INTRODUCTION
When there is a seasonal change, the prevalence of respiratory infections is high in children. Gentamicin is a narrow therapeutic index drug belonging to the class of aminoglycosides which is effective against Gram-negative organisms such as Escherichia coli, Klebsiella species, Pseudomonas aeruginosa, Klebsiella pneumonia, and Staphylococcus aureus, and resistant against Enterobacteriaceae, Enterococci, and Staphylococci (Krause et al., 2016; Lundergan et al., 1999; MacDougall and Chambers, 2011). It is mainly indicated for the treatment of both upper and lower respiratory tract infections, urinary tract infections (UTI), meningitis, bacterial neonatal sepsis, peritonitis, bacterial septicemia, skin, bone, and soft tissue infections (Pacifici, 2015). The mechanism action of gentamicin involves the inhibition of protein synthesis of bacteria by binding with 30S ribosomal subunits, causing the misreading of the mRNA, and facilitating the premature termination of translation (Hahn and Sarre, 1969; Kushner et al., 2016). The available dosage forms of gentamicin are ointment, injections, and drops (Bloomfield et al., 1978). The preferable route of gentamicin administration includes topical routes, intramuscular (IM), intravenous (IV), intrathecal, auricular, and ophthalmic drops (Bloomfield et al., 1978; Haddad et al., 1986). The recommended dose of gentamicin in pediatrics is 2–7.5 mg/kg every 8 hours; with an initial dose of 2–5 mg/kg/day and the maximum dose is 7.5 mg/kg/day. During maintenance dose therapy, dose adjustment may be required for renal failure patients (Taylor and Keane, 1976). The main adverse effects of gentamicin include nephrotoxicity and ototoxicity, wherein nephrotoxicity is reversible and ototoxicity is irreversible (Saleh et al., 2016). Gentamicin is contraindicated in myasthenia gravis, where neuromuscular transmission may be impaired (Garraghan and Fallon, 2015). Gentamicin interacts with penicillin or cephalosporin, cyclosporin, amphotericin B, and furosemide (Garraghan and Fallon, 2015; Noone and Pattison, 1971). Pharmacokinetic parameters of gentamicin include absorption: poorly absorbed orally; volume of distribution (Vd) in infants (0.4 ± 0.1 l/kg), neonates (0.45 ± 0.1 l/kg), and children (0.35 ± 0.15 l/kg); protein binding (0%–30%); half-life in neonates: if <1 week for 3–11.5 hours, 1 week to 1 month for 3–6 hour, infants 4 ± 1 hour, children 2 ± 1 hour, peak serum concentration for IM route within 30–90 minutes; and IV route within 30 minutes, which is excreted through the kidneys (Nocton and Gedeit, 2018). Gentamicin has an average trough concentration of <2 μg/ml and an average peak concentration of <12 μg/ml (Garraghan and Fallon, 2015).
Nowadays, all antibiotics are susceptible to bacteria which lead to irrational use of antibiotics. Antibiotic sensitivity testing (AST) being a scientific tool will be helpful to identify the bacteria and will be helpful in rationalizing the antibiotic treatment.
AST is a test that determines the sensitivity of bacteria to an antibiotic. The two methods of AST are dilution method (broth dilution and agar dilution method) and diffusion method (Stokes’ disk diffusion and Kirby–Bauer disk diffusion method).
The dilution method is used to find out the growth and identification of bacterial populations. Micro-dilution and macro-dilution are the two types of dilutions, wherein broth and agar are the commonly used media. In broth dilution, consecutive twofold dilutions (1, 2, 4, 8, and 12 μl) of antibiotics are made and in agar dilutions and antibiotics are diluted in agar medium (Khan et al., 2019). The disk diffusion method is the gold standard for confirming the susceptibility of bacteria. In this method, an isolated bacteria colony is selected, suspended into growth media, and standardized through a turbidity test (Graham et al., 1985).
Pharmacokinetics refers to the study of the time course of drug absorption, distribution, metabolism, and excretion. Pharmacodynamics refers to the relationship between drug concentration at the site of action and their effect (Tozer and Rowland, 2006).
The pharmacokinetic–pharmacodynamic modeling links the pharmacokinetics and pharmacodynamics to evaluate dose–concentration–response relationships and describe the effect of time courses resulting from a drug dose (Meibohm and Derendorf, 1997). Hence, this review focuses on the pharmacokinetic–pharmacodynamics modeling of gentamicin in pediatrics.
The objective of the study is to correlate pharmacokinetic and pharmacodynamic data of gentamicin for the pediatric population for different disease conditions.
MATERIALS AND METHODS
Types of participants
It includes pharmacokinetics, pharmacodynamics (sensitivity), and Pharmacokinetic- Pharmacodynamic (PK-PD) correlation of gentamicin studies that were carried out in the pediatric population for different disease conditions.
Types of interventions and outcome
Intervention—comparison of pharmacokinetic and pharmacodynamic data of gentamicin. Outcome—to understand the concentration profile of gentamicin showing sensitivity against bacterial infections.
SEARCH METHODS
Electronic searches
A thorough literature search was conducted from the year 1998 to 2020, using the keywords aminoglycosides, gentamicin, population pharmacokinetics, pediatrics, pharmacokinetics and pharmacodynamics correlation, nonlinear mixed effects modeling, therapeutic drug monitoring, and antibiotic sensitivity test in the following database: Cochrane Library, PubMed, Scopus, and Google Scholar.
Data collection and analysis
The collected data was analyzed based on the inclusion and exclusion criteria. The pharmacokinetics and pharmacodynamics studies were categorized, followed by a comparison of the pharmacokinetic and pharmacodynamic profiles of gentamicin.
Data extraction and analysis
Three authors independently extracted all the studies. The following study characteristics were collected: author, study design, country of publication, age range of participants, year of publication, and dose and indication for gentamicin.
A total of 149 articles were identified through searching. 100 articles were screened after removing duplication; 31 articles were excluded from screening; and 69 articles were included. Out of 69 articles, 26 articles were included in the study and 43 articles were excluded. The Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) flow diagram describes the study as shown in Figure 1. All articles included in the review were conducted in pediatrics.
RESULTS AND DISCUSSION
Pharmacokinetic approach in different disease conditions
In Gram-negative bacterial infections, pediatric patients receiving a dose of 7.5 mg/kg/day of gentamicin as the drug of choice. Plasma samples were checked for the targeted trough concentration <2 μg/ml and peak concentration <10 μg/ml. The concentration was found to exceed the therapeutic range. The dose was adjusted to 3 mg/kg/day; their trough and peak concentrations were found to be 1–2 μg/ml and 6 μg/ml, respectively. The adjusted daily dose of gentamicin was 3–5 mg/kg/day which were found to be ideal for Gram-negative bacterial infections (Ismail et al., 1990).
For sepsis, meningitis, and neutropenic fever, neonates received a dose of 5 mg/kg/day of gentamicin every 24 hours as the drug of choice. Blood samples were collected to check for the targeted peak concentration of 8–10 mg/l and trough concentration <2 mg/l. The first dose of gentamicin appears to be more efficient in achieving the target serum concentrations than steady state which would be ideal for sepsis, meningitis, and neutropenic fever (Lim et al., 2020).
For neonatal sepsis and Gram-negative and Gram-positive infections, they received a dose of 4 mg/kg of gentamicin as the drug of choice. The trough concentration should not exceed more than 2 mg/l which can lead to toxicity. A dose 4 mg/kg of gentamicin was found to be sufficient to achieve a Cmax concentration of 5 mg/l with a postnatal age. The dose needs to be increased to 7.5 mg/kg to achieve target peak concentrations of >10 mg/l; trough concentration <2 mg/l in neonates which were effective against sepsis, Gram-negative, and Gram-positive infections (van Donge et al., 2018).
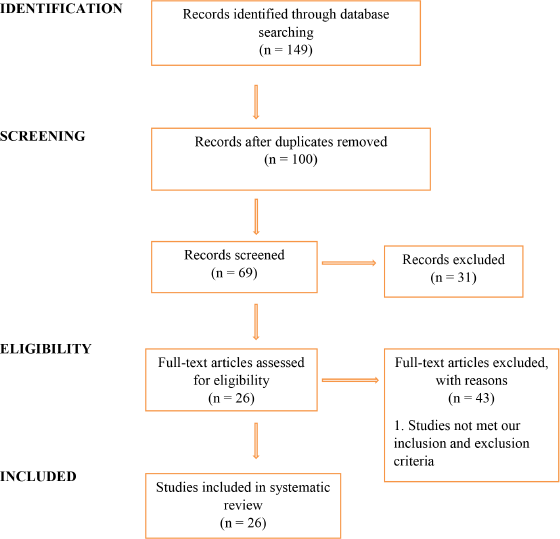 | Figure 1. PRISMA flow diagram. [Click here to view] |
For sepsis, neonates received a dose of 5 mg/kg/day of gentamicin, with a target maximum concentration of 20 mg/l. Low serum levels are achieved with a dosing range of 5–8 mg/kg/day. A once-daily dose is expected to induce a high Cmax/ Minimal Inhibitory Concentration (MIC) level. Micro-organism activity with MICs was as high as 2 mg/l for gentamicin. A lower maximum concentration is achieved by dividing dose and maximum concentration/minimum inhibitory concentration above 8–10 mg/l would be difficult to reach, particularly for Multi-drug Resistance (MDR) P. aeruginosa (Mareville et al., 2012).
For hypoxic ischemic encephalopathy with hyperthermia, neonates received a dose of 5 mg/kg/day of gentamicin every 24 hours. Blood samples were collected and their targeted trough concentration >2 mg/l and peak concentration <6 mg/l were not obtained among the neonates with 24-hour dosing intervals. At 5 mg/kg/day every 36 hours of dosing intervals, the targeted trough concentration <2 mg/l was obtained in neonates which would be effective against hypoxic-ischemic encephalopathy (Frymoyer et al., 2013). The pharmacokinetic studies’ characteristics included in this review are shown in Table 1.
Wound infection
Wound infections are common conditions in pediatrics which occur mainly in the skin for which gentamicin sulfate cream is used as the drug of choice. A wound swab was collected to check for susceptibility. The Gram-negative bacteria such as E. coli (48.3%), Proteus species (74%), K. pneumonia (36%), and P. aeruginosa (86%) show sensitivity toward gentamicin, whereas Gram-positive organism shows resistance against gentamicin (Kibret and Abera, 2011; Mama et al., 2014).
Urinary tract infections
UTI are the most common bacterial infections in neonates and gentamicin is commonly prescribed for the same. The urine samples were collected to check for sensitivity and the following organism such as E. coli, Klebsiella spp., Proteus mirabilis, and P. aeruginosa was identified. Escherichia coli shows a resistance of about 30%–75% for amikacin, gentamicin. Klebsiella (50%), P. mirabilis (52.2%), and P. aeruginosa (100%) are sensitive toward gentamicin (Abuhandan et al., 2013; Garoy et al., 2019; Kibret and Abera, 2011; Rezaee and Abdinia, 2015; Wang et al., 2014).
Community-acquired and persistent nosocomial infections commonly occur in neonates in hospital settings. Gentamicin is mainly used to treat community-acquired infections in neonates. The swab samples for pus, urine, and sputum were checked for susceptibility. The organisms identified were P. aeruginosa, K. pneumonia, and S. aureus. Pseudomonas aeruginosa (83.5%), K. pneumoniae (100%), and S. aureus (92.4%) were sensitive toward gentamicin (Ali et al., 2014; Nwankwo and Nasiru, 2011; Shilpa et al., 2016; Sivanmaliappan and Sevanan, 2011; Yadav et al., 2017).
Typhoid fever
Typhoid fever commonly occurs in pediatrics and is caused by contaminated food and water. Gentamicin is effective against Salmonella typhi mainly in pediatrics. The blood samples were collected for sensitivity and S. typhi was identified; it is susceptible to Ceftriaxone (100%), Cefixime, Gentamicin (99.4%), and Ciprofloxacin (98.6%) (Ali and Sultana, 2016). Probiotics are live micro-organisms that help to provide immunity and prevent the infections in neonates. The swab and sputum samples were checked for sensitivity. Bacteria such as Lactobacillus acidophilus was identified and it is sensitive against gentamicin (Zhou et al., 2005).
Pneumonia
Pneumonia is the second most commonly occurring bacterial infection in children and gentamicin is a commonly used drug in pneumonia. The sputum was collected and Gram-negative organisms, E. coli, Klebsiella spp., Enterobacter spp., Serratia marcescens, and Acinetobacter spp. were identified. Escherichia coli (87%), Klebsiella spp. (93%), Enterobacter spp. (93.9%), S. marcescens (95.8%), Acinetobacter spp. (41%) are sensitive toward gentamicin (Sader et al., 2014).
Severe acute malnutrition (SAM)
SAM is more common in children mainly with pneumonia and UTI conditions. A susceptibility test was carried out with urine and sputum samples. Organisms such as E. coli and nontyphoidal Salmonellae were identified and it shows 100% sensitivity toward gentamicin (Okomo et al., 2011).
Sepsis
Sepsis is more common in neonates; gentamicin is the drug of choice for sepsis. The blood samples were checked for sensitivity and Gram-positive and Gram-negative organisms were identified. Methicillin-resistant S. aureus (MRSA) was sensitive toward gentamicin and Klebsiella and E. coli showed high resistance to Cefotaxime (90.5%), Gentamicin (75%), and Ciprofloxacin (76.2%) (Mokuolu et al., 2002; Pokhrel et al., 2018).
Community-acquired Gram-negative uropathogen infections
Escherichia coli and K. pneumonia were isolated and showed high sensitivity to Ciprofloxacin (95.3%), Amikacin (93.9%), Nalidixic acid (92.2%), Gentamicin (89.2%), and Nitrofurantoin (83.8%) (Mohamed et al., 2012).
The organisms were identified from different diseases and their percentages of sensitivity toward gentamicin are shown in Table 2 and their respective characteristics for pharmacodynamics are shown in Table 3.
Pharmacokinetic–Pharmacodynamic Correlation
The PK-PD model was developed by using in vitro time-kill curve experiments; it is classified into two types: static time-kill curve experiments and dynamic time-kill curve experiments. The correlation was carried out by the semi-mechanistic PK-PD model which describes the time course of the drug concentration and the bacterial growth and killing after antibacterial treatment. For an extremely preterm new-born infant, the highest possible dose is 6 mg/kg for a 36-hour interval, while a dose of 7 mg/kg with a 36-hour interval in the typical term newborn infant. Dosing intervals should longer than 24 hours for extremely preterm infants to reach trough levels of <2 mg/l, unless the dose is reduced to less than 4 mg/kg. To achieve concentrations of <1 mg/l in typical newborn infants, doses of 4 and 5 mg/kg with a 36-hour interval is recommended (Carapetis et al., 2001; Nielsen et al., 2011).
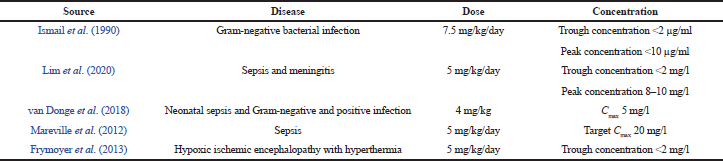 | Table 1. Gentamicin doses concerning the achieved concentration. [Click here to view] |
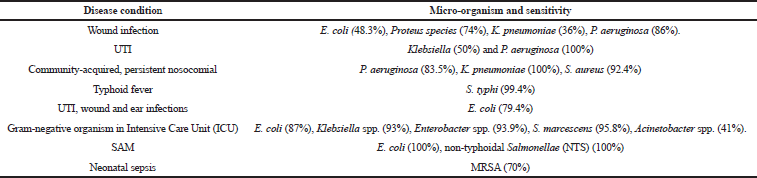 | Table 2. Organisms identified from different disease conditions and its percentage of sensitivity towards gentamicin. [Click here to view] |
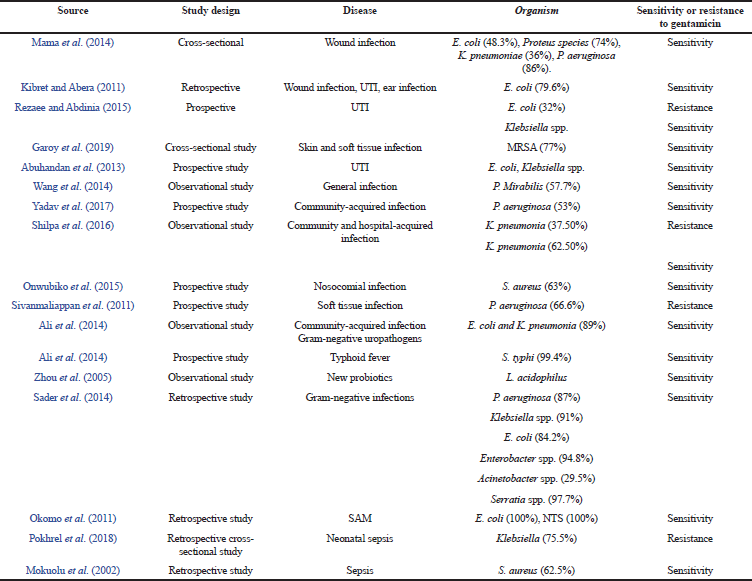 | Table 3. Pharmacodynamic study. [Click here to view] |
In sepsis conditions, 5 mg/kg/day of gentamicin shows a minimum inhibitory concentration of >2 mg/l toward E. coli, P. aeruginosa, Staphylococcus epidermidis, Enterobacter spp., Streptococci, S. aureus, K. pneumoniae, and Citrobacter diversus. For MDR P. aeruginosa, the minimum inhibitory concentration in the range of 8–10 mg/l is not shown (Frymoyer et al., 2013).
In urinary tract infection, 4.5–7.5 mg/kg/day of gentamicin shows minimum inhibitory concentration >2 mg/l toward Gram-negative bacilli, Enterococci, and Staphylococcus spp. (Carapetis et al., 2001). In severe malnutrition, 7.5 mg/kg/day of gentamicin shows a minimum inhibitory concentration of >2 mg/l toward Plasmodium falciparum and E. coli (Seaton et al., 2007).
CONCLUSION
This review focuses on gentamicin correlating the pharmacokinetics and pharmacodynamics data by using the semi-mechanistic PK-PD model. This review guides us about how to correlate the pharmacokinetic and pharmacodynamic data. It will help us find out at what concentration gentamicin shows resistance and sensitivity toward bacteria, especially in pediatric population.
ACKNOWLEDGMENT
The authors would like to thank the staff of the Department of Pharmacy Practice, JSS College of Pharmacy, Ooty, for their support.
AUTHORS’ CONTRIBUTION
Keerthana Chandrasekar and K. P. Arun formulated the hypothesis. B. Vahini, V. Vijay, and R. Shalini carried out the data collection and analysis. All the authors read and accepted the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING
None.
ETHICAL APPROVAL
Not applicable.
REFERENCES
Abuhandan M, Güzel B, Oymak Y, Çiftçi H. Antibiotic sensitivity and resistance in children with urinary tract infection in Sanliurfa. Turk J Urol, 2013; 39(2):106–10. CrossRef
Ali A, Tayebah V, Farid K, Tayebah A, Farhad A, Marziaeh A. Antimicrobial susceptibility patterns of community-acquired Gram-negative uropathogens. Afr J Microbiol Res, 2014; 8:332–6. CrossRef
Ali MK, Sultana S. Antimicrobial sensitivity patterns of Salmonella typhi in children. Bangladesh J Med Sci, 2016; 15(3):416–8. CrossRef
Bloomfield SE, Miyata T, Dunn MW, Bueser N, Stenzel KH, Rubin AL. Soluble gentamicin ophthalmic inserts as a drug delivery system. Arch Ophthalmol, 1978; 96(5):885–7. CrossRef
Carapetis JR, Jaquiery AL, Buttery JP, Starr M, Cranswick NE, Kohn S, Hogg GG, Woods S, Grimwood K. Randomized, controlled trial comparing once daily and three times daily gentamicin in children with urinary tract infections. Pediatr Infect Dis J, 2001; 20(3):240–6. CrossRef
Frymoyer A, Meng L, Bonifacio SL, Verotta D, Guglielmo BJ. Gentamicin pharmacokinetics and dosing in neonates with hypoxic ischemic encephalopathy receiving hypothermia. Pharmacotherapy, 2013; 33(7):718–26. CrossRef
Garoy EY, Gebreab YB, Achila OO, Tekeste DG, Kesete R, Ghirmay R, Kiflay R, Tesfu T. Methicillin-resistant Staphylococcus aureus (MRSA): prevalence and antimicrobial sensitivity pattern among patients-a multicenter study in Asmara, Eritrea. Can J Infect Dis Med Microbiol, 2019; 2019:8321834. CrossRef
Garraghan F, Fallon R. Gentamicin: dose regimens and monitoring. Pharm J, 2015; 295(7874):1–11.
Graham DR, Dixon RE, Hughes JM, Thornsberry C. Disk diffusion antimicrobial susceptibility testing for clinical and epidemiologic purposes. Am J Infect Control, 1985; 13(6):241–9. CrossRef
Haddad NS, Ravis WR, Pedersoli WM, Carson RL Jr. Pharmacokinetics of single doses of gentamicin given by intravenous and intramuscular routes to lactating cows. Am J Vet Res, 1986; 47(4):808–13.
Hahn FE, Sarre SG. Mechanism of action of gentamicin. J Infect Dis, 1969; 119(4):364–9 CrossRef
Ismail R, Sarriff A, Abdul Rahman AF. Therapeutic drug monitoring for gentamicin in Hospital Universiti Sains Malaysia. Med J Malays, 1990; 45(1):57–64.
Khan ZA, Siddiqui MF, Park S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics (Basel), 2019; 9(2):49. CrossRef
Kibret M, Abera B. Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr Health Sci, 2011; 11(Suppl 1):S40–5.
Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: an overview. Cold Spring Harb Perspect Med, 2016; 6(6):a027029. CrossRef
Kushner B, Allen PD, Crane BT. Frequency and demographics of gentamicin use. Otol Neurotol, 2016; 37(2):190–5. CrossRef
Lim WXS, Chua WBB, Chua JM, Lee Q, Chan JW, Sultana R, Poh BH. A retrospective review of the efficiency of first-dose therapeutic drug monitoring of gentamicin, amikacin, and vancomycin in the pediatric population. J Clin Pharmacol, 2020; 60(1):7–15. CrossRef
Lundergan FS, Glasscock GF, Kim EH, Cohen RS. Once-daily gentamicin dosing in newborn infants. Pediatrics, 1999; 103(6 Pt 1):1228–34.
MacDougall C, Chambers HF. Aminoglycosides. In: Brunton LL, Chabner BA, Knollman BJ, (eds.). Goodman & Gilman’s the pharmacological basis of therapeutics, 12th edition, McGraw-Hill, New York, NY, pp 1505–20, 2011.
Mama M, Abdissa A, Sewunet T. Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. Ann Clin Microbiol Antimicrob, 2014; 13:14. CrossRef
Mareville J, Gay J, Cliquennois E, Herbaux C, Pasquier F, Allorge D, Blondiaux N, Berthon C, Alfandari S. Therapeutic drug monitoring of aminoglycosides in acute myeloid leukaemia patients. Scand J Infect Dis, 2012; 44(5):398–401. CrossRef
Meibohm B, Derendorf H. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J Clin Pharmacol Ther, 1997; 35(10):401–13.
Mohamed AF, Nielsen EI, Cars O, Friberg LE. Pharmacokinetic-pharmacodynamic model for gentamicin and its adaptive resistance with predictions of dosing schedules in newborn infants. Antimicrob Agents Chemother, 2012; 56(1):179–88. CrossRef
Mokuolu AO, Jiya N, Adesiyun OO. Neonatal septicaemia in Ilorin: bacterial pathogens and antibiotic sensitivity pattern. Afr J Med Med Sci, 2002; 31(2):127–30.
Nielsen EI, Cars O, Friberg LE. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob Agents Chemother, 2011; 55(10):4619–30. CrossRef
Nocton JJ, Gedeit R. Aminoglycosides. Gentamicin pharmacokinetics. 4th edition, Elsevier Health Sciences, Philadelphia, PA, pp 287–302, 2018.
Noone P, Pattison JR. Therapeutic implications of interaction of gentamicin and penicillins. Lancet, 1971; 2(7724):575–8 CrossRef
Nwankwo EO, Nasiru MS. Antibiotic sensitivity pattern of Staphylococcus aureus from clinical isolates in a tertiary health institution in Kano, Northwestern Nigeria. Pan Afr Med J, 2011; 8:4. CrossRef
Okomo UA, Garba D, Fombah AE, Secka O, Ikumapayi UN, Udo JJ, Ota MO. Bacterial isolates and antibiotic sensitivity among Gambian children with severe acute malnutrition. Int J Pediatr, 2011; 2011:825123. CrossRef
Onwubiko NE and Akande AO. Microbial Contamination In The Operating Theatre Of a Tertiary Health Institution In Kano, Northwestern Nigeria. Nigerian Journal of Microbiology 2015; 27(1):2671–79
Pacifici GM. Clinical pharmacology of gentamicin in neonates: regimen, toxicology and pharmacokinetics. MedicalExpress, 2015; 2(5):M150501
Pokhrel B, Koirala T, Shah G, Joshi S, Baral P. Bacteriological profile and antibiotic susceptibility of neonatal sepsis in neonatal intensive care unit of a tertiary hospital in Nepal. BMC Pediatr, 2018; 18(1):208. CrossRef
Rezaee MA, Abdinia B. Etiology and antimicrobial susceptibility pattern of pathogenic bacteria in children subjected to UTI: a referral hospital-based study in Northwest of Iran. Medicine (Baltimore), 2015; 94(39):e1606.
Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011). Diagn Microbiol Infect Dis, 2014; 78(4):443–8. CrossRef
Saleh P, Abbasalizadeh S, Rezaeian S, Naghavi-Behzad M, Piri R, Pourfeizi HH. Gentamicin-mediated ototoxicity and nephrotoxicity: a clinical trial study. Niger Med J, 2016; 57(6):347–352. CrossRef
Seaton C, Ignas J, Muchohi S, Kokwaro G, Maitland K, Thomson AH. Population pharmacokinetics of a single daily intramuscular dose of gentamicin in children with severe malnutrition. J Antimicrob Chemother, 2007; 59(4):681–9. CrossRef
Shilpa K, Thomas R, Ramyashree A. Isolation and Antimicrobial sensitivity pattern of Klebsiella pneumoniae from sputum samples in a tertiary care hospital. Int J Biomed, 2016; 7(2):53–7. CrossRef
Sivanmaliappan TS, Sevanan M. Antimicrobial susceptibility patterns of Pseudomonas aeruginosa from diabetes patients with foot ulcers. Int J Microbiol, 2011; 2011:605195. CrossRef
Taylor M, Keane C. Gentamicin dosage in children. Arch Dis Child, 1976; 51(5):369–72.
Tozer TN, Rowland M. Introduction to pharmacokinetics and pharmacodynamics: the quantitative basis of drug therapy. Lippincott Williams & Wilkins, Philadelphia, PA, pp 1–13, 2006.
van Donge T, Pfister M, Bielicki J, Csajka C, Rodieux F, van den Anker J, Fuchs A. Quantitative analysis of gentamicin exposure in neonates and infants calls into question its current dosing recommendations. Antimicrob Agents Chemother, 2018; 62(4):e02004–17.
Wang JT, Chen PC, Chang SC, Shiau YR, Wang HY, Lai JF, Huang IW, Tan MC, Lauderdale TL; TSAR Hospitals. Antimicrobial susceptibilities of Proteus mirabilis: a longitudinal nationwide study from the Taiwan surveillance of antimicrobial resistance (TSAR) program. BMC Infect Dis, 2014; 14:486. CrossRef
Yadav VC, Kiran VR, Jaiswal MK, Singh K. A study of antibiotic sensitivity pattern of Pseudomonas aeruginosa isolated from a tertiary care hospital in South Chhattisgarh. Int J Med Sci Public Health, 2017; 6(3):600–5. CrossRef
Zhou JS, Pillidge CJ, Gopal PK, Gill HS. Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int J Food Microbiol, 2005; 98(2):211–7. CrossRef