INTRODUCTION
Clivia miniata (Lindl.) Bosse (syn: C. miniata (Lindl.) Verscaff) is a shade-loving member of the Amaryllidaceae family, whose common names are the Natal lily, bush lily, or Kaffir lily, Boslelie (Afrikaans), or Umayime (Zulu) (Spies et al., 2011). The specific miniata epithet means “cinnabar red” (Harrison, 2012), meaning flowers with a red lead-like color when first found in their natural habitat (Koopowitz, 2002). Clivia miniata is among potential indigenous South African plants for international cut flower trade (Reinten et al., 2011). Due to morphological homogeneity, certain morphological characteristics overlap between some plants, causing taxonomic confusion; however, DNA barcoding has been successfully applied both in the identification of plant species (Bruni et al., 2015) and in the reassessment of taxonomic status and circumscriptions (Rastegar-Pouyani et al., 2014). Cytogenetically studies have reported that the basic chromosome number of C. miniata species is 2n = 2x = 22 (Chen et al., 2003; Spies et al., 2011).
METHODOLOGY AND JUSTIFICATION OF THE STUDY
The literature search for information relevant to the medicinal uses, phytochemistry, and pharmacological properties of C. miniata was conducted between May 2020 and August 2020, using a mixed-method analysis methodology that included the combination of quantitative and qualitative research to compile the study. Data were collected from leading scientific sources online, such as Google Scholar, SCOPUS, Science Direct, SciFinder, and PubMed, while technical reports and other forms of literature were obtained from conference papers, books, theses, websites, and government gazettes. The databases/repositories and sources of literature were selected based on the subject covered and the main search key terms included “C. miniata,” “medicinal properties,” “pharmacological properties,” “taxonomy,” and “botanical descriptions.” Search terms were set to be in the title, keywords, and abstract. Articles published between 1980 and 2020 were included in the search. The Internet search generated 210 articles, then duplicated articles, articles not published in English, and those with limited raw data were excluded, and 30 articles are included in this study. The study focuses in particular on sub-Saharan Africa, but literature and case studies from other regions have been collected and included in the study as well. Clivia miniata is threatened by extinction in nature, and it has been flagged as a priority to study due to its ethnomedicinal value. Documentation of its medicinal uses, phytochemistry, and pharmacological properties is important because this knowledge forms the basic data needed for future health promotion and pharmaceutical research and development.
RESULTS AND DISCUSSION
Botanical descriptions
It is a flowering plant in the genus Clivia and is the only species with trumpet-like flowers, while the other species have pendulous flowers (Spies and Spies, 2018). Clivia's are herbaceous plants with long, narrow (5 cm), smooth-edged, strap-like, slender green leaves (Koopowitz, 2002). Clivia miniata grows as a cluster of plants with a height of about 80 cm, and its flowers range from cream to sporadic occurrences of pure yellow-flowered varieties, sometimes with a faint but very sweet scent (Aubrey, 2001; Koopowitz, 2002). The orange-colored C. miniata flowers often display contrasting cream-yellow form (Fig. 1), illuminating light green features which may differ in color (Conrad and Mathabatha, 2016).
Clivia miniata are colorful plants whose flowers emerge as individual blooms on the tip of an umbel that stands as a hardy stalk above the green foliage below and they have a bell shape and make beautiful additions to the arrangement of the flowers (Koopowitz, 2002). It has a dark green stem and strap-shaped leaves emerging from the fleshy underground stem, and the stem base has compact mass roots that barely shift above ground level (Conrad and Mathabatha, 2016). The shoots emerge from the base of the stem and often form clumps if undisturbed (Conrad and Mathabatha, 2016). Clivia do not form bulbs, but they grow berries because fruits and seeds are enveloped in berries containing one or more than 20 seeds in a berry, while 10 seeds per berry are common in this plant genus (Conrad and Mathabatha, 2016; Koopowitz, 2002).
Clivia miniata is widely distributed across three provinces and two countries (Fig. 2), Eastern Cape, KwaZulu-Natal, and Mpumalanga in South Africa and Swaziland (Spies and Spies, 2018). The species are found in isolated forest habitats along the Kei River and Transkei region, through the provinces of Eastern Cape and KwaZulu-Natal (Conrad and Mathabatha, 2016; Winter, 2000). It is also reportedly established in Mexico, New Zealand, Australian states, Japan, China, and USA and it is a widespread plant for shady areas (Duncan, 2008). Given suitable conditions, it grows into large clumps and is surprisingly water-wise (Duncan, 2008; Koopowitz, 2002; Zwanevelder et al., 2003).
While found throughout South Africa, they prefer shady areas and are often the undergrowth plants in wooded areas or gardens with many trees (Duncan, 2008). Clivia miniata population in the wild has become endangered as many of the large colonies growing in their natural habitat have been destroyed by overharvesting by plant collectors. Also, agriculture and urbanization, as well as the lack of enforcement of legislation, have contributed to the loss of their natural habitat (Aubrey, 2001).
Medicinal uses
The use of traditional remedies is a part of the cultural and religious life of the black South Africans, most especially the people that live in rural settlements. Several studies have identified alkaloids from different parts of C. miniata, and these compounds contribute to both the toxicity and medicinal properties of the plant (Growers, 2017). The leaves, roots, and parts of the corm are prepared using different methods in order to treat a variety of ailments. Traditionally, the black South African women take an infusion of C. miniata leaves as an alternative medicine to facilitate delivery at childbirth or augment labor (Steenkamp, 2003). Rasethe et al. (2019) reported that the bulb of C. miniata is used for the treatment of human immunodeficiency virus, arthritis, skin disorder, and tuberculosis by the people of Limpopo Province of South Africa.
The rhizomes or underground parts of C. miniata are used among the Zulu and other South Africans in general to treat derangement of a febrile (fever), enteric, scarlet and malarial fevers, small-pox and measles, pneumonia, acute bronchitis, and influenza, as well as all catarrhs and bad coughs. It is also used as an emetic, to induce vomiting, and used to relieve pain in general (Tequalelu, 2011).
While the root infusions of C. miniata is used as snakebite antidotes, an effective anodyne, and also applied to the wound, the decoction of the stem is administered orally to cure stomach related ailments and also used in the purification and cleansing of blood (Bhat, 2013; Tequalelu, 2011). A herbal remedy known as isihlambezo, made from the C. miniata plant, was used to treat pregnancy complaints such as indigestion, edema, infection, constipation, and hypertension. However, ingesting too much can overstimulate uterine contractions and cause complications (Tequalelu, 2011).
 | Figure 1. Clivia Miniata. Source: worldoffloweringplants.com. [Click here to view] |
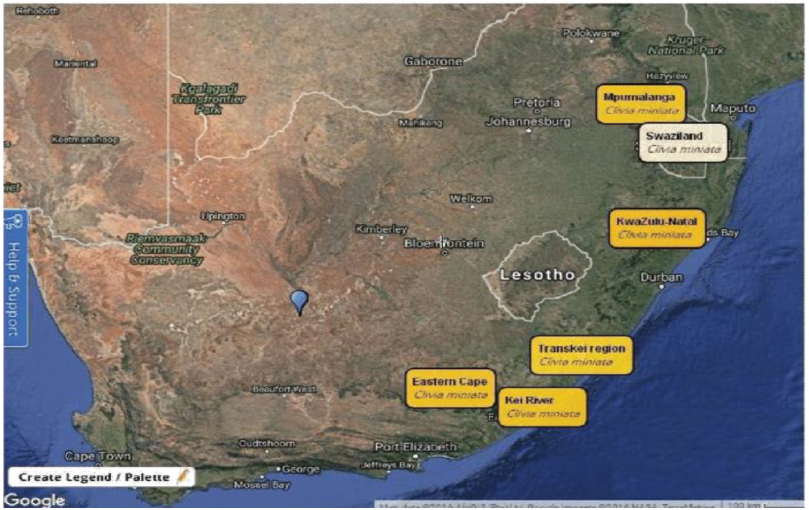 | Figure 2. The distribution of C. miniata in South Africa. Source: Conrad and Mathabatha, 2016. [Click here to view] |
The leaves or roots are usually an ingredient in the herbal remedy called inembe, an infusion taken regularly during pregnancy to ensure an easy childbirth. The leaves are also used in medicines known as isihlambezo which are taken during the last three months of pregnancy and are sometimes used to augment or enhance labor (Hutchings et al., 1996). Likewise, bulb decoctions are used for infertility and urinary complaints in the Transkei region of the Eastern Cape Province of South Africa (Hutchings et al., 1996).
Magical application of C. miniata
Unspecified parts of the plant are used as a protective charm against evil spirits, as well as protection from storms (Growers, 2017; Hutchings et al., 1996). Due to the enormous benefits of this plant, it became the most important component of a traditional healer's pallet of healing plants (Hutchings et al., 1996).
Phytochemistry
Studies have shown that C. miniata contains certain alkaloids that consist of a substance called lycorine, a lactone-containing alkaloid, which would account for the plant’s ability to facilitate childbirth (Pereira et al., 2019). The majority of compounds found in C. miniata are alkaloids and are shown in Tables 1 and 2
Pharmacological properties
The pharmacological importance of C. miniata is discussed below, and they include uterotonic activity, antiviral, and antidiabetic potentials.
Uterotonic effects
Veale et al. (1990) studied the pharmacological effects of an aqueous extract of C. miniata on isolated rat uterus and ileum that were pretreated with diethylstilboestrol. The extracts showed smooth muscle stimulant activity, causing concentration-dependent contractions in the uterus and ileum, and also increased the frequency of spontaneous uterine contractions. Thus, it was concluded that C. miniata seems to act directly as a partial agonist on muscarinic receptors in the uterus and ileum and a competitive antagonist on serotonin creatinine sulphate (5HT) receptors in the uterus. Also, the extract may act indirectly by stimulating prostaglandin synthesis (Veale et al., 1990).
Furthermore, Veale (2000) determined the uterotonic activity of C. miniata in an endometrium-free preparation “stripped” rat myometrium. The study showed that the extract of C. miniata caused a direct contractile response by the isolated tissue, thereby indicating that the extracts exhibited uterotonic activity in the model. The pretreatment of the myometrium with the extract augmented the initial response to acetylcholine. However, the preincubation with atropine had no effect on the response to the plant. Indomethacin administration did not affect the response of the myometrium to the cumulative dosage of acetylcholine, oxytocin, or Clivia extract. Thus, C. miniata appears to act on the myometrium by direct stimulation of smooth muscle contraction.
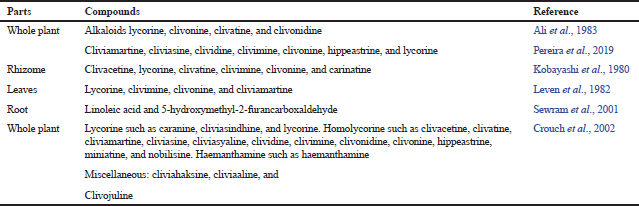 | Table 1. The chemical compounds isolated from C. miniata. [Click here to view] |
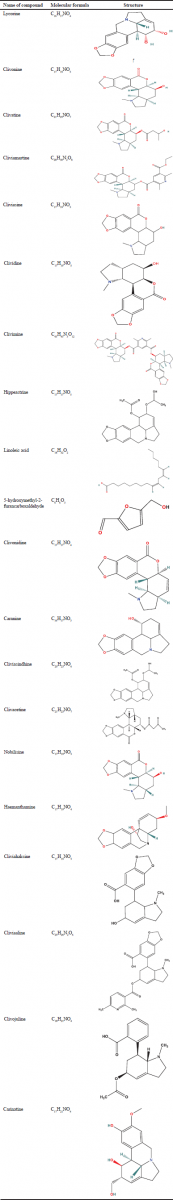 | Table 2. Structures of chemical compounds isolated from C. miniata. [Click here to view] |
In addition, the uterotonic activity of the supercritical fluid extract and isolated compounds from the roots of C. miniata was investigated using guinea pig uterine smooth muscles in vitro. The findings revealed that both the extract and compounds induced uterine contractions at lower doses (Sewram et al., 2001).
Antiviral activity
The study of Leven et al. (1982) and Van Den Berghe et al. (1978) reported that the crude extracts of C. miniata possessed both antiviral and antimicrobial activity, respectively. Among the plants investigated, C. miniata was one of the most active antiviral plants and was found to be active against poliomyelitis, Cocksackie, Semliki forest, measles, and Herpes simplex viruses (Van Den Berghe et al., 1978). Furthermore, Leven et al. (1982) reported that the crude extracts from the roots and leaves of C. miniata showed pronounced antiviral activity. The activity was due to alkaloid lycorine, which decreases the growth of poliomyelitis in VERO cells through its inhibitory action on viral protein synthesis.
Antidiabetic activity
The antidiabetic potential of natural compounds from African medicinal plants was explored with the DIA-DB web server and screened in silico against 17 diabetes targets. C. miniata was among the other plants that were identified as new sources rich in compounds with a potential antidiabetic activity (Pereira et al., 2019).
Health implications of the excessive use of C. miniata
Studies have shown that C. miniata contains lycorine (isoquinoline alkaloid), which makes it poisonous (Crouch et al., 2002; Steenkamp, 2003). The consumption of this plant in large quantities can pose serious health hazards such as collapse, paralysis, and depression of the central nervous system in severe cases. At low doses, it can cause salivation, diarrhoea, nausea, and vomiting (Growers, 2017; Hutchings et al., 1996; Kumbula, 2017). However, lycorine has been shown to have moderate antitumour activity and antiviral properties and to be a weak protozoicide (Crouch et al., 2002; Lamoral-Theys et al., 2009). In cats and dogs, the plant produced symptoms such as convulsions, low blood pressure, tremors, and cardiac arrhythmias in pets with large ingestions (Tequalelu, 2011). The rhizomes and roots are the most poisonous parts; thus, the use of them in high quantity is not encouraged (Growers, 2017).
CONCLUSION
This review summarizes the botany, medicinal uses, phytochemistry, and pharmacological properties of C. miniata, an indigenous perennial herb widely used as herbal medicine in southern Africa. The photochemistry showed that this plant contains certain alkaloids that consist of a substance called lycorine, a lactone-containing alkaloid, which accounts for the plant’s ability to facilitate childbirth or augment labor. Based on the literature search carried out, the plant possesses uterotonic effects, antiviral, antidiabetic, and antimicrobial properties. The leaves, roots, bulbs, and rhizomes are used for the treatment of various human diseases and illnesses such as fever, arthritis, skin disorder, tuberculosis, catarrhs, and bad coughs as well as an antidote for snakebite and in the purification and cleansing of the blood. Due to the ethnomedicinal use and pharmacological properties, it is important to document this plant as it is threatened by extinction in nature and by human activities. Further research on the bioactive properties of the isolated compounds from C. miniata and their modes of action is required in order to illustrate the correlation between ethnomedicinal uses and pharmacological activities. Also, more research should be carried out on the pharmacological properties of the plant using both in vitro and in vivo analysis as it has lead to the discovery and development of new pharmaceutical products. In addition, future research on assessing the toxicological aspects of the leaves, rhizomes, roots, and stems of C. miniata is imperative, as at present, there is not enough systematic information about the toxicity of this plant. The traditional usage of C. miniata by Southern African as an alternative medicine to treat infertility and urinary complaints and induce or augment labor calls for conservation strategies and mechanisms for sustainable utilization of the plant.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Not applicable.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ali A, Ross S, El-Moghazy A, El-Moghazy S. Clivonidine, a new alkaloid from Clivia miniata. J Nat Prod, 1983; 46(3):240. CrossRef
Aubrey A. Clivia miniata [Lindl.] Regel. South Afr Natl Biodivers Inst, 2001;1–5.
Bhat RB. Plants of Xhosa people in the Transkei region of Eastern Cape (South Africa) with major pharmacological and therapeutic properties. Acad J, 2013; 7(20):1474–80.
Bruni I, Galimberti A, Caridi L, Scaccabarozzi D, De Mattia F, Casiraghi M, Labra M. A DNA barcoding approach to identify plant species in multiflower honey. Food Chem, 2015; 170:308–15. CrossRef
Chen R, Song W, Li X, Li M, Liang G, Chen C. Chromosome atlas of major economic plants genome in China. Sci Press Beijing, 2003; 3:809.
Conrad A, Mathabatha M. Elucidating variable traits of flower pigments in clivian plants’ species. Vegetos, 2016; 29(4):4473; doi:10.4172/2229-4473.10002 CrossRef
Crouch NR, Mulholland DA, Pohl TL, Ndlovu E, van Wyk BE. The ethnobotany and chemistry of the genus Clivia (Amaryllidaceae). South Afr J Bot, 2002; 69(2):144–7. CrossRef
Duncan GD. Grow clivias. Kirstenbosch Gardening Series. Cape Town, South Africa: South African Natl Biodivers Institute, 2008.
Growers CJM. Trees, Shrubs, Aloes, Grasses and Ground Covers. KwaZulu-Natal, South Africa. Available via https://cjmgrowers.co.za/clivia-miniata/
Harrison L. RHS Latin for gardeners. United Kingdom: Mitchell Beazley, 2012, 2012.
Hutchings A, Scott AH, Lewis G, Cunningham A. Zulu medicinal plants: an inventory. CAB Direct, 1996; (8):2–3.
Kobayashi S, Ishikawa H, Sasakawa E, Kihara M, Shingu T, Kato A. Isolation of Clivacetine from Clivia miniata REGEL. (Amaryllidaceae). Chem Pharm Bull, 1980; 28(6):1827–31. CrossRef
Koopowitz H. Clivia. Portland, OR, Cambridge, UK: Timber Press, 2002.
Kumbula. A database of Indigenous South African Flora: Clivia miniata. 2000. Available via https://kumbulanursery.co.za/plants/clivia-miniataVeale
Lamoral-Theys D, Andolfi A, Van Goietsenoven G, Cimmino A, Le Calvé B, Wauthoz N, Mégalizzi V, Gras T, Bruyère C, DuBois JC, Mathieu V, Kornienko A, Kiss R, Evidente A. Lycorine, the main phenanthridine amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure-activity relationship and mechanistic insight. J Med Chem, 2009; 52(20):6244–56. CrossRef
Leven M, Vlietinck AJ, Vanden Berghe DA, Totte J, Dommisse R, Esmans E, Alderweireldt FC. Plant antiviral agents. III. isolation of alkaloids from Clivia miniata regel (Amaryllidaceae). J Nat Prod, 1982;45(5):564-73. CrossRef
Pereira ASP, Haan H Den, Peña-García J, Moreno MM, Pérez-Sánchez H, Apostolides Z. Exploring african medicinal plants for potential anti-diabetic compounds with the DIA-DB inverse virtual screening web server. Molecules, 2019; 24(10):1–30. CrossRef
Rasethe M, Semenya S, Maroyi A. Medicinal plants traded in informal herbal medicine markets of the Limpopo Province, South Africa. Evid Based Complement Altern Med, 2019; 2019:2609532. CrossRef
Rastegar-Pouyani E, Oraie H, Khosravani A, Kaboli M, Mobaraki A, Yousefi M, Behrooz R, Fakharmanesh Z, Wink M. A re-evaluation of taxonomic status of Montivipera (Squamata: Viperidae) from Iran using a DNA barcoding approach. Biochem Syst Ecol, 2014; 57:350–6. CrossRef
Reinten EY, Coetzee JH, Van Wyk BE. The potential of South African indigenous plants for the international cut flower trade. 2011; 77(4):934–46. CrossRef
Sewram V, Raynor MW, Mulholland DA, Raidoo DM. Supercritical fluid extraction and analysis of compounds from Clivia miniata for uterotonic activity. Planta Med, 2001; 67(5):451–5. CrossRef
Spies JJ, Spies P. Assessing Clivia taxonomy using the core DNA barcode regions, matK and rbcLa. Bothalia- Bothalia-Afr Biodiver Conservation, 2018; 48(1):1–8. CrossRef
Spies P, van der Westhuizen H, Stegman S, Watson M, Spies J. Barcoding Clivia for species identification. Clivia, 2011; 13:26–37.
Steenkamp V. Traditional herbal remedies used by South African women for gynaecological complaints. J Ethnopharmacol, 2003; 86(1):97–108. CrossRef
Swanevelder ZH, Oberholster A-M, Van Wyk AE, Van der Merwe MM. Diversity and population structure of Clivia miniata Lindl. (amaryllidacea): evidence from molecular genetics and ecology. (2003). Dissertation (MSc (Botany)) - University of Pretoria. Available via http://hdl.handle.net/2263/27805
Tequalelu. Fire Lily – Clivia miniata, 2011. Available via https://herberowe.wordpress.com/2011/02/04/fire-lily clivia-miniata/
Van Den Berghe DA, Ieven M, Mertens F, Vlietinck AJ. Screening of higher plants for biological activities. II. Antiviral activity. Lloydia, 1978; 41(5):463–71.
Veale DJH. The uterotonic mechanisms of the herbal oxytocics Clivia miniata and Agapanthus africanus. 2000. Doctoral dissertation - University of the Witwatersrand, Johannesburg. Available via https://hdl.handle.net/10539/25989
Veale D, Oliver D, Arangies N, Furman K. The pharmacological effects of an aqueous extract of Clivia miniata leaves on isolated rat uterus and ileum. Eur J Pharmacol, 1990; 183(2):570. CrossRef
Winter J. The natural distribution and ecology of Clivia. Clivia Yearbook, 2000; 2:5–9.
Zwanevelder HS, Oberholster A-M, Van Wyk AE, Van der Merwe MM. Diversity and population structure of Clivia miniata Lindl. (Amaryllidaceae): evidence from molecular genetics and ecology, 2003.