INTRODUCTION
Insulin, discovered to be the remedy for high blood glucose level by Frederick Banting and Charles Best in 1921, has been in wide use since then because of the associated tremendous life expectancy. It is an endogenous protein hormone produced by the β-cells of the endocrine pancreas. The human insulin is composed of 51 amino acids in the form of two separate chains, the A chain and the B chain held together by sulfide bonds. The insulin A chain contains 21 amino acids, whereas the B chain contains 30 amino acids (Mane et al., 2012).
Various conditions that are known for high blood glucose level can be managed by using insulin. Such conditions include type 1 diabetes mellitus (DM), various type 2 DM, and gestational diabetes as well as complications of diabetes such as ketoacidosis and hyperosmolar hyperglycemic states. Therefore, insulin is the cornerstone of the management of DM. In addition, the drug is used in combination with glucose for controlling high blood potassium levels (Mahoney et al., 2005).
Since the discovery of this potent agent for managing DM, there have been many advances in its delivery. This report aims to review the various advances in the science and technology of insulin delivery for the purpose of providing an insight into effective and convenient modes of delivery of the drug.
METHODS
This review is based on literature search of the database of PubMed and Google Scholar using the following search strategies: (diabetes or insulin), (insulin or formulation), (insulin or devices), (glucose monitoring or insulin devices), and (insulin or administration or delivery). More articles were identified from the list of references given in those articles. The searches were limited to the publications in English language.
Priority was given to research articles, controlled clinical trials, and reviews. Articles reflecting insulin production, beta cells and pancreatic transplantation, insulin formulations, insulin administration, blood glucose monitoring, and insulin delivery systems were selected from the search results.
OBSERVED ADVANCES
Insulin production
The use of insulin for the management of DM has been for a century, i.e., since 1921. By 1923, two companies, Eli Lilly and Farbwerke Hoechst (one of the progenitors of present-day Sanofi Aventis), had produced large quantities of better quality bovine insulin than what Frederick Banting and coworkers had earlier used in 1922 (Nasrallah and Reynolds, 2012). In the same year, Hagedorn founded the Nordisk Insulin Laboratorium in Denmark, the forerunner of the present-day Novo Nordisk.
Zinc insulin mixture was formulated by D.M. Scott and A.M. Fisher in 1936 and then licensed it to Novo. Hans Christian Hagedorn, in his work the same year, added protamine to insulin which led to the prolongation of the duration of action of the drug (Mccullach, 1938). Nordisk proceeded and formulated isophane porcine insulin also known as Neutral Protamine Hagedorn or NPH insulin (an intermediate-acting insulin) in 1946. Shortly after that (in 1953), Novo formulated lente porcine and bovine insulin by introducing zinc for a longer-lasting effect.
Insulin became the first human protein to be chemically synthesized in 1963. The work started as production of amino acids that were required for the synthesis of insulin. The entire work of insulin synthesis was spearheaded by Wang Yinglai. The work of Wang Yinglai and his group on total synthesis of insulin is seen as being great and as a product of good foresight (Zhang et al., 2011).
Purified monocomponent insulin was introduced in 1973, and the United States officially standardized insulin sold for human use to U-100 (100 units per ml) the same year. Prior to that standardization, insulin was sold in different strengths such as U-80 (80 units per ml) and U-40 formulations (40 units per ml). Forthwith, other countries of the world followed the U-100 form of insulin preparation.
Biotechnology was applied to insulin production as far back as the 1970s. It should be noted that Genentech has the technical know-how of synthesizing human insulin from bacteria using the recombinant DNA technology. In 1978, this biotechnology company produced biosynthetic human insulin in Escherichia coli bacteria using the recombinant DNA techniques and licensed it to Eli Lilly. This made insulin the first human protein to be biotechnologically manufactured.
Human insulin was chemically and enzymatically produced from porcine insulin by Novo Nordisk in 1981. As the research on insulin production using recombinant DNA technology continued, various forms of biosynthetic human insulin were produced. Humulin® was produced by Eli Lilly and Company in 1983, while the recombinant biosynthetic human insulin of Novo Nordisk was developed in 1988.
Insulin analogues are another form of developed insulin. They are a genetically modified form of insulin whereby the amino acid sequences are altered to change the absorption, distribution, metabolism, and excretion patterns of the drug (Jacob et al., 2018). The Eli Lilly Humalog® which is lispro insulin analogue was approved for use and marketing in 1996. In 2000, the Sanofi Aventis Lantus® insulin (glargine analogue) was approved for therapeutic use in the United States and Europe. Furthermore, the Sanofi Aventis Apidra® insulin (glulisine insulin analogue) was approved for therapeutic use in the United States in 2004, while the Novo Nordisk Levemir® (detemir insulin analogue) was approved in 2006.
Transplantation
Pancreatic transplantation provides a self-regulating insulin source that serves as a means of constant supply of insulin without a periodic administration. This is a difficult procedure, relatively uncommon, and usually carried out in conjunction with liver or kidney transplant, although it can be done alone. Thus, researchers have exploited the islet cell transplantation since the beta cell of the pancreatic islet is responsible for insulin production.
Islet cell transplantation was introduced in the year 2000. It provides relief from insulin administration. This procedure is highly beneficial to patients with type 1 DM, provided they take immunosuppressant medication. The approach has recorded great success rates, with a high percentage of patients being kept insulin-free when experimented for a period of 1 year. Further researches are underway to improve its effectiveness (Gangemi et al., 2008; Zhu et al., 2004).
Another approach of transplantation involves the use of genetically engineered non-beta-cells to secrete insulin (Zhu et al., 2004). It has been observed that mice expressing the human transgene produce human insulin specifically in the gut K cells. Hence, a tumor-derived K-cell line has been induced to produce human insulin by providing cells with the human insulin gene linked to the 5′-regulatory region of the gene encoding glucose-dependent insulinotropic polypeptide (Cheung et al., 2000).
Artificial pancreas
This is a non-transplant method of automatic insulin delivery. Research in this method is ongoing in many research laboratories. Artificial pancreas which is a hybrid closed-loop system consists of three parts: an insulin pump, a continuous glucose monitor, and a computer algorithm that analyses the data of the dosing of insulin. A form of artificial pancreas which pairs the technology of an insulin pump with a continuous glucose monitor was developed by the University of Cambridge in 2013.
The “iLet” was introduced by Dr. Edward Damiano of Boston University in 2015. It is a pocket-sized device that automatically controls blood sugar levels in diabetic patients. It consists of a dual-chamber infusion pump which mimics a biological system. The iLet, which is a bionic pancreas system, delivers insulin and glucagon at 5-minute intervals as required by the body. It is also capable of delivering only insulin or only glucagon. The use of iLet was approved under Investigational Device exemption by the Food and Drug Administration (Drug Development and Delivery, 2018).
Beta Bionics entered crucial trials with its final iLet design in 2019. Novo Nordisk is in partnership with Beta Bionics to carry out codevelopment activities. This is a big project as Eli Lilly and Zealand Pharma are also partners in the development (Drug Development and Delivery, 2018).
Insulin preparations
The available monocomponent insulin preparations may be rapid-acting, short-acting, intermediate-acting, long-acting, or ultralong-acting. The different types are shown in Table 1.
Mixtures of two preparations are also available for the purpose of mimicking the activity of the physiological insulin. They are also referred to as combination insulin preparations and include a combination of either rapid-acting or short-acting insulin with longer-acting insulin, such as NPH insulin. They begin to work with shorter-acting insulin (5–15 minutes for rapid-acting and 30 minutes for short-acting) and duration of action of 16–24 hours with the longer-acting. Various preparations with different proportions of the mixed insulin abound, for example, Novolog® Mix 70/30, which contains 70% aspart protamine (similar to NPH) and 30% aspart. Another example is Humulin® 70/30, which contains insulin isophane (intermediate-acting) and insulin regular (short-acting) (Jacob et al., 2018; Nasrallah and Reynolds 2012).
Insulin delivery
The search for an optimal mode of insulin delivery in the management of DM is ever increasing. The initial mode of delivery was parenteral. This mode of administration has witnessed a lot of advancement. Besides, other routes of administrations which are non-invasive have been widely researched.
Parenteral administration
Parenteral administration is associated with pain at the injection site, poor patient compliance, poor social acceptability, inconvenience, and episode of hypoglycemia. There are several technological advances to ease pain and improve convenience and social acceptability. Hence, the means of parenteral administration of insulin has evolved from syringe/needle to pen, jet, and pump (Ogbera and Kuku, 2012; Olamoyegun et al., 2018; Kesavadev et al., 2020). Further advances include sensor-augmented pump hybrid closed-loop systems and cloud-based data systems (Kesavadev et al., 2020).
Syringe and needle
Insulin was first administered using big reusable syringe with plungers, barrels, and large bore needles. A few years after, specifically in 1924, Beckton Dickinson manufactured the insulin-specialized syringe. By the following year, “Novo Syringe” was manufactured by Novo Nordisk and made available in the market (Aronson et al., 2013). In the course of time, there was significant improvement in the quality of syringe produced for the purpose of reducing the bore size of the needle, inflicting less pain, and improving dosing accuracy.
In most developing countries, the commonly used insulin device is insulin syringe, followed by insulin pen. A hospital-based study conducted in Nigeria in 2012 showed that 71% of diabetic patients on insulin therapy used needle and syringe (Ogbera and Kuku, 2012). In 2018, another study in Nigeria reported 55.4% and 44.6% for syringe and pen, respectively. The usage of insulin syringe is almost nonexistent in the Western world such as USA and Germany. The reasons for it being commonly used in developing countries are reduced cost and accessibility (Olamoyegun et al., 2018).
Insulin pen
Insulin pen was first launched by Novo Nordisk in 1985. This was followed by Novo Pen2 (Novo Nordisk Bluesheet, 1988). The insulin pen has three components, namely an insulin cartridge, a disposable short needle, and an incremental “one click per unit” dosing point. Pens can be either disposable or reusable. They are simple to use and flexible and offer accurate insulin delivery and less pain compared to syringe. Studies have shown that the pen has very wide acceptability especially in the developed countries, in China (100%), Germany (95%), Russia (93%), and Saudi Arabia (63%) (Olamoyegun et al., 2018).
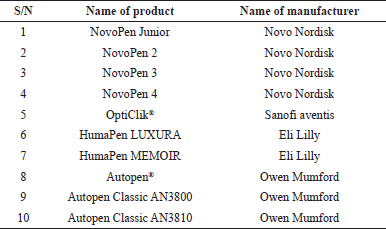
There has been a remarkable evolution in the design of insulin pen with Novo Nordisk, Sanofi, and Eli Lily taking the lead (Kesavadev et al., 2020). At an average of 1 year, a new device is produced with improved delivery of insulin. In 2007, the pen technology was taken to another level. Memory function, which allows the device to retain the date, the time, and the previous doses, was incorporated (Al-Tabakha and Arida, 2008). This has been illustrated in HumaPen MEMOIR. Further advancement in this area includes manufacturing of “smart pen” which can automatically calculate insulin dosage and provide guidance to patients, besides the memory function. Data from this device are transmitted through Bluetooth to produce computer-generated reports (Shah et al., 2016).
Generally, insulin pen delivery devices are either prefilled or refillable (Selam, 2010). Examples of prefilled disposable pens are shown in Table 2, while the refillable pens are shown in Table 3.
Insulin pump
Insulin pump was invented in 1963 and became commercially available in 1979 (Kesavadev et al., 2020). The insulin pump “MiniMed 506” which aids the delivery of meal bolus memory and daily insulin total was introduced by Medtronic in 1992. Insulin pump is an advanced device for the delivery of insulin. The components are insulin reservoir, infusion set, and tubing. The pump dispenses insulin continuously at a preset basal rate and bolus dose before meals (Al-Tabakha and Arida, 2008). The early types were large and had a bag like a backpack. However, there has been significant improvement in the size and programmability of this device. Recent devices can be carried in a shirt pocket. The pump’s working closely resembles normal physiologic secretion.
| Table 1. Types of monocomponent insulin. |
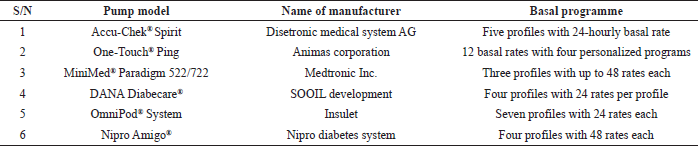
In 2003, Medtronic introduced the first intelligent insulin pump. This pump was wirelessly linked with glucometer and a bolus wizard calculator (Medtronic, 2020). It delivers the required dose and promotes convenience and lifestyle flexibility. Different types of insulin pumps with different basal programs are now available. Some examples as reported by Selam (2010) are shown in Table 4.
Non-invasive administration
The painful, inconvenient, and uncomfortable daily injection of insulin is a major issue and it usually leads to poor compliance (Nolte and Karam, 2004). Even though there are modern injection devices with needles that are thin and sharp, various researches are now directed toward exploiting other routes of administration that are noninvasive.
| Table 2. Examples of prefilled disposable pens. |
| Table 3. Examples of refillable pens |
Intranasal administration
Investigations are underway on intranasal insulin and more so to determine whether intranasal insulin administration can delay or prevent the onset of type 1 diabetes in people who are at risk (Lalej-Bennis et al., 2001; Wherrett, 2014). Insulin in the form of nanoparticles has been investigated for this purpose.
Insulin administered via the nasal route is a way of eliminating the pain of injection. This route has a limitation of small proportion of the administered dose reaching the liver, hence retention in the peripheral circulation which leads to the development of insulin resistance (Wong et al., 2016).
Inhalation
In 2005, inhalable insulin was launched in the United Kingdom, although routine use was not recommended except in cases where there was proven injection phobia diagnosed by a psychiatrist or psychologist. With the exemption of MannKind Corporation which remained optimistic about the concept, all other companies ended their efforts in the production of inhalable insulin. Novo Nordisk and Eli Lily which are well known for the manufacture of insulin preparations ended their efforts at production of inhalable insulin in January and March 2008, respectively.
Exubera®, developed by Inhale Therapeutics, was approved for use by the United States Food and Drug Administration in 2006. It was the first inhalable insulin introduced to the market by Pfizer. It is a rapid-acting form of human insulin that is inhaled as powder through the mouth (Quattrin et al., 2004). Exubera® is an application of Technosphere technology involving dry power formulation of recombinant human regular insulin that can be inhaled and absorbed via the pulmonary tissue with the advantage of not injecting.
Inhalation provides a means of dispelling the pain of injection. The efficacy of inhaled insulin is, however, lower than that of subcutaneous administration. Another drawback to this formulation is drug retention in the peripheral circulation leading to the development of insulin resistance. These limitations of inhaled insulin were responsible for the low sales of Exubera® necessitating its withdrawal (Bailey and Barnett, 2007).
Transdermal administration
Transdermal delivery of insulin has several applications such as pulsatile insulin, ultrasound, and iontophoresis using electric pulses. The physiological secretions of insulin by the pancreas are mimicked by the mechanism of pulsatile insulin injectors called jet injectors or microjets which deliver insulin in pulses into the bloodstream, thus giving rise to varied insulin delivery peaks and durations as such enhancing acceptability by diabetic patients (Arora et al., 2007; Guo et al., 2017).
| Table 4. Examples of insulin pumps. |
Researches have advanced in the manufacture of wrist test appliances for blood glucose levels but work is still in progress to obtain insulin administration (Dixit et al., 2007). In 2015, a similar device that relied on the mechanism of microneedles delivering insulin into the skin pores was in preclinical stage of development (Yu et al., 2015).
A “smart insulin patch” that could dispel the need for painful insulin injection has been developed in United States. The patches that contain numerous hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery (Yu et al., 2015). The beauty of this formulation is that it monitors blood glucose level and releases insulin in a controlled manner to maintain the glucose level within the normal range. The cost of the patch pump is the major challenge with insulin patch. A smart insulin delivery patch is one step close to becoming a reality because researchers are seeking approval for human trial (Watts, 2020).
Oral administration
There is a need for research into oral delivery of insulin because of the limitations of the subcutaneous, nasal, and pulmonary routes. The oral is the most preferred of all routes of drug administration. The route is advantageous in that the pain of injection is eliminated. Also, the peripheral circulation (as in nasal route) with the associated insulin resistance and weight gain is minimized (Khafagy et al., 2007).
Certain hurdles must be overcome before formulating insulin for oral administration. First, the integrity of the drug must be maintained in the face of susceptibility to gastrointestinal enzymatic activities (susceptibility to gastrointestinal enzymes decreases from stomach down to colon) (Ikesue et al., 1993). Secondly, the high molecular weight of insulin and the poor intestinal permeability must be adequately attended to (Yin et al., 2009). The strategies employable to address these delivery challenges are use of enzyme inhibitors, use of absorption enhancers, formulation with hydrogels, and chemical modification of insulin (Yin et al., 2009; Wong et al., 2016).
Many research laboratories have attempted to design mechanisms of transporting sufficient amount of intact insulin from the gut to the portal vein so as to have adequate effect on blood sugar. Many formulation strategies are currently being pursued in an attempt to develop oral insulin preparations. Currently, nanoparticulate delivery systems are being exploited (Card and Magnuson, 2011; Chen et al., 2011) and many clinical trials are ongoing for these preparations (Iyer et al., 2010; Pozzilli et al., 2010).
Oralin®, an oral spray insulin, was approved in 2005 in Ecuador and marketed by Generex. It utilizes RapidMist technology to deliver a mixture of insulin, surfactants, and lipids to the buccal mucosa (Vecchio et al., 2018). From a study conducted by Guevara-Aguirre et al. (2004) on the beneficial effect of Oralin®, it was concluded that the formulation could be used as meal insulin as a complementary therapy to a failing oral hypoglycemic agent in subjects with type 2 diabetes.
The work of Rubin et al. (2013) demonstrated the formulation of insulin as an oral film. The dosage form was formulated using a soluble polymer (to ensure dissolution within 20 minutes) and a hydrophilic bioadhesive polymer (to ensure continuous drug release (over 24 hours). The thin film dosage form was adapted to adhere to a mucous tissue for effective drug delivery.
The work presented by McCourt et al. (2016) from Niagara University, New York, at the 252nd National Meeting and Exposition of the American Chemical Society at Philadelphia United States from August 21 to 25, 2016, showed that the group has developed a new technology for delivering insulin orally. According to the researchers, insulin was encapsulated using their patented cholestosomes (which are neutral, lipid-based particles). The novel vesicles (Cholestosomes®) were made of naturally occurring lipid molecules, which are normal building blocks of fats. It was argued that Cholestosomes® are considered absorbable substances by the gastrointestinal system and that they pass through the gastrointestinal tract without being digested. It was also claimed that intact insulin is released from the Cholestosomes® after they are absorbed.
Nanotechnology
Nanotechnology is one of the approaches to non-injectable formulation of insulin. It is of great importance in antidiabetic research. Chitosan micro- and nanospheres have come up as promising formulations for oral and other types of mucosal transport of large molecules. This is due to the ability of the polymer to enhance absorption of macromolecules. Chitosan nanoparticles were first prepared in 1997 by Fernandez-Urrusuno et al. (1999). The nanoparticles were later used for intranasal administration of insulin in rabbits. The chitosan nanoparticles were found to be efficient vehicles for transporting insulin through the nasal mucosa (McCourt et al., 2016). Nanotechnology is now widely researched and utilized with the expectation that it will eventually provide an effective means of non-invasive administration of insulin (Gupta, 2017).
Blood glucose monitoring
Continuous glucose monitoring (CGM) is necessary for effective blood sugar control. It helps to determine the exact amount of insulin required per time by the patient to achieve and maintain a normal glucose level. One of the ingredients for accurate glucose monitoring is having a good calibration of the glucose monitoring device.
With factory calibration for CGM, user calibration errors and expenses of meter-based calibrations have reduced, sensor accuracy has increased, and the system is more convenient to use. Some currently used sensors may display inaccurate readings with the presence of acetaminophen, ascorbic acid, or acetylsalicylic acid. However, with the advent of Dexcom G6, acetaminophen interference has been overcome. Ongoing works are expected to proffer solution to the interference by other substances (Bailey et al., 2018).
The various targets in the advances in glucose monitoring include accuracy and reduced number of user calibrations, ease of use, longer wear, more impactful data management, and secured connectivity with the insulin delivery devices. Multiple additional improvements have also been explored for CGM, ranging from predictive glucose alerts to integration with artificial pancreas systems or insulin delivery devices (Bailey et al., 2018).
Integrated blood glucose monitoring-insulin delivery technologies
Patients who require insulin administration must engage in frequent glucose monitoring and insulin quantification so as to achieve and maintain optimal glycemic control. Such patients must consistently monitor their glucose levels throughout the day and inject insulin with a syringe, pen, or pump to maintain adequate glucose levels in order to avoid becoming hyperglycemic or hypoglycemic (United States Food and Drug Administration, 2020).
Efforts to combine a CGM sensor with an insulin infusion set have been complicated by the differing wear times being longer for a sensor than an infusion set. The infusion set component may be replaced by automated insertion cannulas to produce a more durable glucose monitoring-insulin infusion system.
Many intelligent pens and pumps are now available for use in computerized systems of CGM, insulin delivery, and data management. Such systems as artificial pancreas are called hybrid closed-loop systems. These are the latest advancements in artificial intelligence-enabled technologies in treating type 1 diabetes (Qian and Schumacher, 2021).
Companion Medical’s Bluetooth LE reusable computerized InPen® was approved by the Food and Drug Administration in July 2016. Its features include a bolus calculator, real-time insulin-on-board tracking, dose history data, reminders to avoid missed meal insulin doses, and an insulin temperature monitor. It also receives CGM data and provides 24-hour glucose averages and summary trend lines. The user can set the app to automatically send a text message with each insulin dose, glucose reading, or carbohydrate entry to as many as five recipients (Bailey et al., 2018).
Integrated sensor-augmented pump therapy systems are comprised of the insulin delivery component and the glucose monitoring component. Examples of such systems are the MiniMed® ParadigmTM Veo system and the VibesTM and G4® Platinum CGM system. These systems are very effective clinically. However, they are more expensive than the stand-alone systems (Riemsma et al., 2016). An integrated system should also have a computerized system for transmitting, storing, and analyzing the data generated.
Integrated blood glucose monitoring-insulin delivery system in pediatrics patients
In August 2020, the United States Food and Drug Administration gave approval for the MiniMed 770G System, a hybrid closed-loop diabetes management device that is intended to automatically monitor glucose and provide appropriate insulin doses with little or no input from the users or their caregivers. The MiniMed 770G System is meant for use by individuals aged 2–6 with type 1 diabetes. It is the first legally marketed device that can automatically adjust insulin delivery based on continuous glucose monitor values for this patient group (United States Food and Drug Administration, 2020).
This new device is a Bluetooth-enabled modified version of the previously approved MiniMed 670G System. It consists of a sensor that attaches to the body to measure glucose levels under the skin; an insulin pump strapped to the body; and an infusion patch connected to the pump with a catheter that delivers insulin. It works by measuring glucose levels in the body every 5 minutes and automatically adjusting insulin delivery by either administering or withholding insulin. One of the drawbacks of this device is that the user needs to manually request insulin doses to counter carbohydrate consumption at mealtime (United States Food and Drug Administration, 2020).
Glucose monitoring technologies and insulin delivery devices under pipeline
Recent years have witnessed an explosion of glucose monitoring technologies, insulin formulations, and delivery. These have also been demonstrated in closed-loop systems of artificial intelligence-enabled glucose monitoring and insulin pumps or pens. There are indications of some lapses which can be addressed in future inventions (Qian and Schumacher, 2021).
There are numerous innovations in the pipeline. These include an updated Insulet OmniPod pump and Cellnovo’s patch pump systems. The former is notable for greater connectivity, user interface improvements, compatibility with concentrated insulin, and artificial pancreas functionality, while the latter is notable for a unique pump mechanism using heat expansion of wax, cell network connectivity, and multiple artificial pancreas collaborations (Bailey et al., 2018).
The Bigfoot Biomedical is developing a Bluetooth insulin pen that connects to the FreeStyle Libre and a mobile app to monitor and automatically adjust long- and short-acting insulin doses. Biocorp is also developing “Datapen” which is a smart reusable injector pen with audio alerts, dose tracking, Bluetooth-enabling cloud connections, reminders, and advice (Bailey et al., 2018).
CONCLUSION
The initial sources of insulin were bovine and porcine pancreas. Insulin preparations from these sources were found to be with a lot of limitations; hence, the development of human insulin through recombinant technology with constant improvement in each preparation. There are various types of formulations (outside of the injectable form) being investigated by different researchers all over the world. Insulin remains the cornerstone of DM management and its delivery has witnessed a lot of advances over the years. It is hereby recommended that researches and clinical trials should be continued and geared toward obtaining safe, effective, and convenient methods of delivering insulin. The target should be to dispel the pain of injection while still retaining the tremendous life expectancy associated with the drug.
AUTHORS’ CONTRIBUTIONS
The conception of the work, the design, analysis, and the drafting of the manuscript were carried out by Emmanuel O. Olorunsola. All the authors were involved in data/information collection and interpretation. Mfonobong F. Alozie, Koofreh G. Davies, and Musiliu O. Adedokun were involved in the critical revision of the manuscript. All the authors gave final approval of the version of the manuscript being published.
ACKNOWLEDGMENT
This work was sponsored by the Nigerian Tertiary Education Trust Fund through the 2019 National Research Grant (Reference number TETFund/DR&D/CE/NRF/STI/37/VOL1).
CONFLICT OF INTEREST
There are no competing interests regarding the publication of this paper.
REFERENCES
Al-Tabakha MM, Arida AI. Recent challenges in insulin delivery systems: a review. Indian J Pharm Sci, 2008; 70:278–86. CrossRef
Aronson R, Gibney MA, Oza K, Berube J, Kassler-Taub K, Hirsch L. Insulin pen needles: effects of extra-thin wall needle technology on preference, confidence, and other patient ratings. Clin Ther, 2013; 35(7):923–33.e4. CrossRef
Arora A, Hakim I, Baxter J, Rathnasingham R, Srinivasan R, Fletcher DA, Mitragotri S. Needle-free delivery of macromolecules across the skin by nanoliter-volume pulsed microjets. Proc Natl Acad Sci U S A, 2007; 104(11):4255–60. CrossRef
Bailey CJ, Barnett AH. Why is Exubera being withdrawn? BMJ, 2007; 335:1156; doi:10.1136/bmj.39409.507662.94 CrossRef
Bailey TS, Walsh J, Stone JY. Emerging technologies for diabetes care. Diabetes Technol The, 2018; 20(S2):S278–84; doi:10.1089/dia.2018.0115 (Accessed 29 March 2021). CrossRef
Card JW, Magnuson BA. A review of the efficacy and safety of nanoparticle-based oral insulin delivery systems. Am J Physiol Gastrointest Liver Physiol, 2011; 301(6):G956–7. CrossRef
Chen MC, Sonaje K, Chen KJ, Sung HW. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials, 2011; 32(36):9826–38. CrossRef
Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe T, Kieffer TJ. Glucose-dependent insulin release from genetically engineered K cells. Science, 2000; 290(5498):1959–62. CrossRef
Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Iontophoresis – an approach for controlled drug delivery: a review. Curr Drug Deliv, 2007; 4(1):1–10. CrossRef
Drug Development and Delivery. Beta bionics receives IDE approval from the FDA to begin a home-use clinical trial testing a new bionics pancreas system. 2018.
Fernandez-Urrusuno R, Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm Res, 1999; 16(10):1576–81. CrossRef
Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, Qi M, Wang Y, Pallan P, Owens C, Bui J, West D, Kaplan B, Benedetti E, Oberholzer J. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant, 2008; 8(6):1250–61. CrossRef
Genentech. Cloning insulin. 2016. Available via www.gene.com>stories>cloning-insulin (Accessed 13 January 2021).
Guevara-Aguirre J, Guevara M, Saavedra J, Mihic M, Modi P. Beneficial effects of addition of oral spray insulin (Oralin) on insulin secreation and metabolic control in subjects with type 2 diabetes mellitus suboptimally controlled on oral hypoglycaemic agents. Diabetes Technol Ther, 2004; 6(1):1–8; doi:10.1089/152091504322783341 CrossRef
Guo L, Xiao X, Sun X, Qi C. Comparison of jet injector and insulin pen in controlling plasma glucose and insulin concentrations in type 2 diabetic patients. Medicine, 2017; 96(1):e5482l; doi:10.1097/MD.0000000000005482 CrossRef
Gupta R. Diabetes treatment by nanotechnology. J Biotechnol Biomater, 2017; 7:268; doi:10.4172/2155-952X.1000268. CrossRef
Ikesue K, Kopeckova P, Kopecek J. Degradation of protein by guinea pig intestinal enzymes. Int J Pharm, 1993; 95:171–9. CrossRef
Iyer H, Khedkar A, Verma M. Oral insulin – a review of current status. Diabetes Obes Metab, 2010; 12(3):179–85. CrossRef
Jacob S, Morsy MA, Nair A. An overview on the insulin preparations and devices. Indian J Pharm Educ Res, 2018; 52(4):550–7. CrossRef
Kesavadev J, Saboo B, Krishna MB, Krishnan G. Evolution of insulin delivery devices: from syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther, 2020; 11:1251–69. CrossRef
Khafagy ES, Morishita M, Onuki Y, Takayami K. Current challenges in non-invasive insulin delivery system: a comparative review. Adv Drug Deliv Rev, 2007; 59:1521–46. CrossRef
Lalej-Bennis D, Boillot J, Bardin C, Zirinis P, Coste A, Escudier E, et al. Efficacy and tolerance of intranasal insulin administered during 4 months in severely hyperglycaemic Type 2 diabetic patients with oral drug failure: a cross-over study. Diabet Med, 2001; 18(8):614–8. CrossRef
Mahoney BA, Smith WA, Lo DS, Tsoi K, Tonelli M, Clase CM. Emergency interventions for hyperkalaemia. Cochrane Database Syst Rev, 2005; 2005(2):CD003235; doi:10.1002/14651858.CD003235.pub2. CrossRef
Mane K, Chaluvaraju, KC, Niranjan, MS, Zaranappa T, Manjuthej T. Review of insulin and its analogues in diabetes mellitus. J Basic Clin Pharm, 2012; 3(2):283–93. CrossRef
McCourt M, Mielnicki L, Catalano J. A cholestosomeTM – new insulin pill for diabetes patients. Paper presented at: the 252nd National Meeting & Exposition of the American Chemical Society (ACS), Philadelphia, PA, 2016.
Mccullach EP. Protamine-Zinc-Insulin in diabetes. Ann Intern Med, 1938. Available via https://doi.org/10.7326/0003-4819-11-11-1979 (Accessed 23 December 2020).
Medtronic. Innovation milestones. 2020. Available via https://www.medtronicdiabetes.com/about-medtronicinnovation/milestone-timeline (Accessed 16 February 2020). CrossRef
Nasrallah SN, Reynolds LR. Insulin degludec: the new generation basal insulin or just another basal insulin? Clin Med Insights Endocrinol Diabetes, 2012; 5:31–7. CrossRef
Nolte MS, Karam, JH. Pancreatic hormones and antidiabetic drugs. In: Katzung BG (ed.). Basic and clinical pharmacology. The McGraw-Hill Companies Inc., Singapore, Singapore, pp 693–715, 2004.
Novo Nordisk Bluesheet. Quarterly perspective on diabetes and chronic diseases. 1988. Available via https://www.press.novonordisk-us.com/bluesheet-issue2/downloads/NovoNordisk_Bluesheet_Newsletter.pdf (Accessed 21 November 2019).
Ogbera AO, Kuku SF. Insulin use, prescription patterns, regimens and costs- a narrative from a developing country. Diabetol Metab Syndr, 2012; 4:50; doi:10.1186/1758-5996-4-50. CrossRef
Olamoyegun MA, Akinlade AT, Ala OA. Audit of insulin prescription patterns and associated burden among diabetics in a tertiary health institution in Nigeria. Afr Health Sci, 2018; 18(4):852–64. CrossRef
Pozzilli P, Raskin P, Parkin CG. Review of clinical trials: update on oral insulin spray formulation. Diabetes Obes Metab, 2010; 12(2):91–6. CrossRef
Qian F, Schumacher PJ. Latest advancements in artificial intelligence-enabled technologies in treating type 1 diabetes. J Diabetes Sci Technol, 2021; 15(1):195–7. CrossRef
Quattrin T, Belanger A, Bohannon NJV. Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patient with type 1 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care, 2004; 27(11):2622–7. CrossRef
Riemsma R, Ramos IC, Birnie R, Buyukkaramikli N, Armstrong N, Ryder S, Duffy S, Worthy G, Al M, Severens J, Kleijnen J. Integrated sensor-augmented pump therapy systems [the MiniMed® ParadigmTM Veo system and the VibesTM and G4® Platinum CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess, 2016; 20(17):v–xxxi. CrossRef
Rubin Y, Cohen S, Ron ES. New oral dissolving films for oral insulin administration for treating diabetes. United State Patent US20130309294A1. 2013. Available via: www.patents.google.com (Accessed 10 January 2021).
Selam JL. Evolution of diabetes insulin delivery devices. J Diabetes Sci Technol, 2010; 4(3):505–13. CrossRef
Shah RB, Patel M, Maahs DM, Shah VN. Insulin delivery methods: past, present and future. Int J Pharm Investig, 2016; 6:1–9. CrossRef
United States Food and Drug Administration. 2020. Available via https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-automated-insulin-delivery-and-monitoring-system-use-young-pediatric (Accessed 28 March 2021).
Vecchio I, Tornali C, Bragazzi NL, Martini M. The discovery of insulin: an important milestone in the history of medicine. Front Endocrinol (Lausanne), 2018; 9:613; doi:10.3389/fendo.2018.00613 CrossRef
Watts M. Initial trials show ‘revolutionary’ insulin patch works – siabetes. Available via https://www.diabetes.co.uk/news/2020/feb/initial-trials-shows-revolutionary-insulin-patch-works.html (Accessed 13 December 2020).
Wherrett DK. Trials in the prevention of type 1 diabetes: current and future. Can J Diabetes, 2014; 38(4):279–84. CrossRef
Wong CY, Martinez J, Dass CR. Oral delivery of insulin for treatment of diabetes: status quo, challenges and opportunities. J Pharm Pharmacol, 2016; 68(9):1093–108. CrossRef
Yin L, Ding J, He C, Cui L, Tang C, Yin C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials, 2009; 30:5691–700. CrossRef
Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A, 2015; 112(27):8260–5. CrossRef
Zhang B, Xue P, Wang E. Yinglai Wang: an admirable biochemist for foresightedness and selflessness. Protein Cell, 2011; 2(7):517–8. CrossRef
Zhu YL, Abdo A, Gesmonde JF, Zawalich KC, Zawalich W, Dannies PS. Aggregation and lack of secretion of most newly synthesized proinsulin in non-beta-cell lines. Endocrinol, 2004; 145(8):3840–9. CrossRef