INTRODUCTION
Obesity is a known risk factor for a number of chronic diseases including, but not limited to, diabetes mellitus (DM), dyslipidemias, cardiovascular disease, cancer, and liver disease (Bhupathiraju & Hu, 2016; Buchwald, 2014). The prevalence of overweight and obesity has reached epidemic levels in the last five decades, necessitating urgent preventive measures. Worldwide, more than 1.9 billion adults aged 18 years and older were overweight and over 650 million were obese in 2016 (WHO, 2020).
Being linked to a spectrum of genetic, environmental, and behavioral risk factors, obesity remains a “wicked problem” as multiple preventive programs have failed to reduce its prevalence among population. Lifestyle modification, including behavioral changes, low-calorie diets, and physical activity, has been recognized as the most known intervention (Wolf & Woodworth, 2009; Yu, 2010). Nevertheless, even if some reduction in the risk of obesity was achieved globally, this would not have a significant impact on individuals who have already developed morbid obesity and remain at higher risk of serious comorbidities as mentioned earlier. Moreover, many patients have found maintaining healthy lifestyle challenging and resorted to clinical interventions to reduce weight (Cannon & Kumar, 2009; Li et al., 2005; Maggard et al., 2005). Bariatric treatment includes pharmacological, minimally invasive, and surgical treatment of obesity (Grandone et al., 2018). The intragastric balloon (IGB) is a modern interventional minimally invasive treatment of obesity, which was first introduced by Nieben and Harboe (1982) (Fernandes et al., 2007). It acts as a space-occupying device that is placed and removed endoscopically. It is indicated in cases where lifestyle and pharmacological therapy has failed and as an alternative to anatomy-altering gastric surgery (Fernandes et al., 2007).
Safety and effectiveness of IGB placement in the treatment of obesity are well established and documented by a number of retrospective studies, prospective clinical trials, and, recently, systematic reviews and meta-analyses (da Silva et al., 2018; Dumonceau, 2008; Imaz et al., 2008; Jamal et al., 2019; Mathus-Vliegen & Tytgat, 2005). Nonetheless, despite their low incidence, some serious complications have been associated with IGB placement, including common complications, such as hemorrhage and ulceration, and life-threatening events, such as gastric and esophageal perforation and bowel obstruction (Stavrou et al., 2019).
So far as the prevalence of obesity increases at an alarming rate, coupled with improved access to health information, it is expected that more patients will be seeking immediate weight reduction strategies in affluent countries. There is scarcity of data on safety, efficacy, and complications associated with the use of IGB from Arabic countries. Thus, the aim of this study was to assess the average weight reduction and complications associated with the use of IGB in a private medical center.
MATERIALS AND METHODS
A retrospective cohort study design involved 64 patients who attended the gastroenterology clinic of Al Madar Private Medical Center, Al Sharjah, United Arab Emirates, between February 2017 and January 2020. Only patients who were registered for IGB therapy alone, without other treatment modalities for obesity, were included in this analysis. All patients underwent full clinical baseline assessment; data obtained from the patients included age, sex, weight, height, Esophago-Gastro-Duodenoscopy (OGD) findings, and the presence of comorbidities. Most of the patients were treated with SPATZ type of IGB except for four patients who were referred from other centers with BioEnterics intragastric balloons. All patients were followed-up until the removal of the IGB 1 year later. Follow-up weight and complications were reported accordingly. Ethics approval was obtained from Al Madar Medical Center with reference no. 4.1/MMC-12/2020. Informed consent was obtained from all individual participants included in the study.
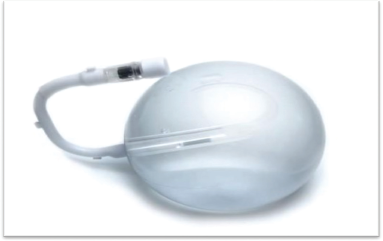
Spatz3 IGB
It is a medical grade, soft, silicone saline-filled gastric balloon that rests in the stomach cavity. The balloon has an inflation tube with a valve that allows inflating and deflating the balloon. The tube is attached to a catheter with a clasp that enables retrieval of the balloon (Fig. 1).
IGB placement
Placement of IGB was carried out at a specialized endoscopy room as a day-only procedure. Patients were fasting for the procedure day. Patients were laid in the lateral position comfortably and sedated using midazolam. Sterile endoscopy probe was advanced through the mouth after application of lubricant. The balloon was introduced in deflated mode into the stomach. After making sure that the balloon is intragastric, it was inflated with an average 400–650 ml of normal saline. Removal of the balloon was carried out in similar environment and technique 1 year later. Using the endoscope, the balloon was ruptured allowing the fluid to drain freely and deflating the balloon to almost its original size prior to placement. As it is a day care procedure, patients were observed to recover from sedation and were discharged on the same day, after ensuring that no major complication has occurred.
Statistical analysis
Data were entered and analyzed using Statistical Package for the Social Sciences V.24. Descriptive statistics were calculated for all variables. The percentage of total weight loss (%TWL) was calculated as the weight reduction achieved relative to the baseline weight. Multiple linear regression was used to identify factors associated with weight reduction beyond the effect of IGB.
RESULTS
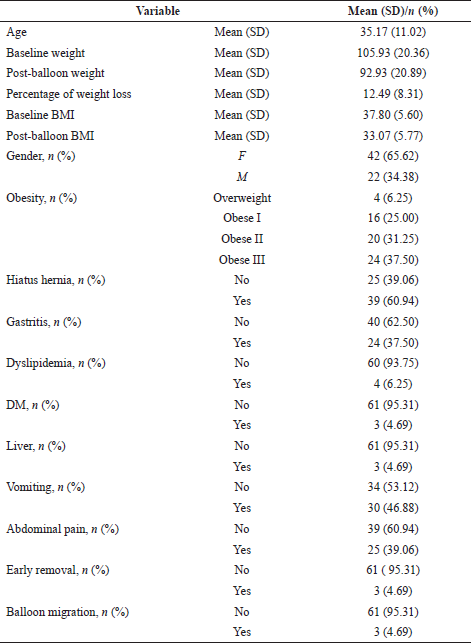
Out of 64 recruited patients, sixty-one completed a 1-year follow-up. Three patients, two females and one male, were taken off the study due to intolerability to IGB, which was removed earlier in the course of treatment. The mean age of participants was 35.17 (±11.02) years with baseline mean weight of 105.51 (±19.93) kg. The majority (65.62%) were female; 4.69% of participants had DM and the same proportion had liver disease at the time of the procedure. Based on the OGD findings, 60.94% were diagnosed with hiatal hernia (HH) and 37.50% had gastritis. Vomiting and abdominal pain were the most commonly reported complications amounting to 46.88% and 39.06%, respectively. Three females (4.69%) experienced balloon migration and spontaneous expulsion and underwent IGB replacement during the follow-up period. As a result of IGB procedure, a mean %TWL of 12.49 (8.31) was achieved (Table 1).
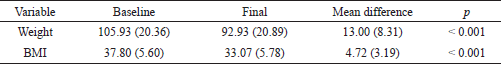
The difference between the baseline and final body weight and BMI is shown in Table 2. The study achieved a statistically significant reduction of mean body weight by 12.49 (±8.31) kg and BMI by 4.72 (±3.19) kg/m2. Furthermore, 54.10%, 72.13%, and 78.69% of participants achieved more than 10%, 7.5%, and 5% TWL, respectively.
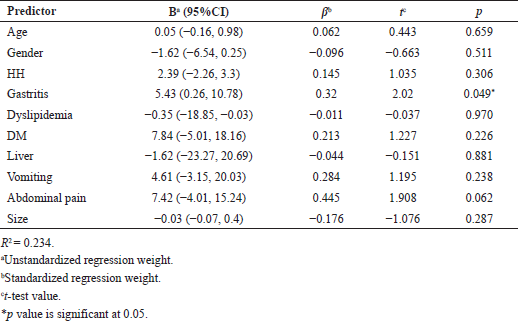
Factors associated with weight loss are shown in Table 3. It was observed that patients with gastritis had higher mean %TWL (B = 5.43, p = 0.045). A positive association, although not significant, was found between abdominal pain after IGB insertion and mean %TWL (B = 7.42, p = 0.062).
DISCUSSION
IGB has gained considerable attention from both treating doctors and patients for its safety, lower risk of complications, relatively faster weight control, and lower cost compared to bariatric surgery. The mechanism of weight reduction involves “stimulation of gastric mechanoreceptors triggering short-acting vagal signals to brain regions implicated in satiety” on top of reducing gastric volume (Tate & Geliebter, 2017). Our results are expected to add to evidence on the safety and complications of using IGB.
 | Figure 1. Spatz3 gastric balloon. [Click here to view] |
 | Table 1. Characteristics of participants. [Click here to view] |
 | Table 2. Baseline and final mean body weight and BMI. [Click here to view] |
The fact that female gender is more represented in this study might be attributed to higher consciousness and importance of body image among females compared to males, leading females to seek weight reduction more often than males (del Mar Bibiloni et al., 2017). Obesity is a well-known risk factor for a spectrum of diseases; however, only a small proportion was found to have dyslipidemia, DM, or liver disease in our sample. The finding that abdominal pain and vomiting are the most common complications is in tandem with other studies (Jamal et al., 2019; Tate & Geliebter, 2017; Yorke et al., 2016). These symptoms are highly expected and reported by most patients as placement of a space-occupying device causes both local irritation and triggering of vomiting center in the brain. Vomiting occurs early in the course of treatment and tends to reduce when the stomach adapts to the IGB (del Mar Bibiloni et al., 2017).
The weight and BMI were significantly reduced by 13 kg and 3.5 kg/m2, respectively (p value < 0.001). This was translated into 12.5% TWL which is considered substantial in reference to other studies. Overall, a greater number (54.1%) of the study samples achieved more than 10% TWL.
 | Table 3. Factors associated with percentage weight loss. [Click here to view] |
The weight reduction achieved in this study is in line with previous research. Several authors reported 12–15 kg weight reduction (da Silva et al., 2018; Fuller et al., 2013; Herve et al., 2005; Ohta et al., 2009; Sallet et al., 2004) and around 10% TWL (Dastis et al., 2009; Dogan et al., 2013; Filip et al., 2019) following IGB procedure. In a larger study by Kotzampassi et al. (2012), out of the 474 patients recruited for IGB, the majority (83%) achieved more than 18% TWL. Similarly, Genco reported a substantial reduction of BMI by around 9 kg/m2 after 6-month balloon placement (Genco et al., 2009).
In concordance with the results of other studies, our findings prove the efficacy and safety of IGB that can be carried out as a day care procedure. The most important argument in support of IGB is maintenance of weight loss after balloon removal. Consulting on lifestyle changes is required to produce long-term results after IGB procedure, as the risk of weight regain is high among obese patients. Several studies have shown reassuring sustainability of weight reduction after removal of the balloon (Carbonelli et al., 2003; Dogan et al., 2013; Herve et al., 2005; Kotzampassi et al., 2012; Mathus-Vliegen & Tytgat, 2005; Sallet et al., 2004).
Given the safety profile, reproducibility, and repeatability of IGB procedure, it is seen as a promising choice in the treatment of obesity that would help to reduce obesity-related morbidities. Guidelines have suggested that 10% TWL should be achieved over a period of 6 months to ensure sustainability and reduction of weight-related morbidities (North American Association for the Study of Obesity et al., 2000).
High proportion of participants was found to have HH which was reported to be a contraindication to IGB placement. However, in our experience, the procedure went smoothly in patients with HH who demonstrated good weight loss outcomes, although lower compared to patients without HH. The effect of having gastritis at baseline and developing abdominal pain after IGB placement on weight reduction might be attributed to the superadded effect of losing appetite and reluctance to eat.
It might be argued that 6 months is the optimal IGB duration whereby serious complications are less likely to happen. In this study, Sptaz3 balloons, known for their longer durability, safety, and adjustability, were used, so the patients managed to complete 1 year of IGB placement which helped in weight reduction. Nonetheless, three of our patients showed early intolerance and were removed from the study. Another three reported balloon rupture, migration, and spontaneous expulsion of the IGB. Those patients were given a 2-week relief and a new balloon was placed back to complete a 1-year follow-up. No serious consequences of expulsion were reported. Our study is consistent with studies that involved 1-year (Brooks et al., 2014; Courcoulas et al., 2017; Machytka et al., 2014; Mathus-Vliegen & Tytgat, 2005; Usuy & Brooks, 2018) and up to 10-months IGB placement duration (El Haddad et al., 2019) in showing favorable safety of the.
Despite all possible precautions taken to report accurate results, some limitations are recognized in our study. First, a larger sample size would make inference about factors associated with weight reduction more plausible. Short duration of follow-up and difficulty to follow up patients after IBG removal might have concealed adequate information on sustainability of weight reduction.
CONCLUSION
IGB is a safe and effective procedure to achieve target weight reduction within a short period of time. Ensuring sustainability of weight reduction requires longer duration of follow-up.
ACKNOWLEDGMENT
The authors would like to thank Associate Professor Dr. Halyna Lugova from the National Defence University of Malaysia for editing the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
FUNDING
None.
REFERENCES
Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res, 2016; 118(11):1723–35. CrossRef
Brooks J, Srivastava E, Mathus-Vliegen E. One-year adjustable intragastric balloons: results in 73 consecutive patients in the UK. Obes Surg, 2014; 24(5):813–9. CrossRef
Buchwald H. (2014). The evolution of metabolic/bariatric surgery. Obes Surg, 24(8):1126–35. CrossRef
Cannon CP, Kumar A. Treatment of overweight and obesity: lifestyle, pharmacologic, and surgical options. Clin Cornerstone, 2009; 9(4):55–71. CrossRef
Carbonelli M, Fusco M, Cannistra F, Andreoli A, De Lorenzo A. Body composition modification in obese patients treated with intragastric balloon. Acta Diabetol, 2003; 40(1):s261–2. CrossRef
Courcoulas A, Dayyeh BKA, Eaton L, Robinson J, Woodman G, Fusco M, Shayani V, Billy H, Pambianco D, Gostout C. Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial. Int J Obes (Lond), 2017; 41(3):427–33. CrossRef
da Silva JR, Proença L, Rodrigues A, Pinho R, Ponte A, Rodrigues J, Sousa M, Almeida R, Carvalho J. Intragastric balloon for obesity treatment: safety, tolerance, and efficacy. GE-Port J Gastroenterol, 2018; 25(5):236–42. CrossRef
Dastis SN, François E, Devière J, Hittelet A, Mehdi AI, Barea M, Dumonceau JM. Intragastric balloon for weight loss: results in 100 individuals followed for at least 2.5 years. Endoscopy, 2009; 41(07):575–80. CrossRef
del Mar Bibiloni M, Coll JL, Pich J, Pons A, Tur JA. Body image satisfaction and weight concerns among a Mediterranean adult population. BMC Public Health, 2017; 17(1):39. CrossRef
Dogan UB, Gumurdulu Y, Akin MS, Yalaki S. Five percent weight lost in the first month of intragastric balloon treatment may be a predictor for long-term weight maintenance. Obes Surg, 2013; 23(7):892–6. CrossRef
Dumonceau JM. Evidence-based review of the bioenterics intragastric balloon for weight loss. Obes Surg, 2008; 18(12):1611. CrossRef
El Haddad, A., Rammal, M. O., Soweid, A., Sharara, A. I., Daniel, F., Rahal, M. A., & Shaib, Y. (2019). Intragastric balloon treatment of obesity: Long-term results and patient satisfaction. Turk J Gastroenterol, 2019; 30(5):461.
Fernandes MAP, Atallah ÁN, Soares B, Saconato H, Guimarães SM, Matos D, Carneiro Monteiro LR, Richter B. Intragastric balloon for obesity. Cochrane Database Syst Rev, 2007; (1). CrossRef
Filip G, Filip S, Dumbrava B. Intragastric fluid filled balloon for weight reduction-A single bariatric center study. Chirurgia (Bucur), 2019; 114(6):739. CrossRef
Fuller NR, Pearson S, Lau NS, Wlodarczyk J, Halstead MB, Tee HP, Chettiar R, Kaffes AJ. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity, 2013; 21(8):1561–70. CrossRef
Genco A, Cipriano M, Materia A, Bacci V, Maselli R, Musmeci L, Lorenzo M, Basso N. Laparoscopic sleeve gastrectomy versus intragastric balloon: a case-control study. Surg Endosc, 2009; 23(8):1849–53. CrossRef
Grandone A, Di Sessa A, Umano G, Toraldo R, Del Giudice EM. New treatment modalities for obesity. Best Pract Res Clin Endocrinol Metab, 2018; 32(4):535–49. CrossRef
Herve J, Wahlen CH, Schaeken A, Dallemagne B, Dewandre JM, Markiewicz S, Monami B, Weerts J, Jehaes C. What becomes of patients one year after the intragastric balloon has been removed? Obes Surg, 2005; 15(6):864–70. CrossRef
Imaz I, Martínez-Cervell C, García-Álvarez EE, Sendra-Gutiérrez JM, González-Enríquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg, 2008; 18(7):841. CrossRef
Jamal MH, Almutairi R, Elabd R, AlSabah SK, Alqattan H, Altaweel T. The safety and efficacy of procedureless gastric balloon: a study examining the effect of elipse intragastric balloon safety, short and medium term effects on weight loss with 1-year follow-up post-removal. Obes Surg, 2019; 29(4):1236–41. CrossRef
Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg, 2012; 22(6):896–903. CrossRef
Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, Suttorp M, Solomon V, Shekelle PG, Morton SC. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med, 2005; 142(7):532–46. CrossRef
Machytka E, Brooks J, Buzga M, Mason J. One year adjustable intragastric balloon: safety and efficacy of the Spatz3 adjustable balloons. F1000Research, 2014; 3(203):203. CrossRef
Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA, Hilton L, Rhodes S, Morton SC, Shekelle PG. Meta-analysis: surgical treatment of obesity. Ann Intern Med, 2005; 142(7):547–59. CrossRef
Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc, 2005; 61(1):19–27. CrossRef
Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet, 1982; 319(8265):198–9. CrossRef
North American Association for the Study of Obesity, National Heart, Lung, and Blood Institute, National Institutes of Health (U.S.), NHLBI Obesity Education Initiative. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health, 2000.
Ohta M, Kitano S, Kai S, Shiromizu A, Eguchi H, Endo Y, Masaki T, Kakuma T, Yoshimatsu H. Initial Japanese experience with intragastric balloon placement. Obes Surg, 2009; 19(6):791–5. CrossRef
Sallet JA, Marchesini JB, Paiva DS, Komoto K, Pizani CE, Ribeiro ML, Ribeiro MLB, Miguel P, Ferraz AM, Sallet PC. Brazilian multicenter study of the intragastric balloon. Obes Surg, 2004; 14(7):991–8. CrossRef
Stavrou G, Tsaousi G, Kotzampassi K. Life-threatening visceral complications after intragastric balloon insertion: is the device, the patient or the doctor to blame? Endosc Int Open, 2019; 7(02):E122–9. CrossRef
Tate CM, Geliebter A. Intragastric balloon treatment for obesity: review of recent studies. Adv Ther, 2017; 34(8):1859–75. CrossRef
Usuy E, Brooks J. Response rates with the Spatz3 adjustable balloon. Obes Surg, 2018; 28(5):1271–6. CrossRef
WHO. Obesity and overweight fact sheet. WHO, Geneva, Switzerland, 2020. Available via https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Wolf AM, Woodworth KA. Obesity prevention: recommended strategies and challenges. Am J Med, 2009; 122(4 Suppl 1):S19–23. CrossRef
Yorke E, Switzer NJ, Reso A, Shi X, de Gara C, Birch D, Gill R, Karmali S. Intragastric balloon for management of severe obesity: a systematic review. Obes Surg, 2016; 26(9):2248–2254. CrossRef
Yu JC. Integrated multidisciplinary treatment modalities for obesity. Zhongguo Yi Xue Ke Xue Yuan Xue Bao, 2010; 32(1):1–3.