INTRODUCTION
Pharmacokinetics (PK) is a scientific domain that deals with the rates of movement of drug and/or its metabolite (s) in the body and forces acting on the process. The intensity of a drug effect in relation to its concentration in a body fluid, usually at the site of drug action is described by pharmacodynamics (PDs). In PKs, the concentration of a drug in plasma or any physiological fluid such as urine, saliva, milk, etc., is determined with respect to time following its administration. The concentration of drug versus time data is used to study the dynamics of the drug in the body with the help of mathematical models that are often derived based on certain set of assumptions. Mathematical models are a collection of mathematical quantities, operations, and relations together with their definitions. Pharmacometrics is an evolving branch of science involving various mathematical models for drugs and diseases. The drug models typically describe and quantify the PK-PD relationships for both desired and undesired effects, and individual patient characteristics while the disease models focus on the quantitative description of the time course of diseases (FDA, 2018). This scientific domain has evolved from the development of the discipline of Population PKs (PopPK) by Lewis Sheiner and Stuart Beal, which was applied into the field of pharmacy in the late 1970s (Bonate, 2005) to primarily address the setback of the lack of effective tools to analyze the sparsely collected samples during the late phase clinical trials and in clinical settings for the PK parameters and the effect of variability on them. The first version nonlinear mixed effects modeling (NONMEM) software was introduced based on first-order approximations to effectively carry out the analysis which later incorporated advance concepts, currently available in its seventh version. The US FDA came into the picture in 1989 when it called for PK screening for study of drugs likely to be used in the elderly. Later, guidance on the PopPK modeling was issued to the industries by the US FDA in the year 1999 revised recently in 2019. The field then witnessed its progression with the PK-PD relationships explained by the scientists which establish and analyze the effect-time courses resulting from a drug dose. The scope of these models extended beyond the small homogenous studies with the adoption of econometric and biometric methods as novel tools in clinical trials. The term Pharmacometrics was first coined in the literature in 1982 by the Journal of Pharmacokinetics and Biopharmaceutics. It is during the late 1990s and the early 2000s, the field of Pharmacometrics went through significant developments and established its values (Geleta et al., 2016). Today it stands as one of the essential aspects in the processes of decision-making in the regulatory approval, labeling of the drug products, advisory for successful trial designs, rendering optimal dosage regimens for patients, etc. This review is intended and presented in the context of the application of pharmacometrics in drug development and regulatory decisions, and focuses on its application in patient care to rationalize the drug therapy and therapeutic decisions.
Applications of Pharmacometrics in Drug Development and Regulatory Approvals
Model-Informed Drug Discovery & Development (MID3) refers to the application of a wide range of quantitative models in drug development that can be effectively used in the process of introducing new therapies to make able decisions across phases of the drug development and potentially eliminate the associated time and cost. They are mainly employed in designing clinical trials, optimizing the doses, and providing supportive evidence to the efficacy of the drug (Wang et al., 2019). Hence, the pharmacometric models can solely be utilized in all facets of the drug development process right from the identification of the right pathway and target; preclinical studies; deriving the safest dose to be used FIHs; to the meticulous analysis of the data from the Phase I/II trials to derive an optimal dose warranting safety and efficacy in Phase III pivotal trials allowing satisfactory preclinical to clinical transition. These models enable the scientists to make efficient and intelligent decisions while progressing from one phase of drug development to the other which contributes significantly to the reduction in the number of late phase failure (Phase 2, Phase 3) of the investigational agent, eliminating the financial loss. Also, the Non-Linear Mixed Effects Modeling (NLME) which is a model-based approach based on sound PK and statistical considerations possesses the advantages of the ability to analyze sparse, non-uniform samples; ability to study multiple factors in one large study, etc. Usually, these models have their quantitative framework built around Lewis B. Sheiner’s learning versus confirming paradigm in the drug development process that lays emphasis on the need for the focus on learning the fundamental science behind molecules such as exposure response (E-R) relationships, factors influencing the causal effect, and how and when to measure these factors to adjust the dose for optimal efficacy; rather than the traditional approach which primarily focuses on establishing a confirmatory trial with statistical significance (Lyauk et al., 2019). Apart from the conventional PK-PD static models that are time independent, non-steady state models that have a more mechanistic and physiology-based approach models happen to be the most reliable models that are widely utilized for this purpose (Wright et al., 2011).
A notable example for the application that enhanced decision-making in the process of drug development is the recently developed mechanism-based, multi-scale PK-PD model for the novel chimeric antigen receptor (CAR)-T cells, which is evolving to be the treatment option for leukemia and other cancers (Figure 1). The investigators adopted a rigorous stepwise approach towards a) Facilitating the design and development of lead CAR-constructs, b) triaging lead CAR-T candidates in preclinical settings, and c) enabling effective preclinical-to-clinical translation (Singh et al., 2020).
These models which were developed for identification and mathematical characterization of the physiological processes associated with the multiphasic CAR-T cell profile, were used in the Development of translational cellular kinetics (CK) and cellular dynamics (CD) relationship which incorporated the system specific and drug specific parameters to describe the a) in vitro functional activities, b) biodistribution and tumor growth inhibition, c) clinical PK and other disease biomarkers.
The developed CK-CD relationship was leveraged to answer critical questions associated with CAR-T cell development such as a) Selection of the Lead Candidate, b) Dose E-R relationship, c) prediction of the First in Human (FIH) threshold dose, and d) Identification of covariates.
Identification of the impact of affinity (preclinical), dose (clinical), patient tumor burden (clinical) on the clinical PK-PD of the CAR-T cells.
Simulated/virtual clinical trials (VCT)
Pharmacometrics has also been utilized for clinical trial simulations to give critical understanding of the factors influencing expected outcome of the study and rationally design the clinical trials. They also provide a prospective of VCT without the involvement of any human subjects which is a rear possibility.
An excellent example for this application is the virtual Tamoxifen Response by CYP2D6 Genotype-based Treatment-1 (TARGET-1) study that simulated the trial using PBPK approach (Figure 2). Though, the trial was carried out in real settings, this simulation was done to predict the outcomes of the study prior to its completion and the likelihood of achieving an optimal efficacy of the proposed dosing versus the traditional standard dosing and the factors influencing this were determined.
This study aimed to predict the outcomes of the TARGET-1 trial (i.e., demonstrating the superior efficacy of 40 mg/day tamoxifen dose—arm A over 20 mg/day tamoxifen dose—arm B in CYP2D6 variant groups) and utilized a physiology-based PK models which used the generated parameters that were optimized with Cluster Newton Method. The outcome of this virtual study spotted partially unfavorable results attributed to the effect of sample size and the fact that individuals of the same CYP2D6 genotype observed significant variability in Endoxifen levels. These findings wait for comparison of the findings of the real study and it is believed that these predictions are commendable in designing a successful clinical trial (Nakamura et al., 2018).
Regulatory perspectives and guidelines
Due to the vast applications of these models during various phases of the drug development as mentioned above, the major regulatory agencies have also started to adopt more scientifically justifiable approaches in which modeling and simulation play a vital role. Some major regulatory agencies such as The US Food and Drug Administration (USFDA) and European Medicines Agency (EMA) started recommending and suggesting explicit guidelines to the industries on MID3 (EMA, 2016; US FDA, 2016, 2014). These models find place in the regulatory-decision making in the areas such as designing a meritorious study, extrapolation of data from adults to children, optimal dose recommendations, assessment of risks of adverse drug reactions (ADRs) such as QT interval interval prolongation, drug–drug interactions (DDI), and medicinal product lifecycle management. A study that reviewed 60 US FDA approved drugs between February 2015 and 2017, for their dose-finding during clinical development using the recommendations of learning versus confirming approach identified that the model-based approach was mentioned in only 2 out of the 60 approval packages (Lyauk et al., 2019). Currently, majority of the regulatory submission of these models is limited to the assessment of DDIs and pediatric modeling by extrapolating the data from adult population. However, the USFDA and EMA are incorporating these models into other aspects of drug development as well. USFDA’s guidance for industries states that E-R information is at the heart of any determination of the safety and effectiveness of drugs (FDA, 2020). There is also an increase in the interest of USFDA in dose–exposure–response characterization which is evident from the launch of a public comment docket for the enhanced incorporation of E-R in drug development and decision-making. Questions raised by the USFDA included the following: “What attributes of an exposure–response analysis are critical to effectively inform a drug development or regulatory decision?”, “What are the main obstacles preventing widespread acceptance of exposure–response analyses?” (Federal Register, 2018). USFDA and EMA have also developed guidance for the PBPK approach (EMA, 2016; US FDA, 2016) and USFDA identifies the application of PBPK models to: 1) integrate the information from multiple studies; 2) determine whether a clinical study is appropriate; and 3) inform the design of clinical studies (FDA, 2018). The EMA hosted a dose-finding workshop in 2014 which recognized the setback of rarely scientifically sound dose selection in drug development and emphasized the need for appropriate design of dose-ranging trials including 4 doses in minimum over at least 10 fold dose range where MID3 can play a potential role (EMA, 2016).
The recent approval of the Nivolumab once every 4 weeks across multiple tumour types supported by the model-based evaluation of its safety and efficacy is a great example for the regulatory application of MID3 (Figure 3).
Nivolumab 3 mg/kg Every 2 weeks was previously studied in registered clinical trials across multiple tumor types and approved.The study aimed to relate the safety and efficacy of with Nivolumab 480 mg Q4W using model-based evaluation. Results revealed the similarity between the 480 mg Q4W and 3 mg/kg Q2W Nivolumab in the safety and efficacy. Thus, this regimen was approved by the major regulatory agencies and is marketed across US, European Union and the other markers providing an extended frequency regimen using robust quantitative models which is more convenient to be used in the healthcare settings without the compromise of the established efficacy and safety (Zhao et al., 2020).
Prediction of pediatric dose based on adult studies
An example of its application in dose-exposure matching from adults to children is the recent pharmacometric analysis-based regulatory approval of weight-based dosing of Eslicarbazepine (ESL) acetate therapy in pediatric patients with partial-onset seizures without clinical data which was supported by modeling and simulation. This model ensured confidence of the efficacy of the proposed dose extrapolated adult dose and led to the FDA approval of ESL in subjects aged 4–17 years without any clinical studies (Sunkaraneni et al., 2018).
Another similar example is the model-based approval of Levofloxacin as a treatment for children following inhalational exposure to anthrax which had no clinical trials for the chosen dose (Li et al., 2010). Other notable examples include the approval of pharmacometric derived doses of Boceprevir and Topiramate. There have also been instances where the FDA personnel have selected the appropriate dosing regimens based on E-R analysis.
Drug interaction studies
Pharmacometrics can also be applied to inform the regulatory agencies about the DDI labels. Exposures can be simulated in conditions with scarce clinical data or infeasibility in special populations using appropriate models and DDIs can be predicted. A recent example for this application is the development, verification, and prediction of DDIs of the anti-cancer drug Osimertinib using PBPK modeling approach to inform drug label. It was identified from in vitro data that metabolism of Osimertinib was majorly by Cytochrome P450 3A (CYP3A) and its inducers and inhibitors possess the potential of interacting with Osimertinib. This study aimed to predict this DDI and suggest the necessary dosage adjustments (Pilla Reddy et al., 2018).
Bioavailability/bioequivalence (BA/BE) studies
Apart from the application of pharmacometrics in the new drug development process, they also can play a crucial role in the BA/BE studies of the generic drugs. This is evident from the USFDA’s public workshop titled “Leveraging Quantitative Methods and Modeling to Modernize Generic Drug Development and Review” (October 2–3, 2017). This workshop highlighted that modeling and simulation is an innovative tool for the effective implementation of the “Drug Completion Action Plan” and aid in improving regulatory decisions. Intentions of the FDA to increase investment in advancing the development of the state-of-the-art modeling and simulation technologies and applying them to generic drug development and review was expressed as these approaches improve the first-cycle generic drug approval rates, reduce cost and time of product development, and expedite approval of safe and effective generic products (FY, 2018).
Development of product specific guidelines (PSGs)
Pharmacometrics can also be utilized by the regulatory authorities to develop PSGs to enhance the process of drug development. An example for this approach is the Quantitative analysis of the PK-PD relationship of abuse-deterrent opioid products. This internal project of USFDA, aimed to aid the development of guidance on the “General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products” and PSGs by informing the regulators about the evaluation of abuse-deterrent properties. The E-R relationships between the various PK metrics and their abuse potential of 3 drugs were evaluated using the available clinical results of the new drug applications. The results recommended that the rational PSGs should contain early partial area under the curve (AUCs) (pAUCs) in addition to the traditional PK parameters to establish BE with reference products as an association between the pAUCs (e.g., pAUC 0–3 hours) and maximum Drug Liking Visual Analogue Scale was established using the E-R modeling (FY, 2018).
Pharmacometrics and regulatory decisions—future scope
A recent survey which was developed in collaboration with the European Federation of Pharmaceutical Industries and Associations MID3 workgroup conducted between May and September 2017 provided an optimistic view of the future of pharmacometrics in the industries and regulatory agencies. This survey was targeted towards the pharmacometricians and clinical pharmacology colleagues across industry, USFDA and EMA. It was found from the consolidated evidence obtained from the target audience that though there was only a modest increase in the degree of impact of MID3 approaches on decision-making in the past 5 years, MID3 is currently being viewed as a growing methodology that is starting to fulfill its promise with respect to advancing research efficiency in the years to come and a substantial increase in its applications and its impact on decision-making is expected in the next 5 years (Marshall et al., 2019).
Other examples for the application of MID3 approach drug development
NONMEM model to estimate the viral kinetic parameters
Though the data was sparse, precise estimates for the fixed effects and the effect of inter-individual variance were provided by the NLME approach. It was identified that this approach provided a high difference in the outcome between the two competing protease inhibitor therapies used in hepatitis C and yielded a high study power that outperformed the empirical approach of obtaining a single time-point change in viral load (VL) and comparison of the mean VL decline using Wilcoxon test. Thus, this approach can be a paradigm for designing pilot studies or small investigator-led trials, provided that the disease/condition has enough data to build a prior PopPK-PD model (Laouénan et al., 2013).
Citrulline-based translational population quantitative system toxicology (QST) model of gastrointestinal (GI) adverse effects
Established a sound basis for the prediction and alleviation of the GI adverse effects that are often dose limiting in the anticancer therapy at the preclinical stage, thus rendering an optimal dosing schedule to be used in humans (Yoneyama et al., 2019).
PK model for the characterization and comparison of the quorum sensitization inhibitor (QSI) compound series (SEN001, SEN019, and SEN032)
Depicted the limited impact of systemic clearance on local drug exposure in the lung after pulmonary delivery, thus elucidating the potential of the pulmonary drug delivery design of these novel QSIs typically with a long residence time in the lung for local efficacy in Pseudomonas aeruginosa lung infection with rapid clearance after systemic absorption for reduced risk of systemic adverse events as targeted treatment for respiratory conditions (Sou et al., 2019).
Translational PK-PD and clinical clamp PK-PD models
Used in various combinations, viz. MK-2640 and regular human insulin; quantitative systems pharmacology (QSP) simulation; diabetes comparator models based on model-based meta-analysis of outcome data from randomized clinical trials in T1DM and T2DM subjects. These models deciphered key explanations in the early drug development phase of MK-2640 insulin and hastened the decision-making process by enabling the understanding of optimal PK-PD properties for therapeutically meaningful glucose-responsive insulin (Visser et al., 2019).
Bottom-up physiology-based PK model
Integrating the processes of esterase mediated conversion of the prodrug Valganciclovir to Ganciclovir, permeability limited distribution, active transport of the drug and taking into account various age-related covariates effectively simulated the measured plasma exposures of the drug in adults, pediatric, infants, and neonatal patients. Therefore, the model can be utilized in challenging drug development conditions where obtaining clinical data is less feasible (Lukacova et al., 2016).
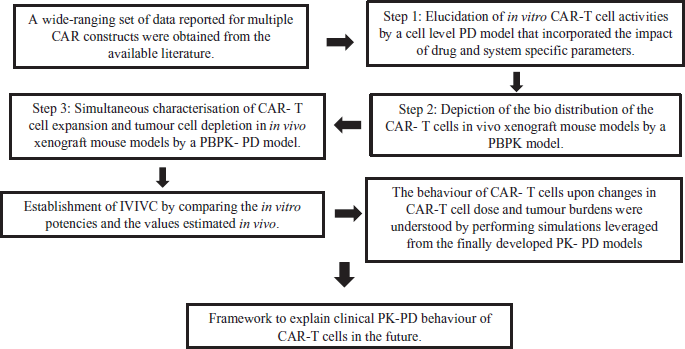 | Figure 1. Overview of the multi scale PK- PDs models for the novel CAR-T cells. (PD—Pharmacodynamics, PBPK—Physiology based pharmacokinetics, IVIVC—In-vitro in- vivo correlation). [Click here to view] |
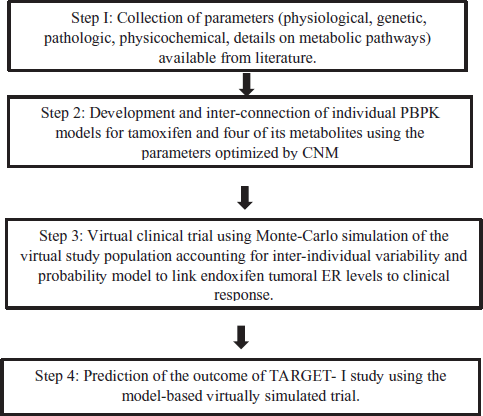 | Figure 2. Overview of the simulated TARGET-1 trial using PBPK approach (PBPK—Physiology-based pharmacokinetic model, CNM—Cluster Newton Method, TARGET-1—Tamoxifen response by CYP2D6 genotype-based treatment-1, ER—Estrogen receptor). [Click here to view] |
Pharmacometrics and pharmacoeconomics (PE)
In the recent years, pharmacometric models have also been applied in PE studies to design PK-PD-PE models as they study the relationship between the costs, adherence, and response to the therapy and the clinical outcomes compared to the PE models that insist only on cost efficacy rendering an ideal framework to assess the influence of drug pharmacology on long-term clinical and economic outcomes, such as cost-effectiveness and value-based pricing (Hill-McManus et al., 2019).
The examples include:
- The application of a linked pharmacometric/PE model to assess the impact of non-adherence and flare resolution on the cost-effectiveness of treatments for gout (Hill-McManus et al., 2018).
- PK/PD/PE analysis of rituximab for follicular lymphoma (Pink et al., 2012).
- Translating pharmacometrics to a PE model of chronic obstructive pulmonary disease (Slejko et al., 2016).
Applications in Clinical Practice
The present medical era compels the need for innovative approaches to optimize the treatment for patients due to the recognition of highly variable health care practices, increasing suboptimal therapies and ADRs associated with the treatment regimens especially in special populations and complex patient characteristics. Undoubtedly, pharmacometrics is one of the tools which can be judicially applied at the bedside to optimally balance risks and benefits and strategize a good treatment option. At present, there is also increasing awareness of personalized medicine opposed to the “one size fits all” approach which reinforces the use of pharmacometrics in clinical settings. Despite the aforesaid benefits, this field is relatively underutilized at the bedside compared to the industrial settings. The factors contributing to this gap could be the lack of robust individual patient specific data, complexity of the user-interface software that are often inconvenient and time-consuming to be learnt and applied by the clinicians, lack of awareness, education, funding, etc. To address these unmet needs and bridge the gap, the International Society of Pharmacometrics and the American College of Clinical Pharmacology jointly created the Clinical Pharmacometrics Special Interest Group (SIG) in the year 2017 with the mission of promoting the use of pharmacometrics at direct patient care and foster an international SIG of scientists and clinicians across a spectrum of therapeutic specialties. The goals of SIG included the enhanced communication between the clinicians with pharmacometric interest and pharmacometricians with clinical interest by the creation and maintenance of a forum and the expand the applications and uses pharmacometric tools to enhance the field of personalized medicine (ACCP, 2017). Model Informed Precision Dosing (MIPD) is an approach inclusive of the pharmacometric models as well as other modeling techniques such as regression models, decision trees, and other algorithms that consider patient specific characters and other available evidences to tailor and optimize therapy to patients. MIPD and pharmacometrics can be integrated into clinical care and can be used at various clinical scenarios such as optimal dosing, oncology, therapy in special populations, management of ADRs and DDIs, managing polypharmacy and associated costs, etc, given that the model is wisely selected according to the clinical setting. These applications have already begun, and a few illustrative examples are described below.
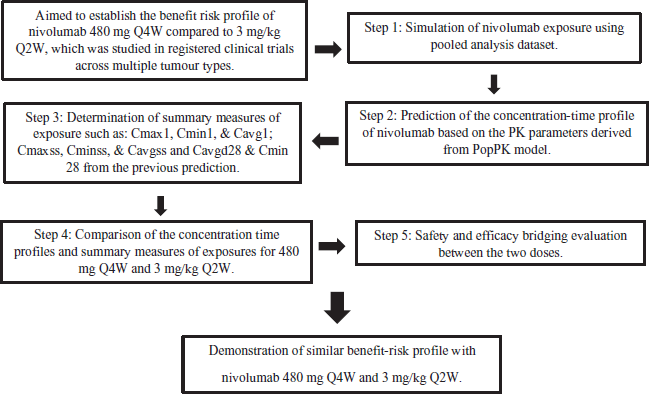 | Figure 3. Overview of the regulatory approval of Nivolumab dosage regimen supported by MID3. (Peak concentration after first dose—Cmax1, trough concentration after first dose- Cmin1, time-averaged concentration after first dose—Cavg1. Peak concentration after steady state- Cssmax, trough concentration after steady state—Cminss, time averaged concentration after steady state—Cavgss, Time averaged concentration over the first 28 days—Cavgd28, trough concentration over the first 28 days—Cmind28). [Click here to view] |
Dosage optimization
Conventional dosing methods utilize the dosing data from the phase 2 and 3 of clinical trials which are mere averages in a controlled population. It is imperative to bear in mind that a patient may not always fit into the average which makes it pivotal to personalize the dose appropriate to the individual factors like age, body weight, ethnicity, co-morbid conditions, co-administered drugs, etc. Although other approaches such as the priori drug dosing that selects the dosage regimen based on the fitting of PopPK model estimates to a patient in the absence of the plasma concentration data and the traditional posteriori drug dosing that selects the dosage regimen based on the patient’s PK parameters derived from the measured plasma concentration provide relatively better outcomes compared to the former dosing method, integration of both priori and posteriori methods to individualize patient’s drug therapy is a superlative approach.
Bayesian approach
The parameters in Bayesian Dosing will incorporate a well-developed PopPK model as the Bayesian prior, with the measured plasma concentration data of an individual patient to calculate a Bayesian posterior parameter value accounting for the variabilities in both the population parameters and measured plasma concentrations (Fig. 4). Thus, the population models are incorporated into the estimation procedure to deduce the PK parameters which in turn aids in designing an ideal dosage regimen for an individual patient.
Some examples of the dosing of drugs based on the Bayesian approach are as follows
1. Revised consensus guidelines (2020) for therapeutic monitoring of Vancomycin and dosing: AUC has been identified as the parameter to guide Vancomycin dosing and the established relationship between Vancomycin dosing to AUC and the minimum inhibitory concentration (MIC) or AUC: MIC is viewed as a useful PD parameter to predict effectiveness. The expert guidelines recommend the daily maintenance of AUCs between 400 and 600 mg* hour/l for optimal efficacy with minimal probabilities of nephrotoxicity. AUC estimation may require calculation by the trapezoid rule using precisely obtained multiple time versus plasma concentration data which can be very cumbersome and impractical in clinical settings. Therefore, Bayesian software programs to calculate Bayesian posterior parameter value distribution for an individual patient using a robust PopPK model and measured concentrations which is then utilized to calculate the optimal dosing regimen for a specific patient is the preferred method based on vancomycin dosing guidelines. DoseMeRx®, PrecisePK®, etc., are examples of such Bayesian dosing softwares. Hospitals and health systems across the United States, Australia, Europe, Africa, and South America use the DoseMeRx® software to guide vancomycin dosing is developed in accordance with FDA regulations and comply with local guidelines in each jurisdiction. The advantages of these Bayesian softwares include:
 | Figure 4. Bayesian approach for dosage optimization. Framework to explain clinical PK-PD behavior of CART cells in the future. [Click here to view] |
- Accounting for the pathophysiological changes in critically ill patients by the integration of covariates, such as creatinine clearance into the population models rendering an optimal dose in both pediatric and adult populations.
- The clinician need not wait for the steady state plasma concentrations (Css) which requires 3 to 4 doses. Rather, vancomycin concentrations measured with 24 to 48 hours itself can be used to design the dosage regimen.
- They can be used to rapidly attain the target concentrations/Css by deriving loading doses followed by low maintenance doses (Rybak et al., 2020).
2. ADVATE® Prophylaxis for Hemophilia A: Hemophilia A is a coagulation disorder with congenital deficiency of factor VIII (FVIII) which requires routine rational prophylactic therapy personalized to a patient with FVIII to prevent bleeding and preserve normal musculoskeletal function. Half-life (t1/2) of the recombinant antihemophilic factor such as ADVATE® is highly variable among the patients making them an essential variable in the PK -guided regimens for successful prophylaxis. Nevertheless, the feasibility of frequent sampling at minimum five time points for the children is questionable as it may be inconvenient for working parents and cause abstinence from schools. Thus, Bayesian models integrating population PK model of FVIII activity and patient’s measured plasma concentration time data to predict PK parameters such as t1/2, volume of distribution (Vd), clearance (CL), etc., to tailor the doses are the most promising approach for personalized dosing with flexibilities in sampling. For this purpose, FDA has approved the PK dosing software myPKFiT® for ADVATE in the USA for patients aged 16 and above with Hemophilia A. This software estimates the PK curve with just two samples which aid the prediction of the elimination rates of FVIII in an individual to design a personalized dosage regimen. This can also enhance patient education to improve patient’s adherence to their treatment (Abrantes, 2019; Álvarez-Román et al., 2017).
A pharmacometric model-based E-R characterization in pulmonary tuberculosis (PTB) patients treated with Rifampicin
Available data from PTB patients previously treated with different doses of Rifampicin was used to derive individual exposure metrics along with the time to stable sputum culture conversion as response measure was used to perform E-R analysis. The results indicated high likelihood of early serum conversions with higher Rifampicin exposure during the first 3 months showing the potential for treatment shortening with higher rifampicin results (Svensson et al., 2018).
Applications in patients with complex characteristics
Challenging patient characteristics and disease conditions often require the best use of personalized medicine. Patients with recent organ transplantations and immunosuppressive therapy often require regular therapeutic drug monitoring to maintain the drug levels within target ranges due to the absence of discrete clinical endpoints which can be a challenging situation in the clinic where pharmacometrics can be employed. An example for this is the prospective randomized study comparing the achievement of tacrolimus target concentrations in renal transplant patients using pharmacometrics-guided dosing in the intervention group and the conventional dosing in the control group. The intervention group used Bayesian dosing of Best Dose software which produced significantly higher proportions of concentrations per patient within the target range compared to the conventional dosing (Størset et al., 2015).
Pregnancy is another complex situation associated with multiple physiological changes that predominantly affect the PK of drugs. Pharmacometric models can be applied in these settings to predict the associated changes in drug disposition and alter the regimen. An illustrative example of this application is the dose optimization of the second-generation antipsychotic Quetiapine in pregnancy. The study applied PK modeling principles and PBPK models to provide insights in Quetiapine in pregnancy that was previously established and derive new dosing strategies to counteract the challenges. The target therapeutic range was set to 50–500 ng/ml throughout the gestational period and deviations of the trough concentrations from the target was observed in all the trimesters in virtual populations of pregnant and non-pregnant women attributed to various mechanisms such as increased Vd increased metabolic clearance and a relative decrease in protein binding. A drastic decline of about 58% in the trough concentration was observed in the second trimester and an increase in the dosing of quetiapine to 500–700 mg twice daily to counteract the sub optimal plasma concentrations was proposed to be a rational approach (Badhan and Macfarlane, 2020). Pharmacometrics have also been applied to optimize pharmacotherapy in the neonatal age group where obtaining adequate samples can be extremely challenging. With the substantial increase in the neonatal opioid withdrawal syndrome (NOWS), pharmacometrics is being employed to determine optimal dosage of the opioids in neonates. One such study estimated the initial dose and up titration of buprenorphine in NOWS to achieve optimum symptom control and higher weaning rates by linking the drug exposure to NOWS symptom scores using a PD model. Simulations revealed that the starting dose did not significantly impact the stabilization time in contrast to the time to wean and time to cessation that were dose dependent. Increase in the up-titration rate by 30% and increasing the starting dose had benefits in time to stabilization and weaning. This model-based approach thus paves way for focused care guidelines which can be used with goals of reducing treatment time and hospital stays in infants with NOWS (Eudy-Byrne et al., 2021). In addition, pharmacometrics can also be used to determine the fetal exposures by linking it to the maternal dosing which is otherwise often illogical and unethical to be determined. A mother-fetus PBPK model has been developed and validated for sensitivity for the purpose mentioned above which can be readily used to predict fetal risk of drug toxicity by estimating the fetal exposure to drugs across gestational ages (De Sousa Mendes et al., 2017; Zhang et al., 2017).
Applications in oncology
Applications of Clinical pharmacometrics in oncology have enormous benefits in cancer precision medicine to aid the clinicians in the identification of predictive biomarkers to hasten clinical interventions, optimal dose selection of cancer chemotherapies, individualizing them in special populations, enabling higher therapeutic success rates and reduction in the rising cancer-care costs.
Identification of disease progression and response to drug therapy using predictive biomarkers
A PK-PD population model was developed to classify response based on the model-based prediction of biomarkers in small cell lung cancer (SCLC) incorporating the disease variables which provide insights into biomarker productions which is presumed to be driven by the tumor size dynamics and exposure to treatment to predict and upgraded to originate accurate patient-specific outcomes by the integration of CT scan data enabling early therapeutic interventions by precise predictions of disease progression. This model successfully prospectively predicted the outcomes in 75% of the follow-up visits when applied to an external dataset of twenty-two treatment-naïve SCLC patients who were administered with the first-line chemotherapy (etoposide plus either carboplatin or cisplatin) whose biomarker samples for serum lactate dehydrogenase and neuron-specific enolase (NSE) were obtained only till the end of treatment. Thus, such models can aid the overall total survival by personalizing therapeutic/monitoring interventions (Buil-Bruna et al., 2015). In another study, pharmacometric model was developed using NONMEM to characterize the unidimensional diameters, 3D measurements (to overcome the inability in the detection of treatment response for tumors with non-uniform size changes by conventional unidimensional size), and densities of tumors in imatinib-treated patients with gastrointestinal stromal tumors (GIST) depicted early clinical judgements and a better overall survival (Schindler et al., 2017). Pharmacometrics is also utilized to explicate chemotherapy-induced toxicities. One such example is the study that used PBPK model to elucidate vincristine-induced peripheral neuropathy following treatment with novel kinase inhibitors revealed the negligible DDI risk between acalabrutinib and P glycoprotein substrates with minor increase in the risk of vincristine-induced peripheral neuropathy when acalabrutinib is added to the therapy (Pilla Reddy et al., 2021).
Pharmacometric model-guided dosing:
A study simultaneously developed models for biomarkers and toxicity response on a novel hypomethylating agent Guadecitabine, by analyzing the E-R measures of interspersed nucleotide element-1 (LINE-1) methylation and absolute neutrophil counts (ANC) from the phase 1 and 2 data to aid the development of an indirect response model to characterize the dynamics of LINE-1 (global DNA methylation metric) and a PK/PD model to link dosing rate to the decline in ANC. This approach observed that the demethylation effect was maximal with minimal cytotoxicity in a 5-day regimen of 60 mg/m2 every 28 days. Thus, this approach could potentially optimize the dosage regimen of the anti-cancer drug (Xu et al., 2017). A community-based multicentre trial of pharmacokinetically guided 5-fluorouracil dosing for personalized colorectal cancer therapy in comparison to the dosing using patient’s body surface area body surface fluorouracil (5-FU) depicted an early achievement of the target AUC (within 4 weeks) in the PK guided dosing translating to the higher response rates and better tolerability. Thus, it is prudent to establish the E-R relationships to develop effective dosing regimens in anti-cancer therapy which is supported by the significant reduction in the number of subtherapeutic concentrations and undesirable gastrointestinal toxicity associated with 5-FU in the personalized colorectal cancer therapy guided by sound PK (Patel et al., 2014).
Dosing in special population
An example for the dosing in special population is a multi-center prospective study which utilized pharmacometric models in children undergoing hematopoietic cell transplantation and receiving fludarabine which suggested reduced morbidity and mortality through improved disease-free survival rates and limited drug-related toxicity achieved by the model-based personalized dosing in infants and young children (Ivaturi et al., 2017).
Applications to manage overdose and ADRs
QSP and QST models with PBPK models can be used for mechanistic insights to identify and manage drug toxicities and overdoses at the bedside. A QSP model which integrated biologics PBPK was used to provide basis for the management of immunogenicity of therapeutic proteins which is a major challenge during patient care (Kierzek et al., 2019). Acetaminophen (APAP) is the most commonly used over the counter analgesic drug but in excess doses it may lead to hepatotoxicity that remains a global issue accounting for more than 50% of overdose-related acute liver failure and approximately 20% of the liver transplant cases in the US (Yoon et al., 2016). Early identification of the liver toxicities and the need for antidote can be assisted by the pharmacometric models. In a study, PK/PD model of APAP was developed to correlate the APAP doses to the biomarker panel which gave mechanistic insights that enhanced the prediction of the hepatotoxicity (Mason et al., 2018). Overdose of APAP is frequently managed using the Rumack-Matthew nomogram to predict the need and doses of N-acetylcysteine (NAC) which often fails to be accurate. Pharmacometrics can be utilized to precisely predict the need and the doses of antidote. In a study PopPK models for APAP overdoses and subsequent Bayesian dosing strategy were developed for APAP overdoses which were used for making early and precise decisions for NAC administration (Desrochers et al., 2017).
CONCLUSION
This narrative review revealed that there is a significant value and a sturdy increase in the application of these pragmatic pharmacometric models into the industries, research as well as the clinics with a basic multidisciplinary knowledge on the vital aspects of modeling such as clinical pharmacology, computational sciences, statistics, regulatory science, etc. Though, currently this field has the support of only a handful number of experts, there is confidence that this domain will gain more popularity among the field of health science and research in the near future.
AUTHOR CONTRIBUTIONS
Bhavatharini P A has done the literature review and manuscript drafting; Arun K P conceptualized the title, supervised the work, corrected & corresponded the manuscript.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Not applicable.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abrantes JA. Pharmacometric approaches to improve dose individualization methods in Hemophilia A. 2019. Available via http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-381218 (Accessed 16 July 2020).
Álvarez-Román MT, Fernandez-Bello I, de la Corte-Rodríguez H, Hernández-Moreno AL, Martín-Salces M, Butta-Coll N, Rivas-Pollmar MI, Rivas-Muñoz S, Jiménez-Yuste V. Experience of tailoring prophylaxis using factor VIII pharmacokinetic parameters estimated with myPKFiT® in patients with severe haemophilia A without inhibitors. Haemophilia, 2017; 23(1):50–4. CrossRef
Badhan RKS, Macfarlane H. Quetiapine dose optimisation during gestation: a pharmacokinetic modelling study. J Pharm Pharmacol, 2020; 72(5):670–81. CrossRef
Bonate PL. Recommended reading in population pharmacokinetic pharmacodynamics. AAPS J, 2005; 7(2):363–73. CrossRef
Buil-Bruna N, Sahota T, López-Picazo J-M, Moreno-Jiménez M, Martín-Algarra S, Ribba B. Early prediction of disease progression in small cell lung cancer: toward model-based personalized medicine in oncology. Cancer Res, 2015; 75(12):2416–25. CrossRef
Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Food and Drug Administration. Guidance for industry: general clinical pharmacology considerations for pediatric studies for drugs and biological products. 2014. Available via https://www.fda.gov/downloads/drugs/guidances/ucm425885.pdf (Accessed 14 July 2020).
De Sousa Mendes M, Lui G, Zheng Y, Pressiat C, Hirt D, Valade E. A physiologically-based pharmacokinetic model to predict human fetal. Exposure for a drug metabolized by several CYP450 pathways. Clin Pharmacokinet, 2017; 56(5):537–50. CrossRef
Desrochers J, Wojciechowski J, Klein-Schwartz W, Gobburu JVS, Gopalakrishnan M. Bayesian forecasting tool to predict the need for antidote in acute acetaminophen overdose. Pharmacotherapy, 2017; 37(8):916–26. CrossRef
EMA. Guideline on the qualification and reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation. Available via http://www.ema.europa.eu/docs/en_GB/docum ent_libra ry/Scientific guide line/2016/07/WC500 21131 5.pdf (Accessed July 11 2020).
Eudy-Byrne R, Zane N, Adeniyi-Jones SC, Gastonguay MR, Ruiz-Garcia A, Kushal G. Pharmacometric dose optimization of buprenorphine in neonatal opioid withdrawal syndrome. Clin Transl Sci, 2021; Article ID:34080312. CrossRef
Federal Register. Exposure-response analysis in drug development and regulatory decision making; establishment of a public docket; request for comments. 2018. Available via https://www.federalregister.gov/documents/2018/04/06/2018-07028/exposure-response-analysis-in-drug-development-and-regulatory-decision-making-establishment-of-a public docket (Accessed 11 July 2020).
FY2018 Regulatory Science Report: Quantitative Clinical Pharmacology. Available via https://www.fda.gov/drugs/generic-drugs/office-generic-drugs-fy-2018-gdufa-science-and-research-report
Geleta B, Makonnen E, Mekonnen H. Pharmacometrics and systems pharmacology: a review on its evolution, development and applications. Clin Pharmacol Biopharm, 2016; 5(1):151–64.
Hill-McManus D, Marshall S, Soto E, Hughes DA. Integration of pharmacometrics and pharmacoeconomics to quantify the value of improved forgiveness to nonadherence: a case study of novel xanthine oxidase inhibitors for gout. Clin Pharmacol Ther, 2019; 106(3):652–60. CrossRef
Hill-McManus D, Marshall S, Soto E, Lane S, Hughes D. Impact of non-adherence and flare resolution on the cost-effectiveness of treatments for gout: application of a linked pharmacometric/pharmacoeconomic model. Value Health, 2018; 21(12):1373–81. CrossRef
ACCP. ISoP ACCP Special Interest Group, 2017. Available via https://www.accp1.org/Members/About/ACCP1/0About/ISoP_ACCP_Special_Interest_Group.aspx%20%5b (Accessed 14 July 2020).
Ivaturi V, Dvorak CC, Chan D, Liu T, Cowan MJ, Wahlstrom J, Stricherz M, Jennissen C, Orchard PJ, Tolar J, Pai SY. Pharmacokinetics and model-based dosing to optimize fludarabine therapy in pediatric hematopoietic cell transplant recipients. Biol Blood Marrow Transplant, 2017; 23(10):1701–13. CrossRef
Kierzek AM, Hickling TP, Figueroa I, Kalvass JC, Nijsen M, Mohan K, Veldman GM, Yamada A, Sayama H, Yokoo S, Gulati A. A quantitative systems pharmacology consortium approach to managing immunogenicity of therapeutic proteins. CPT Pharmacometrics Syst Pharmacol, 2019; 8(11):773–6. CrossRef
Laouénan C, Guedj J, Mentré F. Clinical trial simulation to evaluate power to compare the antiviral effectiveness of two hepatitis C protease inhibitors using nonlinear mixed effect models: a viral kinetic approach. BMC Med Res Methodol, 2013; 13(1):60–70. CrossRef
Li F, Nandy P, Chien S, noel gj, tornoe cw. pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob Agents Chemother, 2010; 54(1):375–9. CrossRef
Lukacova V, Goelzer P, Reddy M, Greig G, Reigner B, Parrott N. A physiologically based pharmacokinetic model for ganciclovir and its prodrug Valganciclovir in adults and children. AAPS J, 2016; 18(6):1453–63. CrossRef
Lyauk YK, Jonker DM, Lund TM. Dose finding in the clinical development of 60 US Food and Drug Administration-Approved Drugs Compared with learning versus confirming recommendations. Clin Transl Sci, 2019; 12(5):481–9. CrossRef
Marshall S, Madabushi R, Manolis E, Krudys K, Staab A, Dykstra K. Model-informed drug discovery and development: current industry good practice and regulatory expectations and future perspectives. CPT Pharmacometrics Syst Pharmacol, 2019; 8(2):87–96. CrossRef
Mason CL, Leedale J, Tasoulis S, Jarman I, Antoine DJ, Webb SD. Systems toxicology approach to identifying paracetamol overdose. CPT: Pharmacometrics Syst Pharmacol, 2018; 7(6):394–403. CrossRef
Nakamura T, Toshimoto K, Lee W, Imamura CK, Tanigawara Y, Sugiyama Y. Application of PBPK modeling and virtual clinical study approaches to predict the outcomes of CYP2D6 genotype-guided dosing of tamoxifen. CPT Pharmacometrics Syst Pharmacol, 2018; 7(7):474–82. CrossRef
Patel JN, O’Neil BH, Deal AM, Ibrahim JG, Sherrill GB, Olajide OA. A community-based multicenter trial of pharmacokinetically guided 5-fluorouracil dosing for personalized colorectal cancer therapy. Oncologist, 2014; 19(9):959–65. CrossRef
Pilla Reddy V, Fretland AJ, Zhou D. Mechanistic physiology-based pharmacokinetic modeling to elucidate vincristine-induced peripheral neuropathy following treatment with novel kinase inhibitors. Cancer Chemother Pharmacol, 2021; 88(3):451–64. CrossRef
Pilla Reddy V, Walker M, Sharma P, Ballard P, Vishwanathan K. Development, verification, and prediction of osimertinib drug–drug interactions using PBPK modeling approach to inform drug label. CPT Pharmacometrics Syst Pharmacol, 2018; 7(5):321–30. CrossRef
Pink J, Lane S, Hughes DA. Mechanism-based approach to the economic evaluation of pharmaceuticals: pharmacokinetic/pharmacodynamic/pharmacoeconomic analysis of rituximab for follicular lymphoma. Pharmacoeconomics, 2012; 30(5):413–29. CrossRef
FDA. Division of pharmacometrics, 2018. Available via https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/division-pharmacometrics (Accessed 21 April 2020).
FDA. Exposure-response relationships—study design, data analysis, and regulatory applications. U.S. Food and Drug Administration, 2020. Available via https://www.fda.gov/regulatory-information/search-fda-guidance-documents/exposure-response-relationships-study-design-data-analysis-and-regulatory-applications (Accessed 11 July 2020).
Rybak MJ, Le J, Lodise TP. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm, 2020; 77(11):835–64. CrossRef
Schindler E, Krishnan S, Mathijssen R, Ruggiero A, Schiavon G, Friberg L. Pharmacometric modeling of liver metastases’ diameter, volume, and density and their relation to clinical outcome in imatinib-treated patients with gastrointestinal stromal tumors: modeling of tumor 1D, 3D, and density measurements. CPT Pharmacometrics Syst Pharmacol, 2017; 6(7):449–57. CrossRef
Singh AP, Zheng X, Lin-Schmidt X, Chen W, Carpenter TJ, Zong A. Development of a quantitative relationship between CAR-affinity, antigen abundance, tumor cell depletion and CAR-T cell expansion using a multiscale systems PK-PD model. MAbs, 2020; 12(1):1688616–21. CrossRef
Slejko JF, Willke RJ, Ribbing J, Milligan P. Translating pharmacometrics to a pharmacoeconomic Model of COPD. Value Health, 2016; 19(8):1026–32. CrossRef
Sou T, Kukavica-Ibrulj I, Soukarieh F, Halliday N, Levesque RC, Williams P, Stocks, M., Cámara, M., Friberg, L. E., & Bergström, C. Model-based drug development in pulmonary delivery: pharmacokinetic analysis of novel drug candidates for treatment of Pseudomonas aeruginosa lung infection. J Pharm Sci 2019; 108(1):630–40. CrossRef
Størset E, Åsberg A, Skauby M, Neely M, Bergan S, Bremer S. Improved tacrolimus target concentration achievement using computerized dosing in renal transplant recipients—a prospective, randomized study. Transplantation, 2015; 99(10):2158–66. CrossRef
Sunkaraneni S, Ludwig E, Fiedler-Kelly J, Hopkins S, Galluppi G, Blum D. Modeling and simulations to support dose selection for eslicarbazepine acetate therapy in pediatric patients with partial-onset seizures. J Pharmacokinet Pharmacodyn, 2018; 45(4):649–58. CrossRef
Svensson EM, Svensson RJ, te Brake LHM, Boeree MJ, Heinrich N, Konsten S. The potential for treatment shortening with higher rifampicin doses: relating drug exposure to treatment response in patients with pulmonary tuberculosis. Clin Infect Dis, 2018; 67(1):34–41. CrossRef
US FDA. Physiologically based pharmacokinetic analyses: format and content, guidance for industry, 2016. Available via https://www.fda.gov/downloads/Drugs/Guida nceCo mplia nceRe gulat oryIn formation/Guidances/UCM53 1207.pdf (Accessed 14 July 2020).
Visser SAG, Kandala B, Fancourt C, Krug AW, Cho CR. A model-informed drug discovery and development strategy for the novel glucose-responsive insulin MK-2640 enabled rapid decision making. Clin Pharmacol Ther, 2020; 107(6):1296–311. CrossRef
Wang Y, Zhu H, Madabushi R, Liu Q, Huang S-M, Zineh I. Model-informed drug development: current US regulatory practice and future considerations. Clin Pharmacol Ther, 2019; 105(4):899–911. CrossRef
Wright DF, Winter HR, Duffull SB. Understanding the time course of pharmacological effect: a PKPD approach. Br J Clin Pharmacol, 2011; 71(6):815–23. CrossRef
Xu C, Goggin TK, Su X, Taverna P, Oganesian A, Lowder JN. Simultaneous modeling of biomarker and toxicity response predicted optimal regimen of guadecitabine (SGI-110) in myeloid malignancies. CPT Pharmacometrics Syst Pharmacol, 2017; 6(10):712–8. CrossRef
Yoneyama T, Abdul-Hadi K, Brown A, Guan E, Wagoner M, Zhu AZX. A citrulline based translational population system toxicology model for gastrointestinal-related adverse events associated with anticancer treatments. CPT Pharmacometrics Syst Pharmacol, 2019; 8(12):951–61. CrossRef
Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen-induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol, 2016; 4(2):131–42. CrossRef
Zhang Z, Imperial MZ, Patilea-Vrana GI, Wedagedera J, Gaohua L, Unadkat JD. Development of a novel maternal-fetal physiologically based pharmacokinetic model i: insights into factors that determine fetal drug exposure through simulations and sensitivity analyses. Drug Metab Dispos, 2017; 45(8):920–38. CrossRef
Zhao X, Shen J, Ivaturi V, Gopalakrishnan M, Feng Y, Schmidt BJ. Model-based evaluation of the efficacy and safety of nivolumab once every 4 weeks across multiple tumor types. Ann Oncol, 2020; 31(2):302–9. CrossRef