INTRODUCTION
According to the World Health Organization (WHO) statistics, an estimate of 350 and 500 million people are suffering with depression and obesity, respectively, and these statics are alarming (World Health Organization, 2000). Worldwide one among six adults obese and approximately each year 2.8 million persons die because of obesity or over weight (Pradeepa et al., 2015). The investigations reported, to achieve and retain weight loss in peoples with obesity or overweight (OW) is arduous; hence, most of the peoples were failure to achieve the goal (Mariman et al., 2012; Rosenbaum et al., 2010; Sumithran et al., 2013). In addition, obesity and depression had contributed to the burden to the public including rise in the healthcare costs, morbidity and mortality (Chapman et al., 2005).
The conflicting outcomes between among obesity (OB) and depression (De Wit et al., 2010; Fabricatore et al., 2011), but some have not supported this conclusion in all subjects (Bin Li et al., 2004; Chang et al., 2012). The management of obesity in patients with depression is a difficult task. However, a moderate decrease in the body weight had contribution positively to improve the depressive symptoms (Jantaratnotai et al., 2011). The aim of this work was to investigate the burden of depression and type-2 diabetes between OW and obese subjects from the Clinical trial of weight loss intervention.
METHODOLOGY
Study population
It was an observational study, the participants were enrolled after taking the informed consent form, and the study was enrolled in clinical trial registry, Reg. No. CTRI/2020/02/023329. Totally 165 subjects of either sex, with aged range from ≥19 years and less than 65 years with non-diabetic OB or OW data were collected at the first visit. The participants were screened for vital and medical examinations and biochemical parameters, such as lipid profile, homeostatic model assessment for insulin resistance (HOMA-IR) were measured at the Department of Endocrinology and Metabolism, India. Before data collection, all participants given informed consent, and the trial protocol was approved by the Institutional Ethics Committee of Endo-Life Specialty Hospital, Guntur, India.
Measurements
Anthropometric measurements
The height and weight of the subjects was measured in centimeters and kilograms using a digital scale. The body mass index (BMI) of the subjects was calculated as the weight of the person in kilograms divided by the square of the height. The OW and obesity subjects were categorized as Class-I, II, and III according to WHO standards (World Health Organization, 2000) as follows: OW (BMI kg/m2 between 25.0and 29.9), and obese (BMI >30.0), obese class-I (30.0–34.9), obese class-II (35.0–39.9) and obese class-III (≥40.0). The waist circumference was measured by using normal conventional (Non-Elastic) measuring tape.
Depression outcomes
In our study, the screening of depression was carried out by Patient Health Questionnaire (PHQ-9) scale. The PHQ-9 scale was used to screen the depressive symptoms (Maurer, 2012), and it is validated (Nease, 2003) and easy to complete by the individuals. Responses are scored from 0 to 3, representing “not at all,” “several days,” “more than half the days,” and “nearly every day,” respectively, with total scores ranging from 0 to 27. Scores ≥ 10 are usually used to describe the depression in clinical studies.
Diabetic risk outcomes
Assessment of diabetic risk was carried out by using a patient self-assessment diabetes screening score which contains total seven questionnaires. If the total score is greater than or equal to five, the subjects are at increased risk for having type-2 diabetes mellitus (T2DM) (American Diabetic Association, 2008). Insulin resistance (IR) was measured with HOMA-IR. This method is used for not only for assessing β-cell function but also for IR from basal (fasting) glucose and insulin. HOMA-IR was calculated using the following formula (Matthews et al., 1985). HOMA-IR = fasting plasma glucose (mg/dl) × fasting insulin (μU/ml)/405. Based on literature (Aydin, 2014; Calori et al., 2011) a cutoff value of ≥2.5 was selected for HOMA-IR to find IR.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) version 22 (IBM Corporation, Chicago, IL) was used for conducting data entry and statistical analysis whereas the frequencies and percentages were determined using descriptive analysis for categorical variables. Central tendency and dispersion were calculated for quantitative variables. Independent sample t-test and Chi-square test or Fisher’s exact test were employed for comparing continuous variables and categorical variables. The results of risk were expressed in 95% confidence intervals (CI) and odds ratio (OR). The level of significance was fixed at p < 0.05 for all the analysis.
RESULTS
The characteristics of the 165 participants (63, male and 103 women) and the participants were stratified by presence of OW (<30 kg/m2) and obesity (≥ 30 kg/m2). Participants of gender, age, height and lipid profile were not significant (p < 0.05) among OW subjects as compared to OB. (Table 1). The continuous variables (Supplementary Table S1) are statistically non-significant and the mean values were nearly equivocal among the both genders, but the mean values of low density lipoprotein (LDL), T2DM risk score was high in males. Table 2 depicts the participants with PHQ-9 score were stratified by moderate to severe depression (≥10) and the non depression to mid depression (<10). Except alcoholics (p < 0.05), remaining all characteristics were significantly (p > 0.05) associated with depressive subjects (PHQ-9 score ≥ 10). The odds of depression were 3–5 times more among the subjects with obesity, hypertension, unmerited status, and smokers, respectively.
Table 3 represents the risk of T2DM, the subjects with low income and alcoholics were not have significant association with T2DM (p < 0.05), remaining all parameters were associated (p > 0.05) with T2DM. The proportionate analysis (Chi square test) explains the highest significant association between BMI and depression (p < 0.05) and significant association between BMI and T2DM (p > 0.05) (Tables 4 and 5). We were also analyzed the prediction of risk of diabetes among the obese subjects with depression (Table 6). The subjects were stratified into PHQ-9 <10 score and ≥10 score respectively. The IR and DM risk (≥ 5) score were not statistically associated with depression.
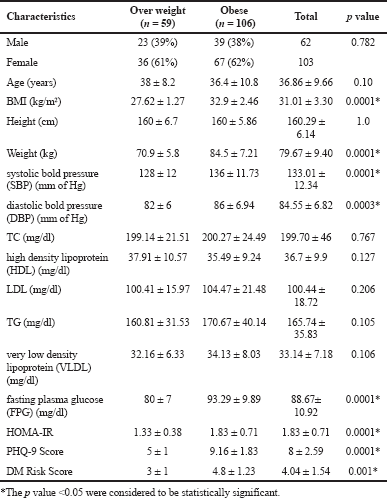 | Table 1. Characteristic of the OW and obese trial participants. [Click here to view] |
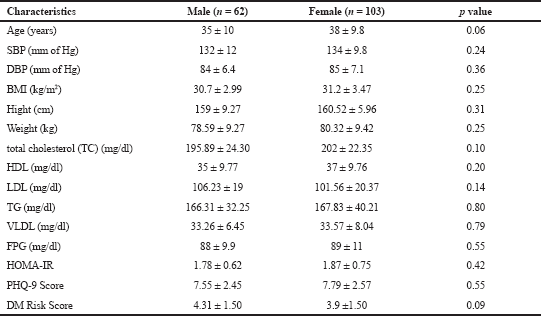 | Table S1. Risk factors among male and females. [Click here to view] |
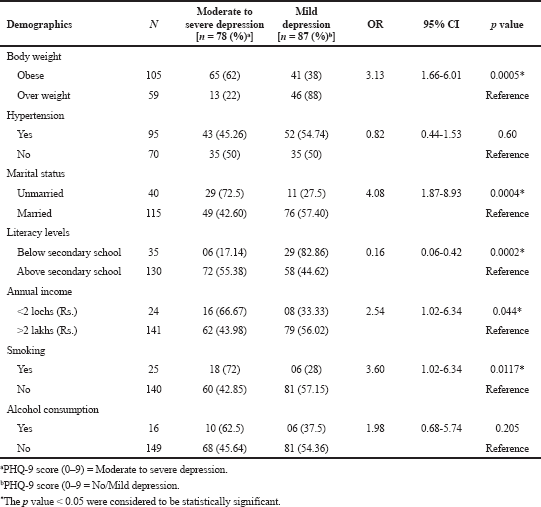 | Table 2. Estimated ORs of depression among study the study subjects. [Click here to view] |
DISCUSSION
OB and OW are common morbidities with overlapping pathophysiology whose co-existence is associated with adverse health outcomes. In fact, depression was improved with successful intervention in many OB or OW subjects after reducing their weight (Jantaratnotai et al., 2017). Present study demonstrates the risk of depression and type-2 diabetes among OW and obese subjects, especially pronounced in the subjects with unsuccessful efforts to reduce their body weight. Additionally we examined, weather the obesity and depression additively associated with risk of diabetes.
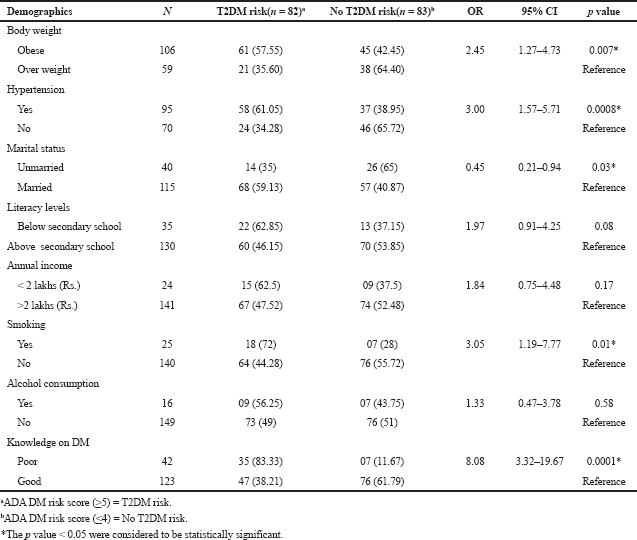 | Table 3. Estimated ORs of T2DM among study the study subjects. [Click here to view] |
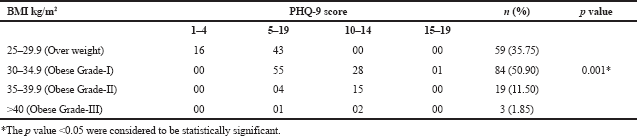 | Table 4. Association between BMI and PHQ-9 score. [Click here to view] |
The mean score of HOMA-IR and PHQ-9 score were significantly high in obese subjects (Table 1) and OW, OB, hypertension, and alcohol consumption were not risk factors for depression in our study subjects (Table 2). The large number 103 (62.43%) of subjects has mild risk of depression and 46 (27.97%) had moderate depression (Supplementary Table S3). The mean PHQ-9 scale was found to be 7.55 ± 2.45 in males and 7.79 ± 2.57 were observed in females (Supplementary Table S1). The similar results were observed in (Cui et al., 2018; Rathee, 2017; Stunkard et al., 2003). The number of subjects with PHQ-9 score (depression severity score) <10 was observed in 44 (37.28%) males and 18 females (38.30%). The depression severity score ≥10 was observed 74 (62.72) in females and 29 (61.70) in males, which indicates the high risk of depression in females. The subjects with <10 PHQ-9 score have the mean BMI of 29.9 ± 2.7 and ≥10 had 33.90 ± 2.88 BMI, respectively. These results suggest, the likely hood of depression was more common in OB individuals (Table S2). Similarly, being OW (OR: 1.39. 95% CI: 1.03–1.87) is a noticeable risk factor for depression (Smarr et al., 2011). The chi-square analysis shown in Table 4 has significant association between BMI and depression score (p = 0.001). Significant relation (p = 0.048) was observed in between PHQ-9 score and DM risk score, and inverse relation with HOMA-IR (Supplementary Tables S6 and S5). The mean FPG value is high 93.70 ± 10.63 among the subjects with ≥10 PHQ-9 score. The similar study conducted in rural China, reported depressive symptoms were negatively associated with metabolic syndrome (MetS) (Yu et al., 2017). The BMI verses PHQ-9 and DM risk were statistically significant (p = 0.0001) (Tables 4 and 5) among our subjects. Recent research indicates that there is a longitudinal association between obesity and depression reported that a higher BMI tended to cause depression and vice versa (Konttinen et al., 2014). The insignificant relation between HOMA-IR and PHQ-9 score (p = 0.31) was showed in (Supplementary Table S4), but the mean value of HOMA-IR was high in ≥10 PHQ-9 score group, which indicates IR is likelihood to associated for depression. The mean HOMA-IR values were equivocal in both genders (Supplementary Tables S1 and S2). In Indian adolescents, the IR was amplified gradually from normal weight to obese in both genders (Singh et al., 2013). There is an inverse relationship of the female gender with obesity and they were more likely to develop MetS of the atherosclerotic risk in community study (Bradshaw et al., 2013).
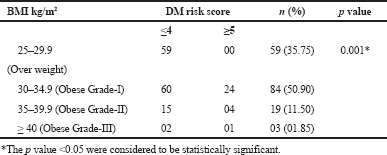 | Table 5. Association between BMI and DM risk score. [Click here to view] |
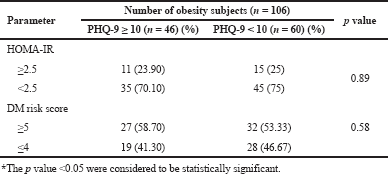 | Table 6. Predicting the risk of diabetes among obese subjects with depression. [Click here to view] |
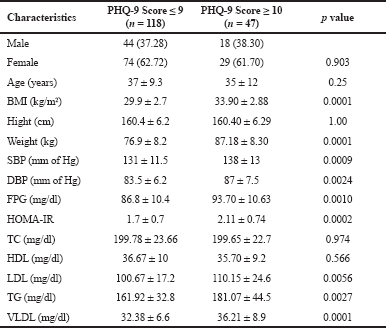 | Table S2. Comparison of variables among the subjects with PHQ-9 Score <10 and ≥ 10. [Click here to view] |
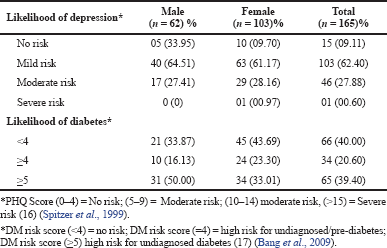 | Table S3. Severity of depression and DM risk among the study subjects. [Click here to view] |
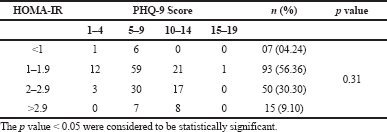 | Table S4. Association between HOMA-IR versus PHQ-9 score. [Click here to view] |
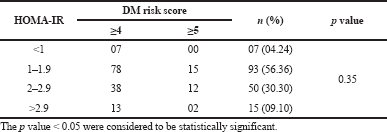 | Table S5. Association between HOMA-IR versus DM risk score. [Click here to view] |
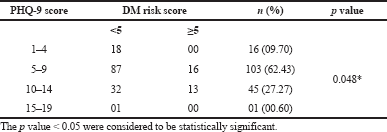 | Table S6. Association between PHQ-9 score versus DM risk score. [Click here to view] |
A strict lifestyle intervention such as regular physical exercise with low carbohydrate and hypo-caloric diet are the cornerstone recommendation to reverse the metabolic complications. Our results have noteworthy implications and may be used for policy formulation as obesity and diabetes has significant health and economic burden. Hence, there is an imperative need to have policies that discourse rising OB and OW prevalence in India.
CONCLUSION
In contrast to our findings, the present study revealed, OW or obesity is jeopardizing to produce pre-diabetic or diabetes, along with sociodemographic risk factors. The gender difference was not significantly associated with depression among the study participants. Therefore, interventions for strengthening the health system through appropriate physical exercise and well-balanced diet and education, would help to reverse the normal BMI but also, overcome depression levels too. Practicing and imbibing such regimen in our daily life style could play crucial role in overcoming metabolic syndrome and their associated complications.
Future perspectives
After end of the trial (CTRI/2020/02/023329), we will further assess the progress of depression, diabetes risk, and vitals parameters.
ACKNOWLEDGMENT
The authors are highly thankful to Endo-life specialty hospital team for providing extensive help and support to acquire the specific data of screened subjects. The authors also extend their appreciation to chairman of Endo-life hospital, Guntur.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
FUNDING
Self funded study.
COMPLIANCE WITH ETHICAL STANDARDS
The study procedure was approved by the Institutional Ethics Committee, Dated:27.02.2020 (IEC/19/Nov/155/65) of Sri Ramachandra Institute of Higher Education and Research (Deemed to be University), Chennai, affiliated Endo-life Specialty Hospital, Guntur, in accordance with the Declaration of Helsinki (Trial registration number: CTRI/2020/02/023329 Dated: 14.02.2020). Informed consent was obtained from all subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
American Diabetic Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care, 2008; 41(Supplement 1):S13–27; doi:10.2337/dc18-S002 CrossRef
Aydin E, Ozkokeli M. Does homeostasis model assessment of insulin resistance have a predictive value for post-coronary artery bypass grafting surgery outcomes? Braz J Cardiovasc Surg, 2014;29(3):360–6. CrossRef
Bang H, Edwards AM, Bomback AS, Ballantyne CM, Brillon D, Callahan MA, Teutsch SM, Mushlin AI, Kern LM. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med, 2009; 151(11):775–83. CrossRef
Bin Li Z, Yin Ho S, Man Chan W, Sang Ho K, Pik Li M, Leung GM, Hing Lam T. Obesity and depressive symptoms in Chinese elderly. Int J Geriatr Psychiatry, 2004; 19(1):68–74. CrossRef
Bradshaw PT, Monda KL, Stevens J. Metabolic syndrome in healthy obese, overweight, and normal weight individuals: the atherosclerosis risk in communities study. Obesity, 2013; 21(1):203–9. CrossRef
Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, Mannino S, Crosignani P, Bosi E, Luzi L, Ruotolo G. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona study. Diabetes Care, 2011; 34(1):210–5. CrossRef
Chang HH, Yen ST. Association between obesity and depression: evidence from a longitudinal sample of the elderly in Taiwan. Aging Ment Health, 2012; 16(2):173–80. CrossRef
Chapman DP, Perry GS, Strine TW. The vital link between chronic disease and depressive disorders. Prev Chronic Dis, 2005; 2(1):1–10. PMID: 15670467
Cui J, Sun X, Li X, Ke M, Sun J, Yasmeen N, Khan JM, Xin H, Xue S, Baloch Z. Association between different indicators of obesity and depression in adults in Qingdao, China: a cross-sectional study. Front Endocrinol, 2018; 9:549. CrossRef
De Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res, 2010; 178(2):230–5. CrossRef
Fabricatore AN, Wadden TA, Higginbotham AJ, Faulconbridge LF, Nguyen AM, Heymsfield SB, Faith MS. Intentional weight loss and changes in symptoms of depression: a systematic review and meta-analysis. Int J Obes, 2011; 35(11):1363–76. CrossRef
Jantaratnotai N, Mosikanon K, Lee Y, McIntyre RS. The interface of depression and obesity. Obes Res Clin Pract, 2017; 11(1):1–0.
Konttinen H, Kiviruusu O, Huurre T, Haukkala A, Aro H, Marttunen M. Longitudinal associations between depressive symptoms and body mass index in a 20-year follow-up. Int J Obes, 2014; 38(5):668–74. CrossRef
Mariman EC. Human biology of weight maintenance after weight loss. Lifestyle Genom, 2012; 5(1):13–25. CrossRef
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985; 28(7):412–9. CrossRef
Maurer DM. Screening for depression. Am Fam Physician, 2012; 85(2):139–44.
Nease DE, Malouin JM. Depression screening: a practical strategy. J Fam Pract, 2003; 52(2):118–26.
Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP, Dhandania VK, Madhu SV, Rao PV, Geetha L, Subashini R. Prevalence of generalized & abdominal obesity in urban & rural India-the ICMR-INDIAB Study (Phase-I)[ICMR-INDIAB-3]. Indian J Med Res, 2015; 142(2):139. CrossRef
Rathee N. Obesity and depression: are they related? Indian J Health Wellbeing, 2017; 8(12):1472–5.
Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes, 2010; 34(1):S47–55. CrossRef
Singh Y, Garg MK, Tandon N, Marwaha RK. A study of insulin resistance by HOMA-IR and its cut-off value to identify metabolic syndrome in urban Indian adolescents. J Clin Res Pediatr Endocrinol, 2013; 5(4):245. CrossRef
Smarr KL, Keefer AL. Measures of depression and depressive symptoms: beck depression inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), geriatric depression scale (GDS), hospital anxiety and depression scale (HADS), and patient health Questionnaire-9 (PHQ-9). Arthritis Care Res, 2011; 63(S11):S454–66. CrossRef
Spitzer RL, Kroenke K, Williams JB, Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient Health Questionnaire. JAMA, 1999; 282(18):1737–44. CrossRef
Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry, 2003; 54(3):330–7. CrossRef
Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin Sci, 2013; 124(4):231–41. CrossRef
World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia, Sydney, Australia, 2000. Available via https://apps.who.int/iris/handle/10665/206936.
Yu S, Yang H, Guo X, Zheng L, Sun Y. Metabolic syndrome and depressive symptoms among rural Northeast general population in China. BMC Public Health. 2017;17(1):1-9. CrossRef