INTRODUCTION
Cancer disease is one of the primary public burdens and is the second leading cause of death worldwide, with 10 million deaths per year and 70% of them occuring in low-middle-income countries. In 2020, the most common causes of cancer deaths were lung, colon and rectum, liver, stomach, and breast with 1.8, 0.935, 0.830, 0.769, and 0.685 million cases, respectively. In terms of new cases of cancer, and also for 2020, the most common were breast, lung, colon and rectum, prostate, skin (nonmelanoma), and stomach with 2.26, 2.21, 1.93, 1.41, 1.20, and 1.09 million cases, respectively (WHO, 2020). In 2018, 9.6 million people globally were estimated to have died from cancer (WHO, 2020). Recently, global cancer statistics including incidence and mortality for 36 types of cancer in 195 countries was presented (Sung et al., 2021). Probably because of the high rate of deaths caused by cancer, many researchers have focused on cancer treatment for decades, applying different methodologies with diverse findings. Thus, new alternative therapies against cancer have emerged using compounds that exhibit good anticancer activity. Among those compounds, hybrids and conjugates based on 5-Fluorouracil (5-FU) are highlighted (see review of Cardona-G et al., 2021).
Since the growth and number of studies available on a specific topic, such as cancer, can be considerably high, the task of structuring a comprehensible review with relevant information could become a challenge for researchers. However, bibliometric analysis allows for scientific mapping of large amounts scientific literature, which could provide macroscopic overview of a topic of interest such as antibiotics, emerging contaminant, antivirals, and multidrug-resistant fungus (Gómez-Ríos, López-agudelo, and Ramírez-Malule, 2020; Gómez-Ríos and Ramírez-Malule, 2019; Liao et al., 2018; Natalia et al., 2021; Ramírez-Malule, 2018; Ramírez-Malule et al., 2020; Ramírez-Malule et al., 2020; Soosaraei et al., 2018). In the case of cancer treatment, there are several reviews available on cancer of breast (Waks and Winer, 2019), prostate (Nevedomskaya et al., 2018), colon (Banerjee et al., 2017), and even survivorship statistics for different cancers (Miller et al., 2016, 2019). Besides, a recent review based on 5-FU was published (Cardona-G et al., 2021). In that review, the authors reported the synthesis of hybrids and derivatives containing 5-FU and their anticancer activity in different cancer cell lines. However, a bibliometric analysis of 5-FU is still lacking. Thus, the aim of this study was to identify and analyze the studies related to 5-FU in the field of chemistry.
METHODS
Database selection
The Scopus database was used as a tool to retrieve the most relevant documents related to 5-FU in the field of “chemistry.” Data collection was acquired from Scopus on March 2, 2021, and comprised records obtained from a systematic search of documents matching the search terms in the fields of article title, abstract, and keywords. The search was refined to article and review as the document types. The resulting search was as follows:
- Search equation: TITLE-ABS-KEY (“5-Fluorouracil”) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”) AND (LIMIT-TO (SUBJAREA, “CHEM”).
- Timespan: 2015–2020. 1,025 articles and reviews were retrieved after removing duplicates.
Data export and analysis
The information retrieved from the Scopus database for all records during the search were (i) both citation and bibliographical information, and (ii) abstract and keywords. Retrieved data were downloaded from Scopus and exported to Microsoft Excel®. Later, VOSviewer 1.6.13 was used for visualization and data analysis. Six was set as the minimum number of occurrences of a keyword. After removing the thesaurus terms, 52 of them met the threshold, which led to 9 clusters.
Bibliometric indicators
The following bibliometric indicators were evaluated:
- Volume and growth of publications related to 5-FU studies.
- Subject areas related to 5-FU studies.
- Co-occurring keywords of network visualization.
- Co-occurrening keywords of overlay visualization.
- Most active countries.
Results
A total of 1,025 documents related to 5-FU in the field of chemistry were indexed in the Scopus database between 2015 and 2020, both included. Figure 1 shows the evolution of the number of publications in this field. As observed, there is an increase in research outputs starting and ending with 137 and 241 records for 2015–2020, respectively. In this time range, the areas of knowledge (excluding chemistry) related to 5-FU studies were (i) Biochemistry, Genetics, and Molecular Biology; (ii) Chemical Engineering; and (iii) Materials Science (Fig 2).
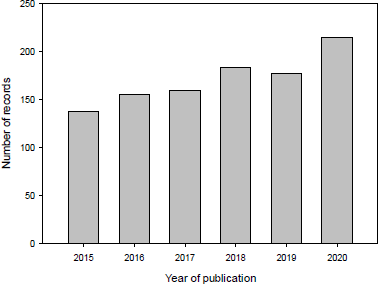 | Figure 1. Evolution of published studies related to 5-FU between 2015 and 2020. [Click here to view] |
Figure 3a shows the co-occurrence of authors’ keywords network, which contains nine clusters easily distinguishable by colors. This figure clearly displays the topic on which the researchers have focused in the last 6 years (2015–2020): (i) drug delivery (blue and purple); (ii) cancer treatment and drug resistance (yellow, orange, and pink); and (iii) anticancer activity (red and blue light) and molecular docking (green). Studies related to drug resistance, chemoresistance, adsorption, mesoporous silica, and molecular docking concentrated the research on 5-FU in 2018 (Fig 3b).
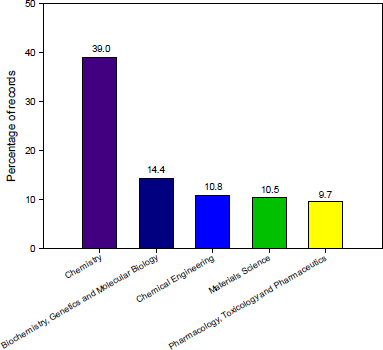 | Figure 2. Summary of publications grouped by areas related to 5-FU studies between 2015 and 2020. [Click here to view] |
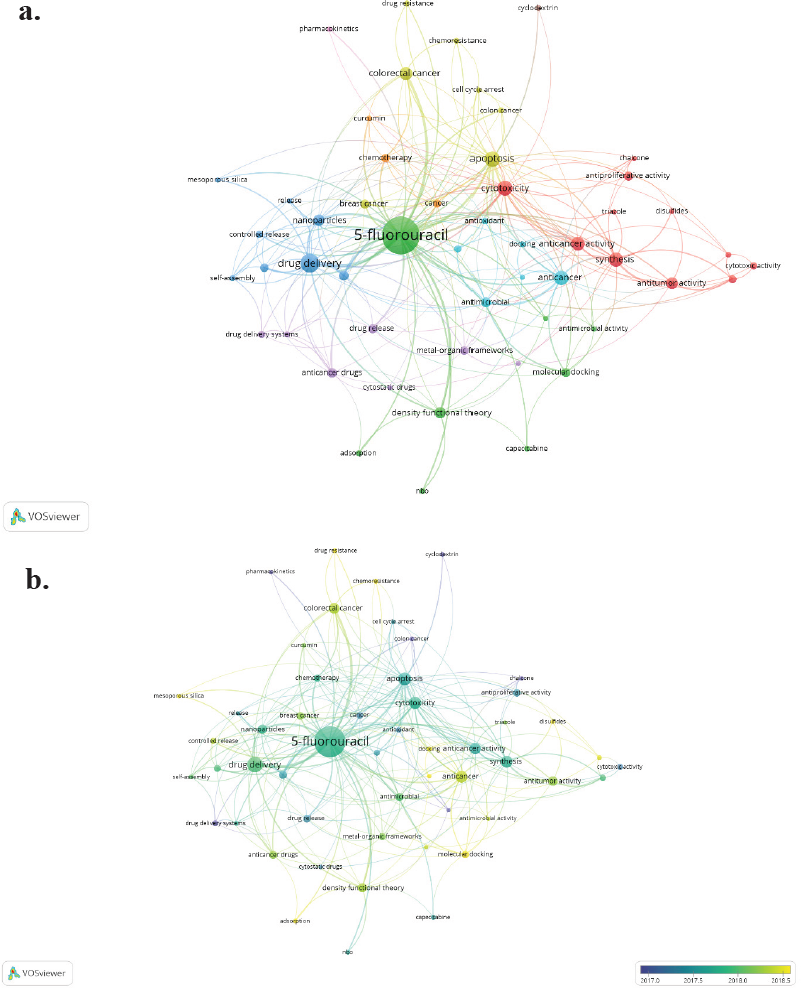 | Figure 3. Bibliometric network of studies related to 5-FU between 2015 and 2020. (a) Research topic map. (b) Research topic map with time overlap. Note: minimum number of occurrences of a keyword is six. [Click here to view] |
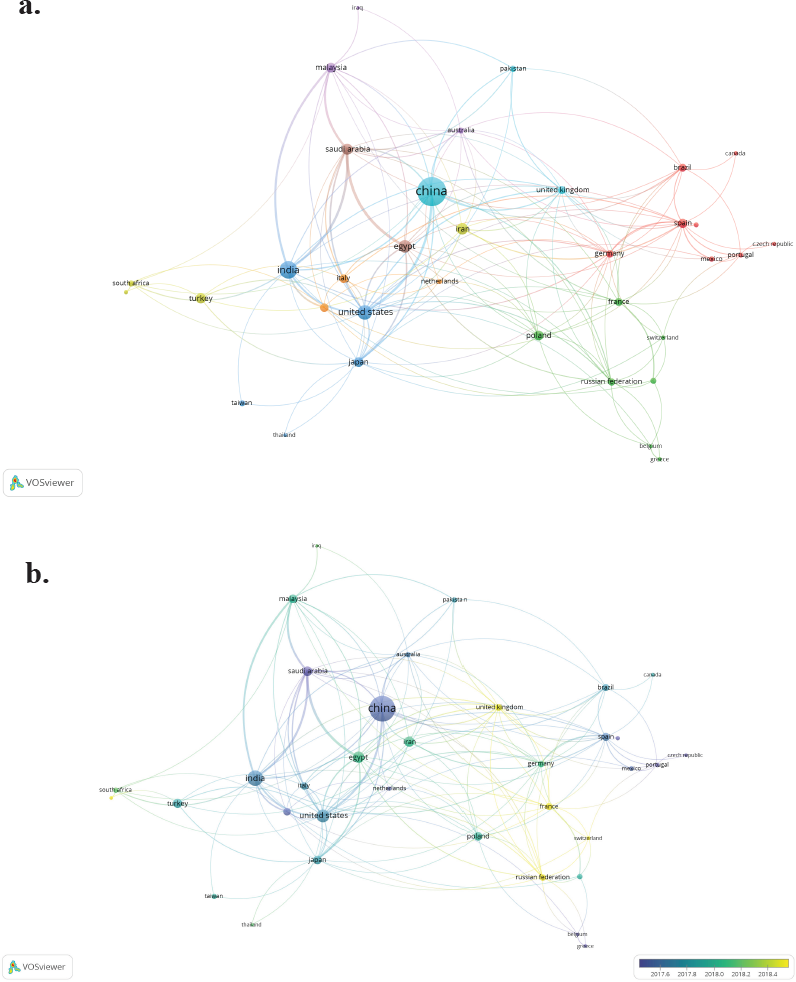 | Figure 4. Countries collaboration network studies related to 5-FU between 2015 and 2020. (a) Network visualization. b) Overlay visualization. Note: countries contributing with a minimum number of both documents and citations of five. [Click here to view] |
In the collaboration networks of countries researching on 5-FU—in the field of chemistry—and during 2015–2020, China ranked first, followed by India and the United States with 330, 121, and 80 records, respectively (Fig 4a). These countries also have the strongest collaboration network. Nevertheless, countries such as the United Kingdom, France, Switzerland, Germany, Poland, Egypt, and Malaysia concentrated the studies between 2017 and 2018 (Fig 4b).
DISCUSSION
Anticancer activity of hybrids and derivatives based on 5-FU
5-FU (Fig 5) is an antimetabolite of the pyrimidine analog type, which is widely used in the first-line treatment for solid tumors, including colorectal being more effective with regard to others, as reviewed by Ribeiro et al. (2016), gastrointestinal tract reviewed by Sara et al. (2018) and breast cancer (Tecza et al., 2018). Although 5-FU is effective, it produces undesirable gastrointestinal and neurological side effects, which in many cases leads to dose limitations or cessation of the anticancer therapy (Rejhová et al., 2018).
In the search to reduce these side effects of 5-FU, two optimization strategies have been proposed: (1) the incorporation of pharmacologically active compounds with anticancer activity (called hybrid molecule) and (2) the functionalization with other groups of compounds (called conjugates). For 5-FU modification and from a synthetic point of view, four important precursors of 5-FU (commonly used in the synthesis of different hybrids or conjugates) have been used: 5-FU acid, 5-FU alkyl bromide, 5-FU-alcohol, and 5-FU alkyne. However, 5-FU acid is the most used (Fig 5).
5-FU colchicine hybrids were obtained via 5-FU acid (Fig 5). These compounds exhibited good cytotoxic activity against four cancer lines cells (R = SMe: 9.5, 7.8, 10.2, and 7.5 μMon A2780, A-549, BEL-7402, and MCF7, respectively; R = N (C2H5)2: 10.2, 10.6, 10.9, and 9.8 μM on A2780, A-549, BEL-7402, and MCF7, respectively) (Shen et al., 2015). On the other hand, 5-FU-1,3,4-selenadiazole conjugate, which was synthetized from 5-FU acid (Fig 5), displayed good activity over kidney-type glutaminase (KGA) and A549 cell lines with IC50 values of 0.02 and 0.46 uM, respectively (Chen et al., 2019).
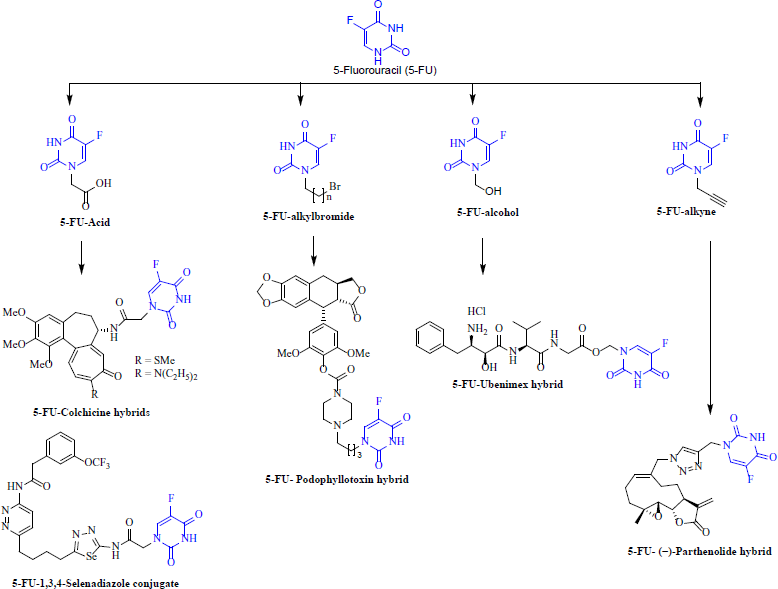 | Figure 5. Chemical structures of 5-FU precursors, 5-FU conjugates, and 5-FU hybrids. [Click here to view] |
5-FU alkyl bromide was used as a precursor to the 5-FU podophyllotoxin hybrid (Fig 5). This compound showed high activity with IC50 values of 0.04 μM against HL-60 and less than 0.01 μM against A-549 (Guan et al., 2016).
FU alcohol was used in the synthesis of 5-FU-Ubenimex hybrid (Fig 5). This hybrid displayed significant in vitro antiproliferation (IC50 = 16.52 uM on PLC/PRF/5), proapoptosis, antimetastasis, antiangiogenesis, and CD13+ cell elimination effects. Moreover, it exhibited further potent in vivo antimetastasis and lifespan extension effects when compared to the approved 5-FU prodrug capecitabine (Jiang et al., 2018).
5-FU-(−)-parthenolide hybrid was obtained by coupling between 5-FU alkyne and (−)-parthenolide azide derivative (Fig 5). This hybrid displayed IC50 values higher than 20 on the two cell lines tested (Bel7402 and Bel-7402/5-FU) (Ding et al., 2019).
Drug resistance of 5-FU-based compounds
Despite the good behavior of 5-FU hybrids and conjugates against diverse types of cancer, there are studies reporting drug resistance to these molecules. In the case of colorectal cancer and using a 5-FU-resistance cell from HTC-8 cells, a high expression of TCF4 and β-catenin indicated an upregulated Wnt pathway. Additionally, the suppression of checkpoint kinase 1 explained the resistance to 5-FU. Both findings led to conclude that the checkpoint kinase 1 pathway was suppressed by the Wnt pathway in 5-FU-resistant cells (He et al., 2018). Kim et al. (2018) investigated the role of the AKT pathway on drug resistance to 5-FU in SNU-C5/5-FU cells, which is also 5-FU-resistant human colon cancer cells. They reported that the overactivation of the AKT pathway was responsible for resistance to those drugs. Since the findings suggested that the inhibition of this pathway may overcome the resistance to 5-FU, the authors proposed it as a target for cancer treatment (Kim et al., 2018).
As reviewed by Marjaneh et al. (2019), miniRNAs have gained attention as strategies to treat and assess prognosis and reduce 5-FU resistance in colorectal cancer since those miniRNAs have an important role in sensitivity to anticancer drugs and even in tumor resistance induction. Thus, detecting, understanding, and targeting those mechanisms have been explored as a novel therapeutic approach to treat colorectal cancer (Marjaneh et al., 2019). MiniRNAs strategies have been also applied to other cancer types, for example, pancreatic cancer. Wang et al. (2016a) reported upregulated miR-320a in 5-FU-resistant pancreatic cancer cells and that its overexpression contributed to the pathogenesis of this cancer. The authors also found that the restoration of PDCD4 expression could in part reduce the function of miR-320a in pancreatic cancer cells, which at the end (by targeting PDCD4) could be an alternative therapeutic (Wang et al., 2016a). 5-FU has been combined with a natural compound such as andrographolide (a bioactive component of the medicinal plant Andrographis paniculata) as a potential adjunctive treatment for colorectal cancer. Andrographolide/5-FU cotreatment increased the apoptosis level of HCT116/5-FUR cells with a high level of BAX. The authors found that interaction between andrographolide and BAX prevented BAX degradation, which led to enhancing mitochondria-mediated apoptosis, leading to a reversed 5-FU resistance. On the contrary, BAX silence reduced the 5-FU effect (Wang et al., 2016b).
Delivery systems and molecular simulations of drugs based on 5-FU
In connection with drug delivery, some nanoparticles have been designed to improve 5-FU loading capacity. In this sense, hollow mesoporous silica nanoparticles have been designed and functionalized using silanes, which led to the insertion of the amine of carboxyl, cyano, and methyl groups onto the nanoparticles. Since amine groups have comparable hydrophilicity but reverse charge to the 5-FU, the presence of this group increased the loading capacity of 5-FU by 28.89% (She et al., 2015). Lollo et al. (2019) synthesized a lipophilic 5-FU derivative (by using lauric acid). The reaction product (named 5-FU-C12) was encapsulated into lipid nanocapsule and this led probably to increased activity of the 5-FU-C12. Higher cytotoxic effect of 5-FU-C12 (loaded into lipid nanocapsules) on different tumors cell lines [glioma (9L) and human colorectal (HTC-116) cancer cell line] was reported when it was compared with 5-FU-C12 alone or 5-FU (Lollo et al., 2019). Safwat et al. (2016) used gold nanoparticles (GNPs) and two thiol-containing ligands, thioglycolic acid (TGA) and glutathione (GSH), to improve the efficacy of 5-FU. In that study, a 5-FU/ligand molar ratio of 1:1 and 2:1 for TGA-GNPs and GSH-GNPs, respectively, led to maximum 5-FU loading (Safwat et al., 2016). 5-FU loading release was pH-dependent, and anticancer effect against colon cancer cells was better for 5-FU/GSH-GNPs when it is compared with free drug.
Molecular simulation approaches have been used to study 5-FU interactions with covalent organic frameworks (COFs) and metal–organic frameworks (MOFs), among others. Safdari et al. (2017) studied the interaction of 5-FU with graphene oxide nanosheet using density functional theory (DFT) and molecular dynamics (MD) simulation. MD simulation results showed that hydrogen bonds among different functional groups on graphene oxide and 5-FU molecules were promoted by increasing the temperature to 400 K (Safdari et al., 2017). Hashemzadeh and Raissi (2018) used DTF and MD to study the adsorption behavior of 5-FU on the COF surface. The authors reported that, after the adsorption on the COF surface, 5-Fu-COF is protected from degradation. Additionally, 5-FU showed high stability on the COF surface (Hashemzadeh and Raissi, 2018). Both experimental and MD studies on MOFs for 5-FU release have been also conducted and led to pH-triggered control (Wang et al., 2017). All those studies have been focused on the design of 5-FU delivery vehicles for cancers.
CONCLUSION
A clear increase in studies related to 5-FU was observed during 2015–2020, with (i) Biochemistry, Genetics, and Molecular Biology; (ii) Chemical Engineering; and (iii) Materials Science as the main areas (excluding chemistry) of this topic. The studies focused mainly on drug delivery, synthesis of anticancer compounds and their activity, molecular simulation, treatment of cancer cells, and drug resistance. China, India, and the United States were the most productive countries on studies related to 5-FU and they also had the strongest collaboration network.
ACKNOWLEDGMENT
Funding: The work has been funded by University of Antioquia, MINCIENCIAS, MINEDUCACIÓN, MINCIT and ICETEX, through the Program Ecosistema Científico Cod. FP44842-211-2018 Project numbers, 58537.
FUNDING
Funding information is not applicable. No funding was received.
AUTHORS’ CONTRIBUTION
Conceptualization: WCG and HRM; methodology: HRM; formal analysis and investigation: WCG and HRM; writing and original draft preparation: HRM; writing and review and editing: WCG and HRM.
REFERENCES
Aragón N, Jaramillo-Echeverry A, Ramirez-Malule H. Bibliometric analysis of bacterial resistance on periodontal disease. J Appl Pharm Sci, 2021; 11(04):118–24.
Banerjee A, Pathak S, Subramanium VD, G D, Murugesan R, Verma RS. Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives. Drug Discov Today, 2017; 22(8):1224–32. CrossRef
Cardona-G W, Herrera-R A, Castrillón-L W, Ramírez-Malule H. Chemistry and anticancer activity of hybrid molecules and derivatives based on 5-Fluorouracil. Curr Med Chem, 2021; 28:1. CrossRef
Chen Z, Li D, Xu N, Fang J, Yu Y, Hou W, Ruan H, Zhu P, Ma R, Lu S, Cao D, Wu R, Ni M, Zhang W, Su W, Ruan B . Novel 1,3,4-selenadiazole-containing kidney-type glutaminase inhibitors showed improved cellular uptake and antitumor activity. J Med Chem, 2019; 62(2):589–603. CrossRef
Ding Y, Li S, Ge W, Liu Z, Zhang X, Wang M, Chen T, Chen Y, Zhang Q. Design and synthesis of parthenolide and 5-Fluorouracil conjugates as potential anticancer agents against drug resistant hepatocellular carcinoma. Eur J Med Chem, 2019; 183:111706. CrossRef
Gómez-Ríos D, Ramírez-Malule H. Bibliometric analysis of recent research on multidrug and antibiotics resistance (2017–2018). J Appl Pharm, Sci. 2019; 9(5):112–16. CrossRef
Gómez-Ríos D, López-Agudelo VA, Ramírez-Malule H. Repurposing antivirals as potential treatments for SARS-CoV-2: from SARS to COVID-19. J Appl Pharm Sci, 2020; 10(5):1–9. CrossRef
Guan XW, Xu XH, Feng SL, Tang ZB, Chen SW, Hui L. Synthesis of hybrid 4-deoxypodophyllotoxin–5-fluorouracil compounds that inhibit cellular migration and induce cell cycle arrest. Bioorg Med Chem Lett, 2016; 26(6):1561–6. CrossRef
Hashemzadeh H, Raissi, H. Covalent organic framework as smart and high efficient carrier for anticancer drug delivery: a DFT calculations and molecular dynamics simulation study. J Phys D Appl Phys, 2018; 51(34):345401. CrossRef
He L, Zhu H, Zhou S, Wu T, Wu H, Yang H, Mao H, SekharKathera C, Janardhan A, Edick AM, Zhang A, Hu Z, Pan F, Guo Z. Wnt pathway is involved in 5-FU drug resistance of colorectal cancer cells. Exp Mol Med, 2018; 50(8):1–12. CrossRef
Jiang Y, Li X, Hou J, Huang Y, Wang X, Jia Y, Wang Q, Xu W, Zhang J, Zhang Y. Synthesis and biological characterization of ubenimex-fluorouracil conjugates for anti-cancer therapy. Eur J Med Chem, 2018; 143:334–47. CrossRef
Kim EJ, Kang GJ, Kang JI, Boo HJ, Hyun JW, Koh YS, Chang WY, Kim YR, Kwon JM, Maeng YH, Yoo ES, Lee CH, Kang HK. Over-activation of AKT signaling leading to 5-fluorouracil resistance in SNU-C5/5-FU cells. Oncotarget. 2018; 9(28):19911–28. CrossRef
Liao H, Tang M, Luo L, Li C, Chiclana F, Zeng XJ. A bibliometric analysis and visualization of medical big data research. Sustainability, 2018; 10(2):166. CrossRef
Lollo G, Matha K, Bocchiardo M, Bejaud J, Marigo I, Virgone-Carlotta A, Dehoux T, Rivière C, Rieu JP, Briançon S, Perrier T, Meyer O, Benoit JP. Drug delivery to tumours using a novel 5-FU derivative encapsulated into lipid nanocapsules. J Drug Target, 2019; 27(5–6):634–45. CrossRef
Marjaneh RM, Khazaei M, Ferns GA, Avan A, Aghaee-Bakhtiari SH. The role of microRNAs in 5-FU resistance of colorectal cancer: possible mechanisms. J Cell Physiol, 2019; 234(3):2306–16. CrossRef
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin, 2019; 69(5):363–85. CrossRef
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin, 2016; 66(4):271–89. CrossRef
Nevedomskaya E, Baumgart SJ, Haendler B. Recent advances in prostate cancer treatment and drug discovery. Int J Mol Sci, 2018; 19(5):1359. CrossRef
Ramirez-Malule H. Bibliometric analysis of global research on clavulanic acid. Antibiotics, 2018; 7(4):102. CrossRef
Ramírez-Malule H, López-Agudelo VA, Gómez-Ríos D. Candida auris: a bibliometric analysis of the first ten years of research (2009–2018). J Appl Pharm Sci, 2020; 10(3):12–21. CrossRef
Ramírez-Malule H, Quiñones-Murillo DH, Manotas-Duque D. Emerging contaminants as global environmental hazards. A bibliometric analysis. Emerg Contam, 2020; 6:179–93. CrossRef
Rejhová A, Opattová A, ?umová A, Slíva D, Vodi?ka P. Natural compounds and combination therapy in colorectal cancer treatment. Eur J Med Chem, 2018; 144:582–94. CrossRef
Ribeiro RA, Wanderley CW, Wong DV, Mota JM, Leite CA, Souza MH, Cunha FQ, Lima-Júnior RC. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: insights into pathogenesis and therapeutic perspectives. Cancer Chemother Pharmacol, 2016; 78(5):881–93. CrossRef
Safdari F, Raissi H, Shahabi M, Zaboli M. DFT calculations and molecular dynamics simulation study on the adsorption of 5-fluorouracil anticancer drug on graphene oxide nanosheet as a drug delivery vehicle. J Inorg Organomet Polym Mater, 2017; 27(3):805–17. CrossRef
Safwat MA, Soliman GM, Sayed D, Attia MA. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int J Pharm, 2016; 513(1–2):648–58. CrossRef
Sara JD, Kaur J, Khodadadi R, Rehman M, Lobo R, Chakrabarti S, Herrmann J, Lerman A, Grothey A. 5-Fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol, 2018: 10:175883591878014. CrossRef
She X, Chen L, Li C, He C, He L, Kong L. Functionalization of hollow mesoporous silica nanoparticles for improved 5-FU loading. J Nanomater, 2015; 2015:1–9. CrossRef
Shen L, Hu J, Wang H, Wang A, Lai Y, Kang Y. Synthesis and biological evaluation of novel uracil and 5-Fluorouracil-1-Yl acetic acid-colchicine conjugate. Chem Res Chinese Univ, 2015; 31(3):367–71. CrossRef
Soosaraei M, Khasseh AA, Fakhar M, Hezarjaribi HZ. A decade bbliometric analysis of global research on leishmaniasis in web of science database. Ann Med Surg, 2018; 26:30–7. CrossRef
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin, 2021; 71(3):201–49. CrossRef
Tecza K, Pamula-Pilat J, Lanuszewska J, Butkiewicz D, Grzybowska E. Pharmacogenetics of toxicity of 5-fluorouracil, doxorubicin and cyclophosphamide chemotherapy in breast cancer patients. Oncotarget, 2018;9(10):9114–9136. CrossRef
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA, 2019; 321(3):288–300. CrossRef
Wang FM, Wang J, Yang SZ, Gu CY, Wu XR, Liu JQ, Sakiyama H, Xu JW, Luo MM, Liu WC. A combination of experiment and molecular simulation studies on a new metal-organic framework showing PH-triggered drug release. J Coord Chem, 2017; 43(2):133–7. CrossRef
Wang W, Zhao L, Wei X, Wang L, Liu S, Yang Y, Wang F, Sun G, Zhang J, Ma Y, Zhao Y, Yu J. MicroRNA-320a promotes 5-FU resistance in human pancreatic cancer cells. Sci Rep, 2016a; 6(1):27641. CrossRef
Wang W, Guo W, Li L, Fu Z, Liu W, Gao J, Shu Y, Xu Q, Sun Y, Gu Y. Andrographolide reversed 5-FU resistance in human colorectal cancer by elevating BAX expression. Biochem Pharmacol, 2016b; 121:8–17. CrossRef
WHO. Cancer. WHO, Geneva, Switzerland. Available via https://www.who.int/news-room/fact-sheets/detail/cancer (Accessed 28 March 2021).