This article is retracted. Please read the retraction notice available here: http://dx.doi.org/10.7324/JAPS.2023.1312.retraction
INTRODUCTION
The most prevalent form of dementia is Alzheimer’s disease (AD) which expresses the features of memory loss and behavioral disturbance (Smith, 2017). Aging, especially people aged 65 years and above are at the risk of various diseases including AD. Every year nearly 1,275 new cases per 10,000 individuals are diagnosed with AD (Querfurth and LaFerla, 2010), and this has become challenging to nursing care facilities and financial burden for care givers.
The increased acetylcholinesterase enzyme (AChE) activity, alteration of acetylcholine (ACh) receptors, or decreased production of ACh in central cholinergic system are all considered as significant hallmarks of AD (Anand et al., 2014). The treatment regimens were developed to target the pathogenesis of AD, but their use is restricted due to unavoidable side effects. The donepezil is being used as a first-line treatment drug; it has undesirable and unavoidable peripheral anticholinergic side effects. Hence, health care professionals are keen to find alternative therapies (Citron, 2010).
Many herbal drugs including extracts are focused to treat AD based on potential effects such as anti-oxidants, AChE enzyme inhibition, secretase enzymes responsible for producing Aβ, chelating heavy metals, or Aβ degradation mechanisms (Eckert, 2010).
The plant genus Sida L. is well documented in Ayurveda, an ancient Indian system of medicine. Around 17 species that occur in India are reported traditionally in Rasayana to treat various ailments including degenerative and musculoskeletal disease (Trikamji, 1984). Sida rhombifolia L. (S. rhombifolia) is a perennial plant that belongs to the family Malvaceae commonly known as arrowleaf sida. The various extracts of S. rhombifolia have been identified as an anti-inflammatory, anti-arthritic, and hepatoprotective (Subramanya et al., 2015). It is also reported as an antidiabetic and poses anti-oxidant property (Chaturvedi and Kwape, 2015).
Traditionally in India, the hot aqueous extracts of dried leaf and root of the S. rhombifolia are used as an aphrodisiac, tonic and is used to treat heart and nervous diseases (Girón et al., 1991).
However, there are limited scientific reports related to claim the effect of S. rhombifolia in the management of AD. Hence, this study was designed to evaluate the hydro-ethanolic extract of S. rhombifolia L. on scopolamine (SCO) induced amnesia in rats.
MATERIALS AND METHODS
Reagents
Quercetin, Donepezil Hydrochloride, Tris-HCl, 2,2-diphenyl-1-picrylhydrazyl (DPPH), H2O2, 5,5'-dithiobis-(2-nitrobenzoic acid), AChE, Acetylthiocholine iodide was procured by Sigma Aldrich, St. Louis, MO. Gallic acid, Ethanol (Hi-media, Amritsar, India), SCO hydrobromide (Vital Laboratories; Vapi, India), potassium dihydrogen phosphate, disodium hydrogen phosphate (SD fine chem. Ltd, Chennai, India), Folin-coicalteau phenol reagent (Fisher scientific, Vantaa, Finland).
Plant collection and authentication
The S. rhombifolia L. the whole plant was collected from the botanical garden, Dharwad University of Karnataka, India. Authenticated by botanist Dr. Harsh V. Hegde (Scientist E) at Indian Council of Medical Research-NITM, Belagavi, India. A voucher specimen (RMRC-1399) was deposited at the herbarium of ICMR-NITM, Belagavi, India.
Method of extraction
The whole plant was washed thoroughly using water and dried at room temperature for 15 days. Using a mechanical blender, plant parts were powdered. The hydro-alcoholic extract was prepared by three-time maceration using ethanol-water (70:30) for 7 days; further Soxhlet extraction was carried out using ethanol (95%) for 8–12 hours. This method allows isolating desirable phytoconstituents. Then extract was cooled, concentrated by the evaporation. Both extracts were combined and stored in a refrigerator until further use (Khanal and Patil, 2020).
Water and alcohol soluble extractive values and, ash values of crude powder were determined to assess the purity, quality as well as to detect adulterations present if any (Kokate, 2017). The S. rhombifolia extract (SRE) was screened for phytoconstituents such as tannins, phenols, flavonoids, saponins, glycoside, alkaloids qualitatively (Oshadie et al., 2017).
Total flavonoid content and phenolic content
The flavonoid content of SRE was quantified and quercetin was used as a standard sample (McDonald et al., 2001). Phenolic compounds of SRE were tested by a spectrophotometric method using Folin–Ciocalteau Reagent (Pawar et al., 2016), and values were reported as mg/g Gallic Acid Equivalent (GAE).
DPPH and H2O2 scavenging activity of SRE
The anti-oxidant activity of SRE was determined by the DPPH scavenging assay method proposed by Teh et al. (2013), and the ability to eliminate hydrogen peroxide was determined by method Ruch et al. (1989). Ascorbic acid was used as standard drugs with three independent tests were carried out to assure the precision of results.
In-vitro estimation of AChE inhibition
Acetylcholinesterase activity of SRE by in vitro was measured by 96 well microplate assay using Ellman’s method. Donepezil was used as a standard control.
Percentage of enzyme inhibited was reported in triplicates to calculate IC50 values by linear regression analysis (Dhanasekaran et al., 2015).
In vivo experimental protocol
Ethical approval and experimental animals
Ethical consent was approved from the Institutional Animal Ethical Committee from KLE College of Pharmacy, Belagavi, an in vivo analysis was carried out (KLECOP/CPCSEA- Res No.221/Po/Res/2000/CPCSEA, Res.25-13/10/2018). Rats were procured by CPCSEA-registered vendor in vivo Biosciences; Bangalore, India.
The healthy Wistar rats of either sex (18 months) weighing 180–280 g used for this study, under standard laboratory conditions rats were housed in cages by grouping them into six in each with the maintenance of natural light and dark condition. Acclimatization was made before the experiment begins. CPCSEA guidelines were followed for experimentation. Rats were fed with standard rat chow pellet and water ad libitum.
Acute oral toxicity study
Organization for Economic Co-operation and Development guidelines, 423 (OECD, 2002) was used to determine the safety and adverse effects of SRE to confirm safe doses for future study. The SRE 2,000 mg/kg orally administered in a single dose to the rats later observed for the next 14 days. After 14 days, there were no side effects, no mortality was observed. Based on the acute oral toxicity study, the geometrical series doses such as 1/10th 1/20th, and 1/40th doses were selected as testing doses.
Preparation of test samples
The SR extract was suspended in 0.5% Sodium carboxy methyl cellulose (CMC) before administration.
Study design
The experimental rats were randomly selected (n=6) and divided them into six groups. Group I: Normal control (administered with normal saline, through i.p route); SCO (1 mg/kg, i.p) was administered for 30 days to group II - VI to provoke cognitive impairment. Group III- Standard control (Donepezil 3mg/ kg, p.o); Group IV- SRE 100mg/kg; Group V- SRE 200mg/kg; Group VI- SRE 400mg/kg were administered orally (test controls). The test doses of donepezil and SRE were given 30 min after SCO administration to rats from 16-30th day to intervene SCO-induced amnesia.
Screening models for amnesia
Morris water maze task
It has a circular pool filled with water (the white dye used to make it opaque), pool was divided into four equal quadrants. Platform immersed 2 cm below the base level (10 × 10 cm) and kept at the center of the Q4 quadrant throughout the testing sessions.
Training trial
Each rat was put in the pool for the 60 seconds 1 day before the test; this free swim allowed the rat to become accustomed to the training setup. Suppose in the 120 seconds, the rat did not find the platform then it was manually guided to reach the platform and allowed to rest on the platform for the 30 seconds.
Acquisition trail
During each day of the study, starting location was randomized but stayed the same for all rats in each experiment. The rat was allowed in the pool with its head pointing toward the sidewall at each trial and given the 90 seconds to search for a hidden platform.
Retention trail
On day 30, the platform was taken away from the position, then time spent to the search of the missing platform is considered as a probe trial referred to as retrieval of memory (Morris, 1984).
Elevated plus-maze
It has four arms (two closed; two open arms where maze was elevated above the floor, and arms extended from a central platform).
Acquisition trial
On day 1, at open arm; the rat was individually placed facing away from a central platform. The acquisition test was considered as transfer latency (TL) by the observation of time taken for the rat to move from open to the closed arm.
Retention of memory of rats was recorded on day 30 using TL and percentage time spends in the open arm (Jafarian et al., 2019).
Passive avoidance test
The apparatus has light and dark compartments in the shuttle box. To separate the compartment, guillotine door is adjusted either lowered or raised by the researcher. The floor has stainless steel rods in which the shock generator has connected dark compartments to transmit shock if the rat stepped into a dark area. Rats were habituated before trial later on day 1 they were placed in the light compartment; the guillotine door was opened for the rat to enter the dark compartment during which foot shock was given for 2 seconds, which is considered as step-through latency as acquisition test. Retention trial was recorded on day 30 including time spent in the dark compartment (Rezvani-Kamran et al., 2017).
Collection of brain sample
After screening behavioral models, animals were
sacrifced with a high dose of anesthetic ether. Brain samples were collected. Later biochemcal parameters such as AChE, β amyloid1–42, Glutathione (Reduced), and lipid peroxide enzyme levels were evaluated.
Acetylcholinesterase inhibition assay
The effect of SRE on AChE level can be detected by the inhibition of AChE level in the brain homogenate as explained by (Ellman et al., 1961). To quantify the rate of moles of substrate-hydrolyzed per min/grams of the tissue, absorbance was noted for 5 minutes at 412 nm.
Aβ1-42 content in brain tissue
The assessment of rat β amyloid1–42 level was done by the Enzyme linked immunosorbent assay sandwich kit manual (Bioassay Technology Laboratory, China). Phosphate buffer (7.4) was used to homogenize the rat brain later, centrifuged at 5,000 G to collect supernatant which was used for the detection of β amyloid1–42. In parallel to the test samples, standard curve analysis was run. A multiscan spectrum spectrophotometer was utilized to check the absorbance at 450 nm OD (Thermo scientific, Multiscan GO); the readings were all carried out in triplicate (Malabade et al., 2015).
Estimation of reduced glutathione
Protocol (Ellman, 1959) was followed to estimate reduced glutathione in brain homogenate. The development of yellow color signifies glutathione (GSH) level which was expressed as nm/mg protein.
Lipid peroxidase assay
The quantity of lipid peroxidation was measured according to the procedure followed by (Wills, 1966). Amount of malondialdehyde formed due to reaction with thiobarbituric acid. Absorbance was measured at 532 nm and expressed as nm/mg protein
Histopathological studies
Rat brain tissue was collected from groups. Neutral buffered formalin 10% was used as a fixation reagent. Processing was done to obtain 4 μm paraffin-embedded sections. Hematoxylin and Eosin staining reagent was used to examine under microscope 40× (Dhalwal et al., 2007).
Statistical analysis
Graph Pad Prism (version 5.0) was used for the data analysis; Analysis of variance and Tukey’s post hoc test using were considered. Statistical difference was considered in terms of mean ± standard errors of the mean (SEM) and p < 0.05. To compute IC50 linear regression, the analysis was used.
RESULTS
The water and alcohol soluble extractive values of the crude powder were obtained as 3.8% and 1.5%, respectively; total ash and acid insoluble ash value were found to 5.9% and 1.5% (W/W). The percentage yield of S. rhombifolia hydroethanolic extract was found to be 10.5% W/W. Phytoconstituents such as flavonoids, saponins, phenols, glycosides, tannins, and steroids were present. The quantitative analysis such as total flavonoid content, total phenolic content of SRE was found to be 87.78 ug/ml quercetin equivalent and 120.77 ug/ml GAE, respectively.
The SRE inhibited DPPH at IC50 value of 175.74 ± 10.51 μg/ml, p < 0.01 compared to ascorbic acid 35.73 ± 0.85 μg/ml. The H2O2 was inhibited at IC50 of 55.84 ± 0.636 μg/ml; p < 0.01 when compared with ascorbic acid 47.81 ± 0.66 μg/ml.
Effect of SRE on AChE inhibition by an in-vitro method
The SRE showed to inhibit to inhibit AChE at IC50 of 53.11 ± 2.39 μg/ml as compared to Donepezil 2.23 ± 0.141 μg/ml.
Effect of SRE on spatial memory
Figure 1 explains escape latency in seconds using the Morris Water Maze task to evaluate the restoration of spatial memory. Figure 1a shows training trials for 7 days before commencing treatment to rats, which expressed significantly decreased escape latency on day 7 as compared to day 1 of the training trial. Figure 1b shows the donepezil and SRE treatment to respective groups intervened and showed a decrease in escape latency significantly compared to SCO-induced rats. Figure 1c shows that the SCO induction elevates escape latency significantly (p < 0.001) as compared to normal control, donepezil (3 mg/kg) and SRE at 100, 200, and 400 mg/kg treatment expressed decreased escape latency significantly (p < 0.001) when compared to rats exposed to SCO. Figure 1d represents a probe trial in which SCO-induced rats showed decreased time spent in the target quadrant as compared to normal control rats; while donepezil and SRE treated rats revealed increased time spent at the target quadrant significantly p < 0.001 compared to SCO-induced rats. These observations indicate improvement in spatial memory.
Effect of SRE on short term learning and memory
Figure 2a shows the effect of SRE on the TL and Figure 2b expresses percentage time spent in open arms following 5 minutes of exploration in an elevated plus-maze. The SCO-induced rats showed increased TL as compared to normal group rats. The donepezil (3 mg/kg) and SRE treatment at 100, 200, and 400 mg/kg doses have shown a marked reduction in TL in contrast to SCO-induced rats.
SCO induced rats markedly (p < 0.001) reduced the percentage of time spent by the rats in the open arms compared to normal control rats. However, donepezil, SRE at 100, 200, and 400 mg/kg showed increased time spent percentage in the open arms, respectively, in comparison to SCO-induced rats indicate retention of short term memory.
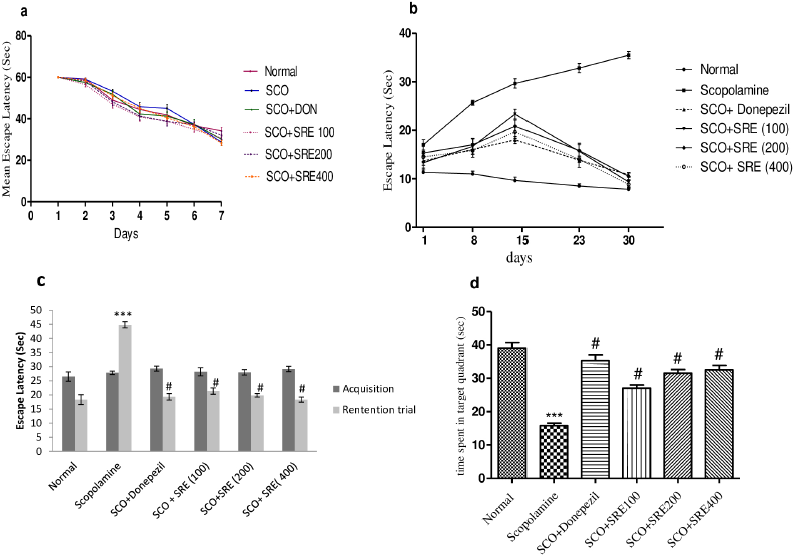 | Figure 1. (a) Escape latency (sec) of rats during training trails. (b) Escape latency expressed by rots for every 7 days of treatment, (c) probe trial (time spent to reach target quadrant) compared to acquisition trial, (d) time spent in target quadrant by rats on probe trial: @ as compared to normal control group, ***compared to scopolamine control group. p < 0.001 was considered as statistically significant. Data were represented as mean ± SEM (n = 6). [Click here to view] |
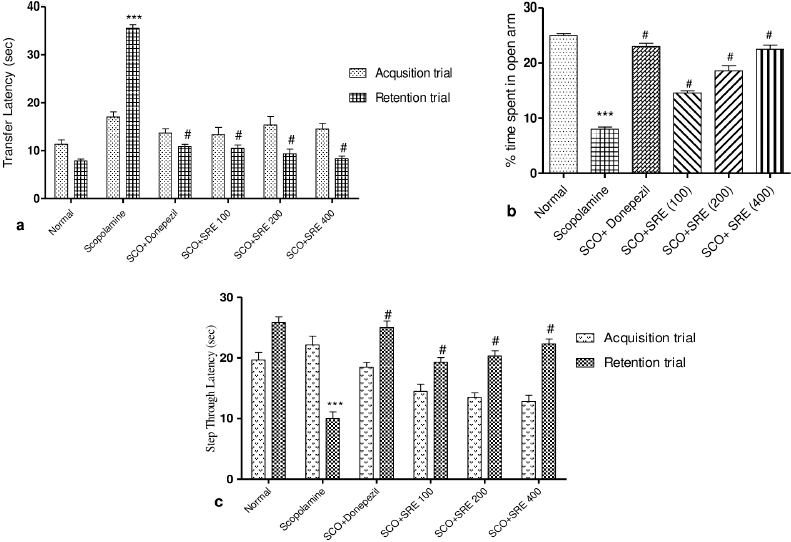 | Figure 2. (a) Transfer latency (sec) of rats on elevated to plus maze, (b) percentage time spent by rats in open arm of EPM, (c) step through latency of rats in passive avoidant test: ***as compared to normal control group, # compared to scopolamine control group. p < 0.001 was considered as statistically significant. Data were represented as mean ± SEM (n = 6). [Click here to view] |
Effect of SRE on fear aggravated related behavior
Memory Retention trial on day 30, the SCO administered rats showed significant (p < 0.001) decreased step-through latency which implies an increase in fear aggravated behavioral task. But, treatment groups viz, donepezil (3 mg/kg), SRE-100, 200, and 400 mg/kg doses showed increased step-through latency indicates the improved non-declarative memory as shown in Figure 2c.
Effect on AChE enzyme level in brain tissue
The rats treated with SRE at 100, 200, 400 mg/kg significantly inhibited Acetylcholinesterase level by (15.27 ± 1.87, 10.62 ± 0.22, 8.71 ± 0.16; p < 0.001), respectively, and Donepezil treated group also showed decreased AChE level (8.09 ± 0.75; p < 0.001) as compared to amnesia induced rats (24.71 ± 0.92). While SCO-induced rats increased AChE enzyme activity (24.71 ± 0.92; p < 0.001) significantly as compared to normal rats (7.26 ± 0.45) as shown in Figure 3a.
β-amyloid1-42 content in brain tissue
In SCO-induced rats, amyloid β1–42 was substantially increased in comparison to normal control (655.7 ± 35.68 pg/ml; p < 0.001). Although a major significant decreased β-amyloid1–42 content was observed in rats treated with SRE, and donepezil (p < 0.001) relative to SCO-induced rats, as shown in Figure 3b.
Antioxidant activity of SRE
Effect of SRE on malondialdehyde content
SCO-induced rats recorded notable changes in malondialdehyde levels (39.53 ± 3.05; p < 0.001) relative to normal group rats (12.8 ± 2.86). Rats administered with SRE (14.96 ± 3.16, 13.89 ± 3.05, 12.29 ± 2.41; p < 0.001) and Donepezil (11.75 ± 1.97; p < 0.001) significantly reduced malondialdehyde (MDA) level which indicates decreased lipid peroxidation in rat brain as compared to SCO-induced rats as shown in Table 1.
Effect of SRE on glutathione (reduced) level
The SCO administered rats showed significantly decreased GSH level (0.53 ± 0.05; p < 0.001) in comparison to normal control rats (1.39 ± 0.05). The rats treated with Donepezil (1.23 ± 0.11; p < 0.001), SRE significantly increased (1.07 ± 0.12, 1.20 ± 0.07, 1.31 ± 0.05: p < 0.001) GSH levels as compared to SCO-induced rats as shown in Table 1.
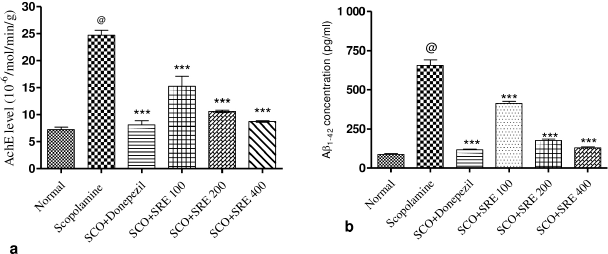 | Figure 3. (a) AchE levels in rat brain homogenate, (b) Aβ1–42 concentration (pg/ml) in rat brain: @ as compared to normal control group, ***compared to scopolamine control group. p < 0.001 was considered as statistically significant. Data were represented as mean ± SEM (n = 6). [Click here to view] |
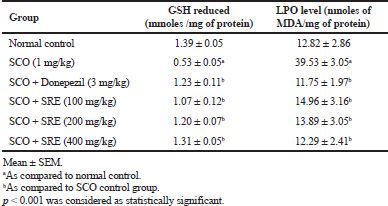 | Table 1. Anti-oxidant activity of SRE in rat brain homogenate. [Click here to view] |
Histopathology of rat brain tissue
SCO-induced rats reflected extreme neurodegeneration with perivascular edema, neuronophagia, and pyknotic nucleus in cortex and hippocampus regions of rat brain at 40× magnification. As observed in Figures 4 and 5, groups treated with donepezil and SRE protected neurons and demonstrated moderate congestion relative to the disease group.
DISCUSSION
The current study dealt to investigate S. rhombifolia hydroethanolic extract on SCO-induced amnesia by evaluating its effect on the short term, spatial memory, and fear aggravated behavior followed by estimation of AChE and antioxidants such as glutathione (reduced) and lipid peroxidation biomarkers using rat brain homogenate. Rat brain histopathology experiments have been performed on the cerebral cortex and hippocampus portions.
The most widely used free radical scavenging activity of potential extracts is tested by DPPH assay (Oroian and Escriche, 2015). The present study exhibits DPPH scavenging activity and this was as per Dhalwal et al. (2007) reports, who explained S. rhombifolia ethanolic extract free radical scavenging activity.
The SRE showed anti-oxidant potential by scavenging hydrogen peroxide; which is supported by a study observed by Chung et al. (1998) who noticed that the phenolic compounds found naturally in herbs are effective to converse deleterious effects of reactive oxygen species.
This research strongly directs us to assess the actions of S. rhombifolia in SCO-induced amnesic rats because an in vitro study reported that S. rhombifolia could inhibit AChE. These findings were correlated with previous reports (Mah et al., 2017).
Memory loss is a common characteristic of the elderly population; to reflect this disease, aged animals may be used as a natural dementia model that has been shown to develop neuropathology, oxidative stress accompanied by memory failure similarly found in patients affected with AD (Li et al., 2016).
SCO has been chosen as an amnesia-causing agent in current research because according to previous studies, SCO injection increases the function of AChE, which metabolizes a significant amount of ACh synapses supply, indicates cognitive imbalance. Repeated dose administration of SCO aggravates the disorder (Rahimzadegan and Soodi, 2018; Soodi et al., 2014).
Spatial memory and learning are essential for identifying changes in the neuronal cholinergic system, which is well recognized by tasks such as the Morris water maze. Rats induced by SCO showed reduced time spent in the target quadrant in the present study, which was similarly observed in previous reports (Kaur and Mehan, 2015). Meanwhile rats treated with donepezil and SRE at 100, 200, 400 mg/kg increase time spent in target quadrant expressively; thus, attenuated behavioral changes caused by SCO.
SCO administered through the i.p. route in single or repeated doses can cause fear, anxiety, and depression-like behavior in rats and this can be prominently predicted by elevated plus-maze test (Aydin et al., 2016). However, rats treated with Donepezil, S. rhombifolia at selected therapeutic doses showed decreased TL and increased percentage time spent in the open arm; this served as improvement in exteroceptive learning and memory.
The treatment groups showed increased step-through latency significantly with less time spent at dark compartment as a compartment to SCO-induced rats. Reports were consistent with (Khurana et al., 2017) which explains increased step-through latency in passive avoidance tests and showed nootropic activity Sida species (S. cordifolia).
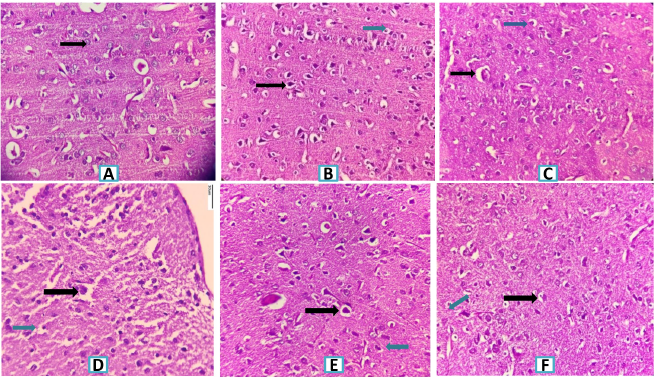 | Figure 4. Histopathology of rat brain (Cerebral Cortex section) with H&E × 400. (A) Normal control group showing normal histological finding (Black arrow-normal neuron). (B) SCO (1 mg/Kg) (Black arrow-severe neuronal degeneration, neuronophagia and blue arrow pyknotic nucleus). (C) SCO + Donepezil (3 mg/kg) group showing minimal neuronal degeneration. (Black arrow-mild congestion, blue arrow minimal neurodegeneration). (D) SCO + SRE (100 mg/kg) group showing moderate neuronal degeneration with neurophagia with congestion of blood vessels. (Black arrow moderate edema and blue arrow shows moderate neurodegeneration). (E) SCO + SRE (200 mg/kg) group showing neuronal degeneration (Black arrow-mild newonal degeneration, blue arrow shows mild neurodegeneration). (F) SCO + SRE (400 mg/kg) group (Black arrow shows mild congestion and blue arrow shows mild neurodegeneration). *SRE = Sida rhombifolia hydroethanolic extract; SCO = Scopolamine. [Click here to view] |
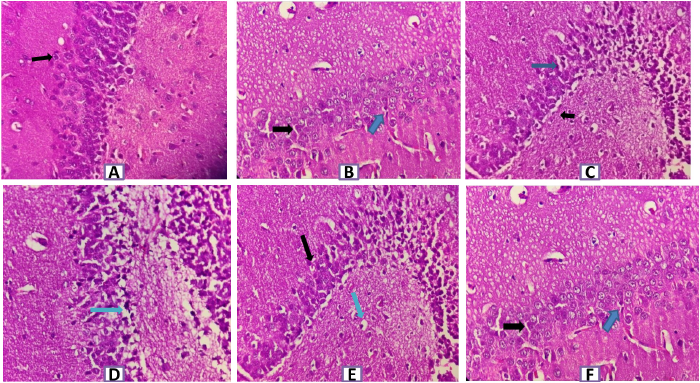 | Figure 5. Histopathology of rat brain (Hippocampus section) with H&E ×400. (A) Normal control group showing normal healthy normochromic neurons with well-outlined cell bodies and absence of intercellular spaces (Black arrow–normal pyramidal cell). (B) SCO (1 mg/kg) induced group showing marked and severe neuronal degeneration with congested blood vesseIs, perivascular edema, neuronophagia, pyknotic cells and g1iosis (Black arrow- edema and blue arrow shows pyknotic cell). (C) SCO+ Donepezi l (3 mg/kg) + SCO group showing mild neuronal degeneration & neuronophagia (Black arrow-mild edema and blue arrow-few cells showing degeneration). (D) SCO+SRE (l00 mg/kg) + SCO showing moderate neuronal degeneration with neuronophagia with congestion of blood vessels and edema (Blue arrow shows intercellular edema). (E) SCO + SRE (200 mg/kg) + SCO showing mild perivascular edema with mild congestion and neuronal degeneration (Black arrow pyramidal cell degeneration and blue arrow-edema). (F) SCO+ SRE (400 mg/kg) + SCO showing mild perivascular edema and minimal pyknosis (Black arrow shows minimal pyknosis, Blue arrow shows mild edema). [Click here to view] |
SCO-induced rats illustrated cholinergic neuronal damage by increasing AChE level in rat brain homogenate sample which is a sign of cognitive imbalance. On the contrary, rats administered with donepezil and SRE at concomitant doses showed improvement with the restoration of learning and memory by blocking the action of AChE and modulating the availability of ACh for synaptic transmission.
In the brain tissue aggregation of β amyloid protein, especially β amyloid1–42 plaque deposition in extracellular space is a characteristic feature considered as a biomarker in cognitive dysfunction (Pattanashetti et al., 2017). The administration of SRE to the rats showed a marked reduction of β-amyloid1–42, which could interferes with aggregation process of beta-amyloid1-42 and protects neurons from damage.
The current study attempted to examine the influence of SRE on reduced GSH levels, which showed increased levels of reduced glutathione and the results were correlated with the previous study on antioxidant properties of S. rhombifolia root and stem extract (Narendhirakannan and Limmy, 2012). A significant increase in lipid peroxidation is measured by MDA levels which are considered as an index of oxidative stress. The activity of SRE resulted in decreasing the lipid peroxidation this was expressed by a decreased level of MDA and correlated with the study that expressed that S. rhombifolia root extract acts as a natural anti-oxidant estimated in rat brain homogenate (Bihaqi et al., 2011; Dhalwal et al., 2007).
Sections of the hippocampus; Cornuammonis (CA field), dentate, subicular complex are anatomical regions of the medial temporal lobe and these play an important role in mammalian memory (Squire, 1992). Histopathological rat brain studies showed that neuronal damage, neural fibrillary tangles hippocampal edema, and pyknotic cells were shown in the SCO-induced group, whereas groups treated with donepezil and SRE showed mild neuronal damage compared to SCO-induced damage.
Earlier scientific reports do confirm the presence of phytoconstituents of plant extract such as Phenolic compounds, flavonoids, alkaloids, β-phenylethylamines, chlorophyll derivatives, steroids phenolic compounds, and these could contribute to cholinesterase enzyme inhibition and improve cognition (Babu et al., 2013; Chaves et al., 2017).
Genus Sida L. revealed its traditional claim for prevention and treatment of neurological disorder in which S. rhombifolia had given attention due to phytoconstituents like flavonoids, alkaloids, other phenolics, and ecdysteroids available in extracts (Dinda et al., 2015). Furthermore, on these presumed reports we planned to explore the possibility of SRE to restore memory and improve learning which was confirmed through decreased AChE; in turn, increases ACh availability at synapse and anti-oxidants present in SRE to combat oxidative stress produced by free radicals. These mechanisms were reported from improvement in spatial, short term memory with a decrease in fear aggravated behavior in rats.
CONCLUSION
The present study confirms the anti-amnesic activity of S. rhombifolia hydro-ethanolic extract through the mechanism of AChE inhibition and antioxidant pathways in SCO-induced rats. This supports the therapeutic value of herbal medicine to enhance memory in AD, yet clinical applications are to be explored.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Study protocol was approved from the Institutional Animal Ethical Committee from KLE College of Pharmacy, Belagavi, an in vivo analysis was carried out (KLECOP/CPCSEA-Res No.221/Po/Res/2000/CPCSEA, Res.25-13/10/2018).
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer's disease: past, present, and future. Neuropharmacology, 2014; 76(Part A): 27–50; doi:10.1016/j.neuropharm.2013.07.004 CrossRef
Aydin E, Hritcu L, Dogan G, Hayta S, Bagci E. The effects of inhaled pimpinellaperegrina essential oil on scopolamine-induced memory impairment, anxiety, and depression in laboratory rats. Mol Neurobiol, 2016; 53(9):6557–67; doi:10.1007/s12035-016-9693-9 CrossRef
Babu PVA, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem, 2013; 24(11):1777–89; doi:10.1016/j.jnutbio.2013.06.003 CrossRef
Bihaqi SW, Singh AP, Tiwari M. In vivo investigation of the neuroprotective property of convolvulus pluricaulis in scopolamine-induced cognitive impairments in Wistar rats. Indian J Pharmacol, 2011; 43(5):520–5; doi:10.4103/0253-7613.84958 CrossRef
Chaturvedi P, Kwape TE. Attenuation of diabetic conditions by Sida rhombifolia in moderately diabetic rats and inability to produce similar effects in severely diabetic in rats. J Pharmacopuncture, 2015; 18(4):12–9; doi:10.3831/kpi.2015.18.032 CrossRef
Chaves OS, Teles YCF, De Oliveira Monteiro MM, das Graças Mendes Junior L, de Fátima Agra M, de Andrade Braga V, Silva TMS, de Fátima Vanderlei de Souza M. Alkaloids and phenolic compounds from Sida rhombifolia L. (Malvaceae) and vasorelaxant activity of two indoquinoline alkaloids. Molecules. 2017; 22(1):94; doi:10.3390/molecules22010094 CrossRef
Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr, 1998; 38(6):421–64; doi:10.1080/10408699891274273 CrossRef
Citron M. Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov, 2010; 9(5):387–98; doi:10.1038/nrd2896 CrossRef
Dhalwal K, Deshpande YS, Purohit AP. Evaluation of in vitro antioxidant activity of Sida rhombifolia (L.)Ssp. retusa (L.). J Med Food, 2007; 10(4):683–8; doi:10.1089/jmf.2006.129 CrossRef
Dhanasekaran S, Perumal P, Palayan M. In-vitro screening for acetylcholinesterase enzyme inhibition potential and antioxidant activity of extracts of ipomoea aquatic forsk: therapeutic lead for Alzheimer’s disease. J Appl Pharm Sci, 2015; 5(2):12–6. CrossRef
Dinda B, Das N, Dinda S, Dinda M, Sarma IS. The genus Sida L. – a traditional medicine: tt's ethnopharmacological, phytochemical and pharmacological data for commercial exploitation in herbal drugs industry. J Ethnopharmacol, 2015; 176:135–76. CrossRef
Eckert GP. Traditional used plants against cognitive decline and Alzheimer disease. Front Pharmacol, 2010; 1:138; doi:10.3389/fphar.2010.00138 CrossRef
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys, 1959; 82(1):70–7; doi:10.1016/0003-9861(59)90090-6 CrossRef
Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol, 1961; 7(2):88–90; doi:10.1016/0006-2952(61)90145-9 CrossRef
Girón LM, Freire V, Alonzo A, Cáceres A. Ethnobotanical survey of the medicinal flora used by the Caribs of Guatemala. J Ethnopharmacol, 1991; 34(2–3):173–87; doi:10.1016/0378-8741(91)90035-C CrossRef
Jafarian S, Ling KH, Hassan Z, Perimal-Lewis L, Sulaiman MR, Perimal EK. Effect of zerumbone on scopolamine-induced memory impairment and anxiety-like behaviours in rats. Alzheimer’s Dement Transl Res Clin Interv, 2019; 5:637–43. CrossRef
Kaur, R., Mehan S, Khanna D, Kalra S. Ameliorative treatment with ellagic acid in scopolamine induced Alzheimer's type memory and cognitive dysfunctions in rats. Austin J Clin Neurol. 2015; 2(6):1053. CrossRef
Khanal P, Patil BM. α-Glucosidase inhibitors from Durantarepens modulate p53 signaling pathway in diabetes mellitus. Adv Tradit Med, 2020; 20:427–38; doi:10.1007/s13596-020-00426-w CrossRef
Khurana N, Sharma N, Patil S, Gajbhiye A. Evaluation of nootropic activity of sidacordifolia in mice. Int J Green Pharm, 2017; 11(3):S417–22.
Kokate CK. Practical pharmacognosy. 53rd edition, Vallabh Prakashan Publication, New Delhi, India, pp 7.19–7.25, 2017.
Li X, Bao X, Wang R. Experimental models of alzheimer ’s disease for deciphering the pathogenesis and therapeutic screening (review). Int J Mol Med, 2016; 37(2):271–83. CrossRef
Mah SH, Teh SS, Ee GCL. Anti-inflammatory, anti-cholinergic and cytotoxic effects of Sida rhombifolia. Pharm Biol, 2017; 55(1):920–8; doi:10.1080/13880209.2017.1285322 CrossRef
Malabade R, Ashok T. Cassia tora a potential cognition enhancer in rats with experimentally induced amnesia. J Young Pharm, 2015; 7(4):455–61; doi:10.5530/jyp.2015.4s.7 CrossRef
McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem, 2001; 73(1):73–84; doi:10.1016/S0308-8146(00)00288-0 CrossRef
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods, 1984; 11:47–60. CrossRef
Narendhirakannan RT, Limmy TP. Anti-inflammatory and anti-oxidant properties of Sida rhombifolia stems and roots in adjuvant induced arthritic rats. Immunopharmacol Immunotoxicol, 2012; 34(2):326–36; doi:10.3109/08923973.2011.605142 CrossRef
OECD. Test No. 423: acute oral toxicity - acute toxic class method. OECD Guidel Test Chem, 2002; 4:1–14. doi:10.1787/9789264071001- en
Oroian M, Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res Int, 2015; 74:10–36. CrossRef
Oshadie G, Silva D, Abeysundara AT, Minoli M, Aponso W. Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants. Am J Essent Oils Nat Prod, 2017; 5(2):29–32.
Pattanashetti LA, Taranalli AD, Parvatrao V, Malabade RH, Kumar D. Evaluation of neuroprotective effect of quercetin with donepezil in scopolamine-induced amnesia in rats. Indian J Pharmacol, 2017; 49(1):60–4; doi:10.4103/0253-7613.201016
Pawar RS, Kumar S, Toppo FA, PK L, Suryavanshi P. Sida cordifolia Linn. accelerates wound healing process in type 2 diabetic rats. J Acute Med, 2016; 6(4):82–9; doi:10.1016/j.jacme.2016.08.004 CrossRef
Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med, 2010; 362:329–44. CrossRef
Rahimzadegan M, Soodi M. Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus. Basic Clin Neurosci, 2018; 9(1):5–14; doi:10.29252/nirp.bcn.9.1.5 CrossRef
Rezvani-Kamran A, Salehi I, Shahidi S, Zarei M, Moradkhani S, Komaki A. Effects of the hydroalcoholic extract of Rosa damascena on learning and memory in male rats consuming a high-fat diet. Pharm Biol, 2017; 55(1):2065–73; doi:10.1080/13880209.2017.1362010 CrossRef
Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from chinese green tea. Carcinogenesis, 1989; 10(6):1003–8; doi:10.1093/carcin/10.6.1003 CrossRef
Smith M. Genetics of Alzheimer’s disease. Dementia, 2017; 23(4):519–27; doi:10.1201/9781315381572.
Soodi M, Naghdi N, Hajimehdipoor H, Choopani S, Sahraei E. Memory-improving activity of Melissa officinalis extract in naïve and scopolamine-treated rats. Res Pharm Sci, 2014; 9(2):107–14.
Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev, 1992; 99(2):195–231; doi:10.1037/0033-295X.99.2.195 CrossRef
Subramanya MD, Pai SR, Upadhya V, Ankad GM, Bhagwat SS, Hegde HV. Total polyphenolic contents and in vitro antioxidant properties of eight Sida species from Western Ghats, India. J Ayurveda Integr Med, 2015; 6(1):24–8; doi:10.4103/0975-9476.146544. CrossRef
Teh SS, Ee GCL, Mah SH, Yong YK, Lim YM, Rahmani M, Ahmad Z. In vitro cytotoxic, antioxidant, and antimicrobial activities of Mesua beccariana (Baill.) Kosterm., Mesua ferrea Linn., and Mesua congestiflora extracts. Biomed Res Int, 2013; 2013:1–10. doi:10.1155/2013/517072 CrossRef
Trikamji Y. Vatavyadhichikitsa: ayurvedadipika of chakrapanidatta on charakasamhita of agnivesa, In Charaka and Dridhabala. Chikitsasthana. 1st edition, Varanasi, India: Chaukhambha Prakashan,pp 623, 1984.
Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J, 1966; 99(3):667–76; doi:10.1042/bj0990667. CrossRef