commonly used by menopausal women. Based on the study by Meissner et al. (2006), the headache symptoms of postmenopausal women were improved between the first month and the second month of intervention. The mean value was reduced from 0.20 to 0.14 after the consumption of 2 g/day of vegetable hard gel capsules with pre-Gelatinized Organic Maca (Maca-GO), twice per day with meals. Thus, based on a previous report, it is postulated that maca was responsible for relieving headaches among the respondents in the present study.
CONCLUSION
The effects of psyllium husk and selected mixed herbal supplement on health indicators have not been studied by others. However, psyllium husk has been reported to have beneficial effects on BMI, fasting blood glucose, blood pressure, and lipid profile. In the present study, the supplemental group showed improvement in health indicator parameters of “bowel movement” and “headache.” Total bilirubin levels also showed improvement after short-term supplementation of 1 month. Therefore, it can be concluded that the 25 g/day of psyllium husk and selected mixed herbal supplement with 1-month intervention period had shown the same benefits in health indicator parameters, specifically in improving bowel movement and reducing headache.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to all the young adult respondents for their participation and full cooperation. Special thanks are due to Mrs. Hassan, Z. for providing supplemental products in this study.
AUTHOR CONTRIBUTIONS
TAll authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The present study was approved by the Research Management and Innovation Centre Human Ethics Committee, Universiti Malaysia Terengganu with reference number UMT/JKEPHMK/2020/43. Consent forms were signed and collected from all study participants.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
TThis journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Anastasi J, Michelle Chang M, Capili B. Herbal supplement: talking with your patients. J Nurse Pract, 2011; 3(1):29–35. CrossRef
Bhadoriya SS, Ganeshpurkar A, Narwaria J, Rai G, Jain AP. Tamarindus indica: extent of explored potential. Pharmacol Rev, 2010; 5(9):73–81. CrossRef
Blackwood AD, Salter J, Dettmar PW, Chaplin MF. Dietary fiber, physiocochemical properties and their relationship to health. J R Soc Health, 2000; 120(4):242–7. CrossRef
Bonnie, R. J., Stroud, C., Breiner, H. 2015. Investing in the Health and Well-Being of Young Adults. National Academies Press (US).
Brum JM, Gibb RD, Peters JC, Mattes RD. Satiety effects of psyllium in healthy volunteers. Appetite, 2016; 105:27–36. CrossRef
Center for Disease Control and Prevention. Health indicators warehouse. Center for Disease Control and Prevention, Atlanta, GA, 2012. Available via https://www.cdc.gov/nchs/ppt/nchs2012/LI-18_CHURCHILL.pdf (Accessed 31 December 2019).
Charan J, Biswas T. Calculate sample size for different study design in medical research. Indian J Psychol Med 2013; 35(2):121–8. CrossRef
Choi E, Kang J, Cho J, Lee S, Kim T, Yeo I. Supplementation of standardized lipid-soluble extract from maca (Lepidium meyenii) increases swimming endurance capacity in rats. J Funct Food, 2012; 4(2):568–73. CrossRef
Cicero A, Derosa G, Manca M, Borghi C, Gaddi A. Different effect of psyllium and guar dietary supplementation on blood pressure control in hypertensive overweight patients: a six-month, randomized clinical trial. Clin Exp Hypertens, 2007; 29(6):383–94. CrossRef
Danielsson J, Kangastupa P, Laatikaineen T, Aalto M, Niemela O. Impacts of common factors of life style on serum liver enzymes. World J Gastroenterol, 2014; 20(33):11743–52. CrossRef
Doughari JD. Antimicrobial activity of Tamarindus indica L. Trop J Pharm Res 2006; 5(2):597– 603. CrossRef
Erdogan A, Rao SSC, Thiruvaiyaru D, Lee YY, Adame EC, Valestin J, O’ Banion M. Randomised clinical trial: mixed soluble/ insoluble fiber vs. psyllium for chronic constipation. Aliment Pharmacol Ther, 2016; 44(1):35–44. CrossRef
Feinglos M, Gibb R, Ramsey DL, Surwit R, McRorie J. Psyllium improves glycemic control in patients with type II diabetes mellitus. Bioact Carbohydr Diet Fiber, 2013; 1(2):156–61. CrossRef
Forey BA, Fry JS, Lee PN, Thornton AJ, Coombs KJ. The effect of quitting smoking on HDL-cholesterol: a review based on within subject changes. Biomark Res 2013; 1(1):32–6. CrossRef
Galisteo M, Sanchez M, Vera R. A diet supplemented with husk of Plantago ovata reduces the development of endothelial dsyfunction, hypertension and obesity by affecting adiponectin and TNF-(Alpha) in obese zucker rats. J Nutr, 2005; 135(10): 2399–404. CrossRef
Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab, 2012; 10(2):486–9. CrossRef
Ghosh DK, Mukherjee PK. Natural medicines: clinical efficacy, safety and quality. Boca Rotan, FL: CRC Press, p 605, 2019. CrossRef
Girish K, Machiah K, Ushanandini S. Antimicrobial properties of a non-toxic glycoprotein (WSG) from Withania somnifera (Ashwagandha). J Basic Microbiol, 2006; 46:365–74. CrossRef
Hamza A, Amin A, Daoud S. The protective effect of a purified extract of Withania somnifera against doxorunicin-induced cadiac toxicity in rats. Cell Biol Toxicol, 2008; 24(1):63–73. CrossRef
Jo JS, Kimm HJ, Yun JE, Lee KJ, Jee SH. Cigarette smoking and serum bilirubin subtypes in healthy Korean men: the Korea medical institute study. J Prev Med Public Health, 2012; 45(2):105–12. CrossRef
Jorge F, Lilian MF. How sample size influences research outcomes. Dental Press J Orth, 2014; 19(4):27–9. CrossRef
Lembe D, Gasco M, Gonzales G. Fertility and estrogenic activity of Turraeanthus africanus in combination with Lepidium meyenii (Black maca) in female mice. J Integr Med, 2012; 4(3):345–51. CrossRef
Masoob R, Miraftab M. Psyllium: current and future applications. Woodhead Publishing Limited, Sawston, UK, pp 244–53, 2010. CrossRef
Mehrotra V. Mehrotra S, Kirar V. Antioxidant and antimicrobial activities of aqueous extract of Withania somnifera against methicillin-resistant Staphylococcus aures. J Microbial Biotechnol 2011; 1(1):40–5.
Meissner HO, Mscisz A, Reich-Bilinska H, Mrozikiewicz P, Bobkiewicz-Kozlowska T, Kedzia B, Lowicka A, Barchia I. Hormone-balancing effect of pre-gelatinized organic maca (Lepidium perivianum Chacon): (iii) clinical responses of early postmenopausal women to Maca in double blind, randomized, placebo-controlled, crossover configuration, out-patient study. Int J Biomed Sci 2006; 2(4):375–94.
Mensah A. (2016). Ashwagandha danger: this herbal remedy may have repercussions. Mensah Medical, Warrenville, IL, (2016). Available from http://www.mensahmedical.com/ashwagandha-dangers/ (Accessed 1 April 2019).
Nelofer J, Sabeena R, Zubia M, Zahoor AB, Sana A, Musarat R, Adila H, Mehrunisa A, Mehwish N, Ayesha M, Muzaffar K. (2015). To estimate the level of bilirubin in the blood of male cigarette smokers. Am Eurasian J Toxicol Sci, 7(3):177–9.
Parkash J, Prasad DN, Shahnaz M, Dev D. Herbs as traditional medicines: a review. J Drug Delivery Therapeutics, 2018; 8(5):146–50. CrossRef
Semeco A. Seven benefits of psyllium. Medical News Today, Brighton, UK, 2017. Available via https://www.medicalnewstoday.com/articles/318707.php (Accessed 14 April 2019).
Soltanian N, Janghorbani M, Adibi P. Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: a randomized trial in patients with type 2 diabetes and chronic constipation. Complement Ther Med, 2018; 40:1–7. CrossRef
Song YJ, Sawamura M, Ikeda K, Igawa S, Yamori, Y. Soluble dietary fiber improves insulin sensitivity by increasing muscle GLUT-4 content in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol, 2000; 27(1–2):41–5. CrossRef
Wang Y, Wang YC, McNeil B, Harvey L. Maca: Andean crop with multi- pharmacological functions. Food Res Int, 2007; 40(7):783–93. CrossRef
Yonei Y, Mizuno Y, Katagiri E. Effects of cosmetics therapy using isoflavone and pine bark extract on the skin and QOL: a double-blind placebo- controlled trial. Anti-Aging Med Res, 2004; 1:48–58.
Yu LL, Lutterodt H, Cheng H. (2009). Beneficial health properties of psyllium and approaches to improve its functionalities. Adv Food Nutr Res, 2009; 55:193–221.
Yusof MA (2018). Malaysian developing heart disease at younger age. New Straits Time, Kuala Lumpur, Malaysia, p 3, 2018.