INTRODUCTION
The World Health Organization has reported that hepatocellular carcinoma (HCC) was the 5th most common cancer worldwide and the 2nd most predominant cause of death related to cancer in 2012 (World Health Organization, 2012). HCC is the most common liver cancer and may result from chronic hepatitis B virus infection, post-hepatitis C virus (HCV) liver cirrhosis, smoking, excess drinking of alcohol, and aflatoxin intake (American Cancer Society, 2016).
Viral hepatitis is the 7th leading cause of death worldwide (Stanaway et al., 2016). Approximately 50% of deaths related to chronic HCV infection that led to liver cirrhosis progressed to HCC development (Lavanchy, 2011; Mohd Hanafiah et al., 2013). HCV infection is a public health problem in Egypt, where it has the highest prevalence among nations according to the WHO (WHO, 2016). It is known that post-viral hepatitis HCC is mainly a result of hepatocellular inflammation, oxidative stress, abnormal signaling pathways with activation of oncogenes, and integration of viral DNA into the host genome (Sukowati et al., 2016; Tokino et al., 1991).
The intestinal barriers that prevent the passage of microbes and their metabolites across the mucosa include the intact intestinal mucosal barrier, the mucus layer, secretion of immunoglobulin A, and associated lymphoid tissues (Peterson and Artis, 2014). A close relationship exists between the gut microbiome and the liver, mainly due to their anatomical positions and the flow of nutrients and blood supply from the gut via the portal vein. The liver is the most vulnerable organ to gut-derived toxins, dangerous metabolites, bacteria, and their metabolites (Miele et al., 2013; Schnabl and Brenner, 2014). Growth and alteration in gut microbiota cause damage to the intestinal wall, promoting the transmission of bacteria and metabolites that elicit systemic endotoxemia and disease in distant organs, including the liver (Qin et al., 2010; Yu and Schwabe, 2017).
Dysbiosis is the loss of beneficial microorganisms and overgrowth of harmful microbes, changing the overall microbial profile (Petersen and Round, 2014). Dysbiosis of gut microbiota favors chronic hepatic inflammation with subsequent carcinogenesis, which can be elicited by the interaction between the liver, abnormal gut microbiota, and their metabolites together with the immune system via macrophages and Kupffer cells that secrete inflammatory cytokines, IL-8, tumor necrosis factor α, and interleukin 1 beta (Brandi et al., 2017; Chassaing et al., 2014; Rivera et al., 2007).
Logically, dysbiosis of gut microbiota augments the pathophysiology of viral hepatitis by inducing chronic inflammation of the liver with subsequent hepatic HCC (Fattovich et al., 2004). Mechanisms by which viral hepatitis induces disturbance of gut microbiota are unknown; however, maintaining gut homeostasis can prevent viral hepatitis-induced hepatic disease progression and HCC development. Therefore, in this study, we explored changes in gut microbiota between healthy volunteers, patients with chronic HCV infection, and patients with HCV-induced HCC.
PATIENTS AND METHODS
Study design and participants
This cross-sectional cohort study was carried out on 400 individuals with average body volume recruited from the Internal Medicine Department and Tropical Medicine and Gastroenterology Department of two dedicated centers (Helwan University Hospital and South Valley University Hospital) in Egypt, in collaboration with the Medical Biochemistry Department of the Faculty of Medicine, South Valley University, Qena, Egypt. The study period lasted from January 2017 to January 2020. The individuals were divided clinically into three groups. Group I consisted of 100 patients with HCC on top of liver cirrhosis caused by chronic HCV infection. The diagnosis of HCC was made according to American Association for the Study of Liver Diseases (AASLD) guidelines, based on clinical, laboratory, and imaging data and liver biopsy when required (Marrero et al., 2018). Group II consisted of 200 chronic HCV-infected patients. All patients were infected with HCV genotype 4. Group III consisted of 100 healthy control subjects negative for hepatitis markers and with normal abdominal ultrasounds. Patients below 18 years of age who underwent antibiotic therapy within the preceding 2 weeks, those who had autoimmune hepatitis, human immunodeficiency virus, or hepatitis B virus coinfection, those who reported excessive alcohol intake, and those with other etiologies of hepatic affection were excluded from the study. The study protocol was approved by the Qena Faculty of Medicine Ethical Committee, and written informed consent was obtained from each subject.
Clinical assessments
Complete medical histories were taken, and full clinical examinations and laboratory and radiological investigations were performed on each included subject. Serum alpha-fetoprotein (AFP) determination and multislice abdominal computed tomography (MSCT) were performed for patients with suspected HCC. For cirrhotic patients with hepatic focal lesions of >1 cm on abdominal ultrasound and AFP >20 ng/ml, MSCT imaging was performed. If typical features of HCC were not observed but HCC was still suspected, the liver was biopsied to confirm the diagnosis per AASLD guidelines. All patients with HCC were evaluated using a Child–Pugh score (Pugh et al., 1973), and tumor staging was assessed using tumor (T), nodes (N), and metastases (M) (TNM) and Barcelona Clinic Liver Cancer (BCLC) scoring systems (Llovet et al., 1999; Vauthey et al., 2002).
Investigatory workup
Blood samples and biochemical and molecular assays
We obtained 10 ml blood samples from each included subject under sterile conditions and transported them to the laboratory for routine laboratory investigations, including complete blood counts using Sysmex, serum creatinine, complete liver function tests [alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, and total bilirubin] using an autoanalyzer (Dialab 450 system), prothrombin time, prothrombin concentration, and international normalized ratio (INR). HCV genotype estimation and HCV polymerase chain reaction (PCR) assessment were performed using reverse transcription PCR (RocheCOBAS TaqMan HCV assay version 2.0, lower limit of detection 15 IU/ml). hepatitis B surface antigen estimation and HCV antibody detection were performed using the automated MiniVidas immunoassay system (Biomerieux, Marcy l'Etoile, France). AFP assessment was also performed for patients with suspected HCC (Hassan et al., 2018).
Polymerase chain amplification of stool microbiota
Stool samples were collected from each participant and rapidly transported to the laboratory. At the laboratory, an aliquot was taken from stool in a 1.5 ml Eppendorf tube and stored at −80°C. DNA was extracted from the stored sample after thawing using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer`s instructions.
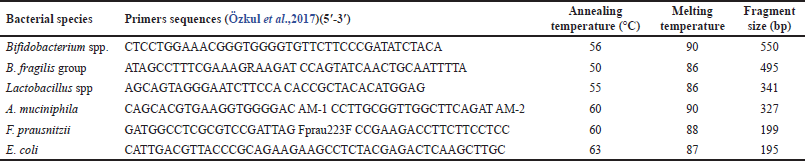
Positive control strains for PCR analysis included Akkermansia muciniphila (ATCC BAA-835), Faecalibacterium prausnitzii [ATCC (American type culture collection) 27766], Bifidobacterium breve (ATCC 15700), Lactobacillus acidophilus (ATCC 4356), Bacteroides fragilis (ATCC 25285), and Escherichia coli (ATCC 25922). These control strains were cultured according to the standard microbiological recommendations, and DNA from these strains was suspended in 1 ml phosphate buffer and extracted using the Qiagen extraction kit (Özkul et al., 2017).
The amplification of different bacterial species of microbiota was performed using the primers listed in Table 1. Amplification reactions were carried out on a total volume of 20 μl and consisted of 4 mM MgCl2, 0.25 μM of each primer, and 2 μl of DNA template. Amplification involved an initial denaturation step at 95°C for 10 minutes, followed by 45 cycles of denaturation at 95°C for 10 seconds, annealing at the specific annealing temperature (Table 1) for 5 seconds, and extension at 72°C for 10 seconds. The products of the amplified PCR were analyzed using gel electrophoresis (Özkul et al., 2017).
Abdominal multislice CT (HCC radiological hallmark)
The typical feature used for HCC diagnosis is arterial phase hyperintensity (washin), followed by portal venous washout to iso- or hypodensity (delayed phases) (European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer, 2012).
Statistical analysis
Data were analyzed using the Statistical Program for Social Science program version 24. Qualitative data are expressed as frequencies and percentages; quantitative data are expressed as means ± standard deviation. A chi-square test was used when comparing gut microbiota between groups. p-values of <0.05 were considered significant.
RESULTS
Demographic data of the study groups
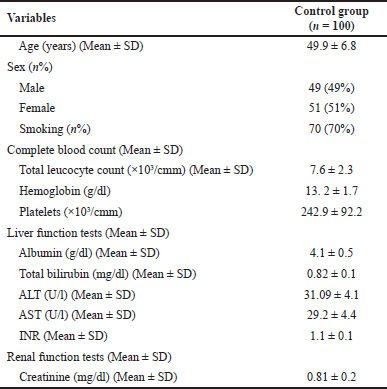
This descriptive cross-sectional cohort study included 300 patients and 100 healthy controls. The first group included 100 patients with HCC, and their basic demographic and laboratory data are shown in Table 2. The second group included 200 patients with chronic HCV infection; their basic demographic data are shown in Table 3. The third group included 100 healthy volunteers whose gut microbiota were evaluated for comparison with patients from groups I (HCC) and II (HCV). Their demographic data are shown in Table 4.
Profile of gut microbiota among patients with HCC compared with the control group
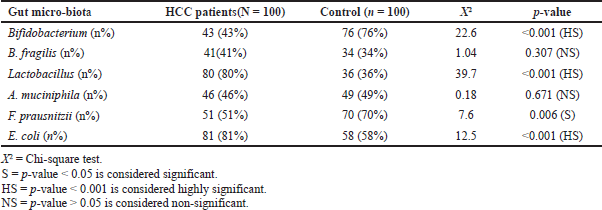
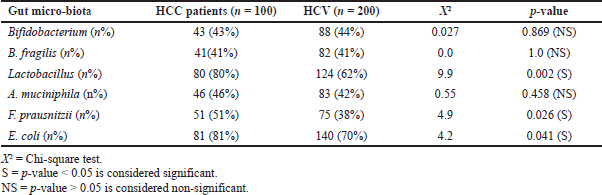
Differences were detected between patients with HCC and controls (p < 0.001) in the levels of Bifidobacterium, which was detected in fewer patients with HCC (43%) compared with healthy controls (76%). However, Lactobacillus and E. coli species were detected in more patients with HCC (80% and 81%) compared with healthy controls (36% and 58%, resp.). Statistically significant differences were also detected between patients with HCC and controls (p < 0.05) in F. prausnitzii, which was detected in fewer patients with HCC (51%) compared with healthy controls (70%). No statistically significant differences were detected between patients with HCC and controls (p > 0.05) regarding B. fragilis and A. muciniphila (Table 5).
| Table 1. Bacterial species, primers sequences, annealing and melting temperatures of PCR, and the result size of bp. |
| Table 2. Demographic, clinical, and routine laboratory data of HCC patients. |
| Table 3. Demographic, clinical, and routine laboratory data of chronic HCV infected patients. |
| Table 4. Demographic and routine laboratory data of healthy control subjects. |
Profile of gut microbiota among patients with chronic HCV infection compared with the control group
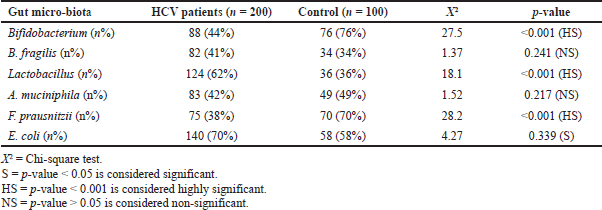
A statistically significant difference was detected between patients with HCV and controls (p < 0.001) regarding Bifidobacterium and F. prausnitzii, which were detected in fewer patients in the HCV group (44% and 38%) compared with healthy controls (76% and 70%, resp.). On the other hand, Lactobacillus was detected in more patients in the HCV group (62%) compared with healthy controls (36%). Statistically significant differences were detected between patients with HCV and controls (p < 0.05) of E. coli which was detected in more patients with HCV (70%) compared with healthy controls (58%). However, no statistically significant differences were found between patients with HCV and healthy volunteers (p > 0.05) in B. fragilis and A. muciniphila species (Table 6).
Comparison between patients with HCC and chronic HCV infection
Statistically significant differences were detected between the HCC and HCV groups (p < 0.001) regarding F. prausnitzii in the gut microbiota, which was detected in a greater number in patients with HCC (51%) than in patients with HCV (38%). Lactobacillus and E. coli were also detected in more patients in the HCC group (80% and 81%) than those in the HCV group (62% and 70%, resp.). No statistically significant differences were detected between patients with HCC and HCV (p > 0.05) regarding Bifidobacterium, B. fragilis, and A. muciniphila (Table 7).
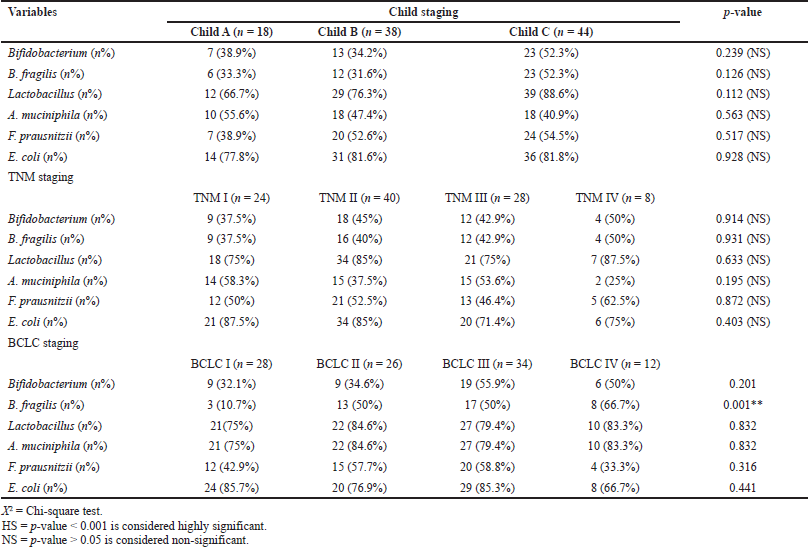
Correlation between gut microbiota and clinical scores in the HCC group
No significant correlations could be detected between gut microbiota and either Child–Pugh grade or TNM stage. A correlation was detected, however, between the presence of B. fragilis and BCLC staging, with the highest detection in stage IV patients (66.7%) (Table 8).
DISCUSSION
The gut and the liver are physiologically and anatomically connected, known as the gut–liver axis. Gut microbiota and their metabolites affect both hepatocytes and stromal cells (hepatic stellate and Kupffer cells) (Ohtani and Kawada, 2019). Intestinal dysbiosis is also observed in different chronic hepatic diseases, which has caused concern on the role of the gut microbiota in the development and progression of hepatic diseases and aggravation of related complications (Sandler et al., 2011; Konturek et al., 2018). Few studies have evaluated the role of gut dysbiosis in patients with viral hepatitis. Therefore, in this study, we sought to compare changes that occur in the gut microbiota of patients with chronic hepatitis C, HCC, and normal healthy controls.
The different species of the genus Bifidobacterium are reported to have beneficial health effects, including the regulation of homeostasis of other intestinal microbes, the suppression of pathogens and harmful bacteria that colonize the gut mucosa, the modification of local and systemic immune responses, the inhibition of procarcinogen enzymatic activities of the gut microbiota, and the mending of the gut mucosal barrier by lowering the levels of lipopolysaccharides (Mayo and van Sinderen, 2010; Pinzone et al., 2012). In our study, less than half of patients with HCC and chronic HCV infection had detectable Bifidobacterium in their stool samples, compared with 76% of healthy volunteers. This finding agrees with Yu et al. (2010), who found that decreased Bifidobacterium species led to the accumulation of lipopolysaccharides, which act as pathological mediators of inflammation-associated HCC. In addition, impairment of gut wall integrity due to the absence of Bifidobacterium may allow translocation of pathogenic bacteria and endotoxins, which can lead to chronic hepatic inflammation with a subsequent risk of the development of HCC.
| Table 5. Comparisons between HCC patients and control as regards studied gut micro-biota. |
| Table 6. Comparison between chronic HCV infected patients and control as regards studied gut micro-biota. |
| Table 7. Comparison between HCC patients and HCV patients as regards gut micro-biota. |
It is known that Lactobacillus species are important for human health, as they can decrease gastrointestinal pH to protect the host against invasion by pathogens (Martín et al., 2013). On the other hand, Lactobacilli may also be pathogenic in susceptible patients via several mechanisms, including the ability of some strains to bind to the intestinal wall and translocate into the bloodstream, leading to bacteremia. Also, some can adhere to collagen in the extracellular matrix and produce glycosidase, an enzyme that contributes to the damage of affected tissues (Apostolou et al., 2001; Harty et al., 1994; Oakey et al., 1995). In this study, most patients with HCC and chronic HCV infection had greater amounts of Lactobacillus in their stool than healthy controls, with more detected in patients with HCC than in chronic HCV-infected patients. This agrees with Sherid et al. (2016) who reported the involvement of Lactobacillus in the development of bacteremia and liver abscesses. On the contrary, Zhang et al. (2012) reported that disturbance of gut microbiota homeostasis with decreased Lactobacilli led to the damage of mucosa, endotoxemia, systemic inflammation, and tumor formation.
| Table 8. Correlation between the studied gut- microbiota and different scores in the HCC group. |
In healthy individuals, F. prausnitzii represented >5% of the gut flora. This species plays an important role in improving the immune system, as it has anti-inflammatory activities such as promoting IL-10 secretion and inhibiting IL-12 and interferon-γ expression (Fukui, 2019; Miquel et al., 2013). In this study, F. prausnitzii was detected in about half of the patients with HCC and 38% of the patients with chronic HCV infection compared with 70% of healthy controls. This agrees with Munukka et al. (2017) who found mice treated with F. prausnitzii had improved hepatic ALT and AST levels and decreased adipose tissue inflammation. Also, in a study on patients with HCC, Liu et al. (2019) found that HCC is caused by different factors that decreased levels of Faecalibacterium, which resulted in reduction in the levels of anti-inflammatory short-chain fatty acids.
The family Enterobacteriaceae includes medically relevant species such as Salmonella, E. coli, Yersinia pestis, Klebsiella, Shigella, Proteus, Enterobacter, Serratia, and Citrobacter. Many members of species of this family are normally present in humans as gut microbiota. Some Enterobacteria are pathogens because they produce endotoxins; when released into the bloodstream, they can cause a systemic inflammatory and vasodilatory response (Brenner et al., 2005; Paterson and Doi, 2017). In this study, E. coli was found in stool samples of about 80% of patients with HCC and 70% of patients with chronic HCV infection. This finding agrees with many studies that reported that increased Enterobacteriaceae was linked to the progression of liver cirrhosis and development of cirrhosis-related complications (Bajaj et al., 2014; Chen et al., 2011; Lax et al., 2012; Sanduzzi Zamparelli et al., 2017).
Bacteroides is the most predominant bacteria in colon, with B. fragilis being the most abundant among all Bacteroides species (Ahmed et al., 2018). Bacteroides, together with other gut commensal bacteria, provides the human body with energy through carbohydrate fermentation, producing a pool of volatile fatty acids that are absorbed by the colon (Lopez et al., 1996). Akkermansia muciniphila is one gut microbiome known to have an anti-inflammatory effect in humans by improving hepatic inflammation and protecting liver cells against damage via an immune cell-mediated mechanism. Also, this species is believed to have a role in cancer response to immunotherapy (Routy et al., 2018; van Passel et al., 2011; Wu et al., 2017). In our study, we found no significant difference between patients with HCC and chronically HCV-infected patients in comparison with the control group regarding stool isolates of B. fragilis. However, Chen et al. (2011) found a significant decrease in Bacteroides levels in patients with liver cirrhosis-related complications. On the other hand, Xie et al. (2016) reported a marked increase in Bacteroides species levels in a mouse model of HCC development post-nonalcoholic steatohepatitis.
Also, in this study, no significant difference was detected between HCC patients and chronic HCV patients compared with the control group regarding the stool isolate of A. muciniphila. Akkermansia muciniphila was detected in the stool of less than half of patients with HCC and HCV. To some extent, different experimental studies on animal models are in accordance with our results. They have demonstrated that the presence of gut A. muciniphila can enhance the anticancer effect of T-cell-based immunotherapies (Matson et al., 2018; Routy et al., 2018; Sivan et al., 2015; Vétizou et al., 2015).
Though many staging systems for HCC are used worldwide, no system is considered superior in evaluating suitable treatment and patient prognosis (Wildi et al., 2004). Child–Pugh and TNM have a better predictive ability for overall survival than BCLC (Sirivatanauksorn and Tovikkai, 2011). However, TNM fails to evaluate patient prognosis accurately because it only evaluates tumor extension, and BCLC has demonstrated better prognostic ability than the TNM staging system (Cillo et al., 2006). Therefore, in this study, we used different systems to evaluate patients with HCC. No significant differences were detected between gut microbiota and HCC progression with respect to Child–Pugh or TNM scoring systems. However, a significant difference was detected between the number of positive stool isolates of B. fragilis and the BCLC staging system; B. fragilis was isolated from 66.7% of patients with BCLC stage IV compared with 10.7% of patients with BCLC stage I. This agrees with Guoxiang et al. who found a marked increase in Bacteroides species along with increased lipopolysaccharide levels correlated with the progression of liver disease from steatosis, leading to HCC development (Xie et al., 2016).
CONCLUSIONS
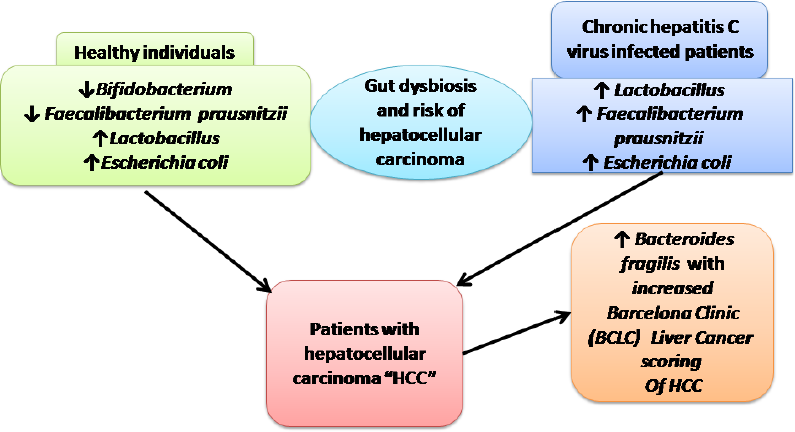
A characteristic pattern of Bifidobacterium, Lactobacillus, and E. coli species was detected in patients with chronic HCV and HCC. Dysbiosis may, therefore, be associated with the progression of liver disease and hepatic carcinogenesis. It may also suggest that a probiotic can help prevent the progression of liver disease. However, the role of dysbiosis in the development of liver disease and hepatic carcinogenesis is under debate. Further studies are needed in a large cohort of patients to derive additional meaning from these results.
DECLARATION
This research article was posted as a preprint prior to publication in the preprint research square server (https://doi.org/10.21203/rs.3.rs-29305/v1).
AUTHOR'S CONTRIBUTION
Study concept and design: KM, HAO, HA, MHH, and AMMS. Patient selection and clinical assessments: HAO and AMMS. Biochemical and molecular analysis of stool and blood samples: KM, HA, and MHH. Statistical analysis: KM, HAO, HA, MHH, and AMMS. Literature search: KM, HAO, HA, MHH, and AMMS. All authors revised and approved the final manuscript version.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
FUNDING
None.
REFERENCES
Ahmed Z, Ahmed U, Walayat S, Ren J, Martin DK, Moole H, Koppe S, Yong S, Dhillon S. Liver function tests in identifying patients with liver disease. Clin Exp Gastroenterol, 2018; 11:301–7. CrossRef
American Cancer Society. Liver cancer risk factors. American Cancer Society, Atlanta, GA, 2016. Available via http://www.cancer.org/cancer/livercancer/detailedguide/liver-cancer-risk-factors. (Accessed 8 August 2016).
Apostolou E, Kirjavainen PV, Saxelin M, Rautelin H, Valtonen V, Salminen SJ, Ouwehand AC. Good adhesion properties of probiotics: a potential risk for bacteremia? FEMS Immunol Med Microbiol, 2001; 31(1):35–9. CrossRef
Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol, 2014; 60(5):940–7. CrossRef
Brandi G, De Lorenzo S, Candela M, Pantaleo MA, Bellentani S, Tovoli F, Saccoccio G, Biasco G. Microbiota, NASH, HCC and the potential role of probiotics. Carcinogenesis, 2017; 38(3):231–40. CrossRef
Brenner DJ, Krieg NR, Staley JT, Garrity. (eds.). Bergey's manual of systematic bacteriology. 2nd edition, Springer, New York, NY, 2005. CrossRef
Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology, 2014; 59(1):328–39. CrossRef
Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology, 2011; 54(2):562–72. CrossRef
Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D'Amico F, Ciarleglio FA, Bridda A, D'Amico DF. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol, 2006; 44(4):723–31. CrossRef
European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol, 2012; 56(4):908–43. CrossRef
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology, 2004; 127(5):S35–50. CrossRef
Fukui H. Role of gut dysbiosis in liver diseases: what have we learned so far? Diseases, 2019; 7(4):58. CrossRef
Harty DW, Oakey HJ, Patrikakis M, Hume EB, Knox KW. Pathogenic potential of lactobacilli. Int J Food Microbiol, 1994; 24(1–2):179–89. CrossRef
Hassan AM, Osman HA, Mahmoud HS, Hassan MH, Hashim AA, Ameen HH. Sofosbuvir-daclatasvir improves hepatitis C virus-induced mixed cryoglobulinemia: upper Egypt experience. Infect Drug Resist, 2018; 11:895–901. CrossRef
Konturek PC, Harsch IA, Konturek K, Schink M, Konturek T, Neurath MF, Zopf Y. Gut liver axis: how do gut bacteria influence the liver? Med Sci (Basel), 2018; 6(3):79. CrossRef
Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect, 2011; 17(2):107–15. CrossRef
Lax S, Schauer G, Prein K, Kapitan M, Silbert D, Berghold A, Berger A, Trauner M. Expression of the nuclear bile acid receptor/farnesoid X receptor is reduced in human colon carcinoma compared to nonneoplastic mucosa independent from site and may be associated with adverse prognosis. Int J Cancer, 2012; 130(10):2232–9. CrossRef
Liu Q, Li F, Zhuang Y, Xu J, Wang J, Mao X, Zhang Y, Liu X. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog, 2019; 11:1. CrossRef
Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis, 1999; 19(3):329–38. CrossRef
Lopez JB, Balasegaram M, Thambyrajah V, Timor J. The value of liver function tests in hepatocellular carcinoma. Malays J Pathol, 1996; 18(2):95–9.
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology, 2018; 68(2):723–50. CrossRef
Martín R, Miquel S, Ulmer J, Kechaou N, Langella P, Bermúdez-Humarán LG. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microb Cell Fact, 2013; 12:71. CrossRef
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science, 2018; 359(6371):104–8. CrossRef
Mayo B, van Sinderen D, (eds.). Bifidobacteria: genomics and molecular aspects. Caister Academic Press, Wymondham, UK, 2010.
Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C, Grieco A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des, 2013; 19(29):5314–24. CrossRef
Miquel S, Martín R, Rossi O, Bermúdez-Humarán LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol, 2013; 16(3):255–61. CrossRef
Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology, 2013; 57(4):1333–42. CrossRef
Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, Hänninen A, Vuopio J, Huovinen P, Jalkanen S, Pekkala S. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J, 2017; 11(7):1667–79. CrossRef
Oakey HJ, Harty DW, Knox KW. Enzyme production by lactobacilli and the potential link with infective endocarditis. J Appl Bacteriol, 1995; 78(2):142–8. CrossRef
Ohtani N, Kawada N. Role of the gut-liver axis in liver inflammation, fibrosis, and cancer: a special focus on the gut microbiota relationship. Hepatol Commun, 2019; 3(4):456–70. CrossRef
Özkul C, Yalınay M, Karakan T, Yılmaz G. Determination of certain bacterial groups in gut microbiota and endotoxin levels in patients with nonalcoholic steatohepatitis. Turk J Gastroenterol, 2017; 28(5):361–9. CrossRef
Paterson DL, Doi Y. Enterobacteriaceae. In: Mayers D, Sobel J, Ouellette M, Kaye K, Marchaim D. (eds.). Antimicrobial drug resistance. Springer, Cham, Switzerland, 2017; doi:10.1007/978-3-319-47266-9_8
Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol, 2014; 16(7):1024–33. CrossRef
Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol, 2014; 14(3):141–53. CrossRef
Pinzone MR, Celesia BM, Di Rosa M, Cacopardo B, Nunnari G. Microbial translocation in chronic liver diseases. Int J Microbiol, 2012; 2012:694629; doi:10.1155/2012/694629 CrossRef
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg, 1973; 60(8):646–9. CrossRef
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature, 2010; 464(7285):59–65. CrossRef
Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol, 2007; 47(4):571–9. CrossRef
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science, 2018; 359(6371):91–7. CrossRef
Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T, Douek DC. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology, 2011; 141(4):1220–30. CrossRef
Sanduzzi Zamparelli M, Rocco A, Compare D, Nardone G. The gut microbiota: a new potential driving force in liver cirrhosis and hepatocellular carcinoma. U Eur Gastroenterol J, 2017; 5(7):944–53. CrossRef
Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology, 2014; 146(6):1513–24. CrossRef
Sherid M, Samo S, Sulaiman S, Husein H, Sifuentes H, Sridhar S. Liver abscess and bacteremia caused by Lactobacillus: role of probiotics? Case report and review of the literature. BMC Gastroenterol, 2016; 16(1):138. CrossRef
Sirivatanauksorn Y, Tovikkai C. Comparison of staging systems of hepatocellular carcinoma. HPB Surg, 2011; 2011: 818217. CrossRef
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science, 2015; 350(6264):1084–9. CrossRef
Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, Forouzanfour MH, Groeger J, Hanafiah KM, Jacobsen KH, James SL, MacLachlan J, Malekzadeh R, Martin NK, Mokdad AA, Mokdad AH, Murray CJL, Plass D, Rana S, Rein DB, Richardus JH, Sanabria J, Saylan M, Shahraz S, So S, Vlassov VV, Weiderpass E, Wiersma ST, Younis M, Yu C, El Sayed Zaki M, Cooke GS. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet, 2016; 388(10049):1081–18. CrossRef
Sukowati CH, El-Khobar KE, Ie SI, Anfuso B, Muljono DH, Tiribelli C. Significance of hepatitis virus infection in the oncogenic initiation of hepatocellular carcinoma. World J Gastroenterol, 2016; 22(4):1497–512. CrossRef
Tokino T, Tamura H, Hori N, Matsubara K. Chromosome deletions associated with hepatitis B virus integration. Virology, 1991; 185(2):879–82. CrossRef
van Passel MW, Kant R, Zoetendal EG, Plugge CM, Derrien M, Malfatti SA, Chain PS, Woyke T, Palva A, de Vos WM, Smidt H. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS One, 2011; 6(3):e16876. CrossRef
Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A, Cleary KR, Nagorney DM. Simplified staging for hepatocellular carcinoma. J Clin Oncol, 2002; 20(6):1527–36. CrossRef
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science, 2015; 350(6264):1079–84. Available via http://www.who.int.library.aucegypt.edu:2048/countries/egy/en/ (Accessed 9 January 2016). CrossRef
Wildi S, Pestalozzi BC, McCormack L, Clavien PA. Critical evaluation of the different staging systems for hepatocellular carcinoma. Br J Surg, 2004; 91(4):400–8. CrossRef
World Health Organization. Estimated cancer incidence, mortality and prevalence worldwide in 2012. World Health Organization, Geneva, Switzerland, 2012. Available from http://www.globocan.iarc.fr/Pages/fact_sheets_population.aspx
World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. World Health Organization, Geneva, Switzerland; 2016 (http://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf;jsessionid=10F21056DC7293B9C437F10FF7465E60?sequence=1).
Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, Li Y, He X, Li L. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front Microbiol, 2017; 8:1804. CrossRef
Xie G, Wang X, Liu P, Wei R, Chen W, Rajani C, Hernandez BY, Alegado R, Dong B, Li D, Jia W. Distinctly altered gut microbiota in the progression of liver disease. Oncotarget, 2016; 7(15):19355–66. CrossRef
Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol, 2017; 14(9):527–39. CrossRef
Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, Wang C, Zhang HL, Cao GW, Wu MC, Wang HY. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology, 2010; 52(4):1322–33. CrossRef
Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, Zhai B, Tan YX, Shan L, Liu Q, Chen HY, Dai RY, Qiu BJ, He YQ, Wang C, Zheng LY, Li YQ, Wu FQ, Li Z, Yan HX, Wang HY. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol, 2012; 57(4):803–12. CrossRef
GRAPHICAL ABSTRACT
|