INTRODUCTION
Sleep deprivation leads to significant changes in hippocampal activity during episodic memory encoding, which results in impaired subsequent retention (Havekes and Abel, 2017). Situations like shift works and jet lags are associated with sleep deprivation and fatigue (Thomas et al., 2020), which leads to decrements in cognition as well as in psychomotor vigilance response speed or the reaction time to sustained attention to visual stimulus (Belenky et al., 2003; Gregory et al., 2015; Waser et al., 2019). Nonprescription supplements, such as nootropics, have been increasingly commonplace in these situations to combat the effects of sleep deprivation (Klumpers et al., 2015). Nootropics, also known as memory-enhancing drugs, are purported to boost levels of neurotransmitters in the brain for better learning and memory retention (Brondino et al., 2013; Yang et al., 2020). However, the link between these compounds and benefits to cognition still remains unestablished (Medina et al., 2015).
The plant Bacopa monnieri is a commonly used natural nootropic in Ayurvedic medicine. Bacopa monnieri is also used either alone or in combination with other herbs, as a memory and learning enhancer, sedative, and antiepileptic (Bhattacharya et al., 2000; Dubey and Chinnathambi, 2019). Studies of B. monnieri whole plant or alcohol extracts used in rats have reported cognition-enhancing effects, including improved motor learning and acquisition, consolidation, and retention of memory (Abdul Manap et al., 2019; Bhattacharya and Ghosal, 1998; Chaudhari et al., 2017).
The use of Drosophila melanogaster as an organism for the study of learning and memory has been established extensively. They can be reliably trained to learn and remember the association between stimuli through both classical and operant conditioning (Foley et al., 2017; Dissel, 2020; McGuire, 2005). Many of the molecular mechanisms underlying learning and memory in mammalian systems were first elucidated on the fly (Dag, 2019; Dissel, 2020; Margulies, 2005). The pattern of learning and memory functions is conserved between mammals and Drosophila (Guarnieri and Heberlein, 2003; Tsuda and Lim, 2018). The fly has short- and long-term memory involving acquisition, consolidation, and recall. Importantly, its cognitive capacities are shown to be highly amenable to experimental alterations on the genetic, biochemical, environmental, and pharmacological levels.
The behavioral performance of D. melanogaster individuals in the aversive phototaxic suppression (APS) assay provided information on the effects of B. monnieri supplementation on non-sleep-deprived (slp+) and sleep-deprived (slp−) Drosophila. Future efforts on the animal model may relate the results obtained to better evaluate the effects of these nootropics among humans in conditions like jet lag, sleep deprivation, and night shift-requiring jobs.
This study tested the potential effects of B. monnieri supplement on the learning capacities and short-term memory (STM) retention in non-sleep-deprived and sleep-deprived D. melanogaster subjected to the APS assay.
MATERIALS AND METHODS
Fruit fly breeding and handling
Wild-type Drosophila melanogaster (Oregon-R-P2 strain) were obtained from the Bloomington Drosophila Stock Center (Indiana, USA). The flies were reared based on the protocol by Medina et al. (2015) at room temperature on a sweet potato medium. A bottle containing 1-day-old flies from hatched pupae was immediately transferred to two separate bottles containing freshly prepared sweet potato medium. The flies were maintained at 24°C–28°C and in a 12-hours light/12-hours dark cycle, with the 40 W fluorescent light. Only female flies were used in the experiments since they exhibit sustained activity during the day, whereas male flies spend considerable time sleeping during both day and night (Huber et al., 2004). Thus, female flies are more consistent models for sleep-related studies (Dissel, 2020; Li et al., 2009).
Carbon dioxide gas was used to anesthetize the flies during the segregation of males and females based on the presence or absence of sex combs. Newly enclosed flies were separated from breeding bottles and transferred and kept in vials with media for 7 days to ascertain that all flies that were used in the experiment were 7 days old.
Determination of B. monnieri experimental doses
A lethality test was carried out to determine the experimental doses of B. monnieri supplement (Memo Plus Gold, Puducherry, India). The baseline dosages of the supplements for Drosophila were calculated based on a study by Nair and Jacob (2016) for dose conversion between humans and animals. We focused our doses based on a rough extrapolation from the prescribed human dosing. Additionally, we used flanking doses both 10 times greater and 10 times less than our estimated human dose. An assumption for extrapolation was that a 70 kg human equals a 1 mg fly. To evaluate the effect of B. monnieri supplementation, a 4.62 g/ml value was computed relative to the weight of the flies, equivalent to 125 mg for human (the dose is administered to humans twice a day). Two tenfold higher values and two tenfold lower values were included in the series of test dosages for the lethality test. Three sublethal concentrations were chosen for the experiment proper: 4.62 × 10-4 g/ml, 4.62 × 10−3 g/ml, and 4.62 × 10−2 g/ml, and were labeled as low, mid, and high, respectively.
Flies were raised in the media containing a specific dose of the B. monnieri supplement at 25°C. The supplement was added into the media when it cooled down to 60°C and before it solidified.
Sleep deprivation procedure
Prior to sleep deprivation, 7-day-old female flies were individually selected for positive phototaxis. The flies, fed with and without B. monnieri supplement, were sleep-deprived using a sleep nullifying apparatus (SNAP) adapted from Shaw et al. (2000). The flies were individually placed inside test tubes that contain appropriate media and were positioned on the rack of the SNAP. The SNAP moves the rack up and down every 4 seconds such that flies in the test tubes were displaced during the dropping movement and prevented from sleeping. The sleep deprivation process was done for 10 hours (12:00 AM to 10:00 AM).
Sensitivity tests for confounding stress
Quinine sensitivity
A 1 × 4-cm filter paper was soaked in 1 µM quinine solution (Sigma-Aldrich, St. Louis, MO) and placed inside the lighted tube of the T-maze, two centimeters from the opening of the tube. The other tube was covered in aluminum foil to serve as the darkened tube. These two tubes were screwed on opposite sides of the center column of the T-maze.
A fly was placed inside the dark tube for 5 minutes before being allowed to move to the lighted tube. The time in seconds it took for the fly to move toward the lighted tube with quinine was recorded. This time is the quinine sensitivity index (QSI). The QSI was determined by calculating the time in seconds that the fly spent on the dry side of the tube when the other side had been wetted with quinine, during a 5-minutes period.
Velocity stress
Seven-day old female flies were continuously displaced using the SNAP, 30 times per minute for 10 hours from 12:00 AM to 10:00 AM. After the SNAP treatment, the flies were then given 30 minutes to recover before being subjected to the APS assay.
APS assay
Learning phase
A fly that demonstrated positive phototaxis was tapped back into the dark chamber. Thirty seconds was allowed for acclimatization but during this time, a filter paper with a quinine solution was put inside the lighted chamber. Then, the trap door was slowly opened as well as the lights. The fly was given 10 seconds to walk toward the quinine coated filter paper in the lighted tube. This was repeated nine times. Failure to walk to the lighted tube was recorded as “Pass,” which is equivalent to “task learned through reinforcement.” The right–left double alternation of the tubes was executed in an RRLLRRLLRR manner, switching every two trials.
STM retention phase
To assess STM retention in flies, each fly was placed back into its original food tube and remained there for 3 hours. Nootropic feeding was resumed during this resting period. Three hours after training, each fly was put in the T-maze again in the same way as before, and the number of times the fly avoids (pass) or goes into (fail) the lighted chamber was recorded. A total of five trials were conducted to test STM retention. Alternation of the tubes was carried out after every trial.
Average pass rates
Flies from the darkened tube which went through the lighted tube were recorded and tallied per trial except during photopositive screening. A score of “1” was given for a fly exhibiting a photonegative choice. A score of “0” was noted for a fly entering the lighted tube. From a series of nine trials, any consecutive trials that obtained a score of “1” at least four consecutive times, starting from the last trial (ninth trial), were summed and divided by the total number of trials to get the pass rate. The APR or the mean of all the pass rates of the 30 flies per treatment group was then obtained together with its standard deviation and error. These values were then used to represent the percent learning and memory retention in the descriptive statistical methods that were carried out.
Statistical analysis
A two-way analysis of variance (ANOVA) was utilized to detect if the differences in obtained APRs were significant due to B. monnieri supplementation, sleep deprivation, and the interaction of the two. In addition, the Bonferroni method was used as a pairwise comparison for each treatment group using the level of significance (α) which was set at 0.05 for all statistical tests used.
RESULTS AND DISCUSSION
This study presents the first report, to our knowledge, on the effects of B. monnieri supplementation in learning and in STM of sleep-deprived flies. In this study, we utilized Drosophila as a model of learning and memory impairment caused by sleep deprivation and hypothesized that B. monnieri may counter the impairment brought by sleep deprivation. Our study showed that sleep deprivation reduced both learning and STM retention in flies. With B. monnieri treatment, results showed that B. monnieri supplement improved the learning and the STM retention of both sleep-deprived and non-sleep-deprived flies. Future efforts on the animal model may then relate the results obtained to better evaluate the effects of these nootropics on humans in conditions like jet lag, sleep deprivation, and night shift-requiring jobs.
Bacopa monnieri supplementation converted to fly dosages is sublethal
The lethality test showed that the negative control (i.e., no B. monnieri supplement) had a 100% survival rate after seven days (Figure 1). The three lower concentrations, 4.62 × 10−4 g/ml, 4.62 × 10−3 g/ml, and 4.62 × 10−2 g/ml, among the five concentrations tested exhibited survival rates close to the negative control, with the highest of these three concentrations obtaining 86.67% survival rate or 26 out of 30 flies. These three concentrations were determined to be sublethal and used further in the next phase of the experimentation. The remaining two higher concentrations, 4.62 × 10−1 g/ml and 4.62 g/ml, of the five concentrations tested, had survival rates of 60.00% and 53.33%, respectively. In addition, the pupae formation from these two higher concentrations was approximately 50% less compared to the three lower concentrations, suggesting that these two concentrations may have negative effects on fertility and thus they were not used further in succeeding experiments.
Although the use of B. monnieri has been reported to exert different pharmacological actions, there is evidence that some can cause toxicity (Singh and Dhawan, 1982; Singh and Singh, 2009). The lethality test is intended to give information on the safety dosage of B. monnieri before further evaluation of its benefits in fruit flies. Several studies have demonstrated the safety profile of B. monnieri in mammals (Rauf et al., 2012; Sireeratawong et al., 2016), similar to the sublethal results in our study. The decrease in pupae formation is similar to the studies on mammals (Singh and Singh, 2009). Constant high dose administration of B. monnieri demonstrated a decrease in sperm motility, viability, and sperm count. It also showed minute histological changes intraepithelial vacuolation, loosening of germinal epithelium, exfoliation of germ cells, and occasionally giant cell formation. However, it is noteworthy that libido remained to be unaffected in B. monnieri-treated rats.
Quinine sensitivity and velocity stress does not affect learning capacity and memory formation
Quinine sensitivity was tested in order to assess its induction of avoidance behavior in both slp+ and slp− flies. It was observed that when quinine was used in the T-maze test, flies briefly crossed from the dark chamber of the tube to the quinine portion of the lighted chamber and then quickly came back to the dry side of the chamber. There was no significant difference (p < 0.05) on the QSI between slp+ and slp− fruit flies (Table 1). All flies in both treatment groups displayed QSI scores well within ranges that permit normal learning together.
 | Table 1. Difference on the QSI of both undeprived and sleep-deprived D. melanogaster. [Click here to view] |
Since there is no difference in quinine sensitivity of slp+ and slp− flies, these data suggest that contact chemosensation of the bitter-tasting compound is not significantly altered by sleep deprivation.
Studies have revealed that varying intensity of stress can both improve and impair learning and memory (Duncko et al., 2007; Fukui et al., 2001). When the velocity stress test was carried out, the APR of the fruit flies that had undergone the stress test was similar to the APR values of the slp−/Bm− treatment group. ANOVA results indicated that the increased rotations per minute of the sleep deprivation machine had no significant difference (p < 0.05) on the learning and STM retention capacity of the fruit flies (Fig. 2). This indicates that the reduction of learning capacity and STM retention is specifically due to sleep deprivation alone. Despite velocity stress having the same intensity as sleep deprivation in flies (Shaw et al., 2000), our results showed that learning and STM capacity of flies that had undergone the test remained intact compared to the slp−/Bm− treatment group. Flies are known to sleep mostly at night (Huber et al., 2004); hence, learning and STM impairment are likely to be secondary from sleep deprivation rather than from mechanical stress.
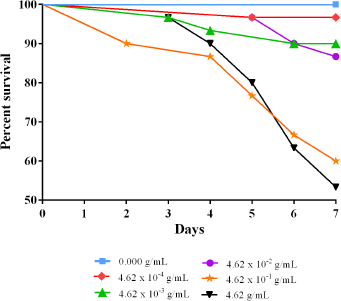 | Figure 1. Percent survival of Drosophila melanogaster on the difference of tenfold concentration of Bacopa monnieri supplementation over the course of 7 days. The three lowest among the five tested concentrations showed the highest survival rate next to the no supplement treatment group. [Click here to view] |
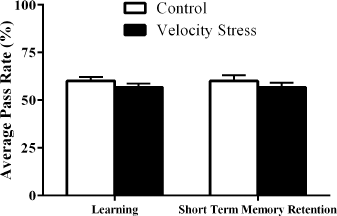 | Figure 2. Comparison of the effect of velocity stress caused by mechanical stimulation of the machine on the learning and STM retention of D. melanogaster (n = 30). The results indicate that stress did not affect both learning and memory (p < 0.05). [Click here to view] |
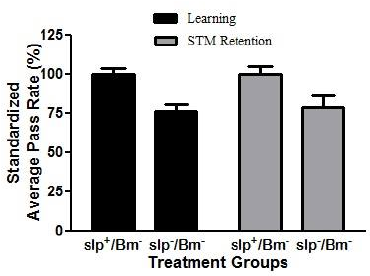 | Figure 3. APR values for learning and STM retention of sleep-deprived D. melanogaster show impaired learning and STM in Drosophila. There is a decrease (p < 0.05) in learning and STM retention in sleep-deprived flies compared to non-sleep-deprived flies (n = 30). [Click here to view] |
Sleep deprivation reduces learning capacity and STM retention
Reduction of memory retention has been previously documented in flies (Dag, 2019; Bai and Sehgal, 2015). However, these studies did not account for learning alongside memory retention. The APS assay was used to assess learning capacity along with STM in the context of sleep deprivation. APR values of all sleep-deprived treatment groups for both learning and STM retention significantly decreased (p < 0.05) in comparison with the APR values of non-sleep-deprived groups. The APR values of the slp−/Bm− treatment group for learning and STM retention were 45.92% and 47.33%, respectively (Fig. 3).
The number of trials it takes for a specific fly to learn in the slp−/Bm− treatment group was significantly lower (p < 0.05) from the slp+/Bm− group. This finding indicates that low motivation is an unlikely reason for the impairment of learning. On the other hand, the decline in STM retention may be attributed to the deficit in mushroom body (MB) plasticity (Seugnet et al., 2009) during episodic memory encoding, resulting in impairment of subsequent retention. Two factors are most likely to contribute to learning and memory deficits following sleep loss: excessive synaptic potentiation and dysregulated reinforcement from dopaminergic neurons in the MB. In Drosophila, dopaminergic neurons project arborizations to the MB neuropile where they influence aversive learning (Seugnet et al., 2009). Previous studies have shown that dopaminergic neurons in the MBs play a role in decision-making under conflicting situations (Tang and Guo, 2001) and may signal the aversive stimulus to the MBs in olfactory conditioning (Riemensperger et al., 2005). Interestingly, flies in the APS assay also face a conflicting choice between their prepotent attraction toward light and the aversive stimulus. The decrease in learning and STM retention in sleep-deprived fruit flies is similar to cognitive impairments observed in sleep-deprived humans (Belenky et al., 2003).
Supplementation of B. monnieri increases learning in non-sleep-deprived and sleep-deprived flies
The B. monnieri supplement is purported to enhance learning. All treatment groups with B. monnieri supplementation had increased APR values in comparison to the one without B. monnieri supplementation. The slp+/Bm+ mid concentration-treatment group obtained the highest APR of 81.48% (Fig. 4). The mid and high concentration APR values were significantly higher (p < 0.05) than the APR values of the low concentration. However, the difference between the mid and high concentrations was found to be not significant (p > 0.05). Although the APR values obtained in the slp−/Bm+ groups were lower compared to the APR values of the slp+/Bm+ groups (Fig. 5), this difference was not significant (p > 0.05). There was no interaction between sleep deprivation and B. monnieri supplementation that may affect the rates obtained (p < 0.01).
The B. monnieri supplement increases learning in sleep-deprived flies at the same level as their non-sleep-deprived counterparts even at low concentration. The results demonstrate that B. monnieri supplement makes up for the deficiency of learning caused by sleep deprivation. The increase in learning of flies fed with B. monnieri was dose-dependent, where the optimal effect was reached at the mid concentration used. The groups fed with B. monnieri supplement had a faster learning rate in the learning phase (phase I), where evidence of learning was observed as early as trial 2. This is indicative of better learning capacities of flies supplemented with B. monnieri than flies without B. monnieri supplement.
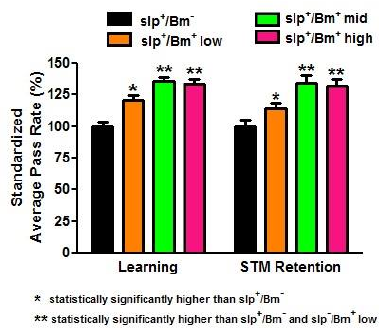 | Figure 4. APR values for learning and STM retention of non-sleep-deprived D. melanogaster show an increase (p < 0.05) in learning and STM retention in slp+/Bm+ compared to slp+/Bm− (n = 30). Low dosage corresponds to 4.62 × 10−4 g/ml, middle dosage corresponds to 4.62 × 10−3 g/ml and high dosage corresponds to 4.62 × 10−2 g/ml. Error bars represent standard error (SE) values. [Click here to view] |
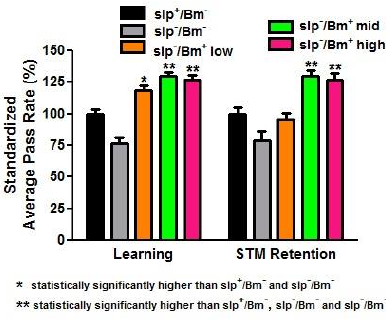 | Figure 5. A B. monnieri altered learning and memory of D. melanogaster. APR values of all sleep-deprived treatment groups decreased in comparison to the APR values of non-sleep-deprived groups. All treatment groups with B. monnieri supplementation had increased (p < 0.05) APR values in comparison to the no supplementation group (n = 30). Low dosage corresponds to 4.62 × 10−4 g/ml, middle dosage corresponds to 4.62 × 10−3 g/ml, and high dosage corresponds to 4.62 × 10−2 g/ml. Error bars represent SE values. [Click here to view] |
Phytochemical studies have revealed different secondary metabolites in B. monnieri, like alkaloids, saponins, and sterols (Deepak and Amit, 2004; Mahato et al., 2000). Previous studies of B. monnieri have identified its effects on learning acquisition. Learning ability in rats has been significantly enhanced by Bacopa extract as it facilitated acquisition, consolidation, and retention of three newly learned behavioral responses (Singh and Dhawan, 1982). Additionally, this study showed that the bacosides caused enhanced levels of protein kinase activity and increased protein levels in the hippocampus, cerebral cortex, and hypothalamus regions of the brain. The findings indicated positive implications for improved neurotransmission and repair of damaged neurons via enhanced regeneration of nerve synapses. A study by Hosamani and Muralidhara (2009) elucidated that B. monnieri prevented apoptosis in neurons from oxidative stress, a process that may be induced by sleep deprivation in learning of fruit flies.
Supplementation of B. monnieri increases STM in non-sleep-deprived flies and sleep-deprived flies
The determination of STM retention in slp+ and slp− flies fed with B. monnieri also used the APS assay similar to the learning phase done earlier. In this assay, a period of 3-hours posttraining was allowed to pass before starting the memory retention phase. There was a significant increase (p < 0.001) in the APR values of the groups slp+/Bm+ low, slp+/Bm+ mid, and slp+/Bm+ high in comparison to slp+/Bm− (Fig. 4). This implies that STM retention in flies fed with B. monnieri had retained the learned behavior for a longer time compared to the group without B. monnieri supplementation.
Sleep-deprived flies fed with B. monnieri, when compared to those that are not fed with the supplement, retained the behavior longer indicating that the supplement compensates for the deficiency caused by sleep deprivation. The STM retention rates of the two highest concentrations were higher compared to that of the lowest concentration (p < 0.001). Looking at the mid and high concentrations, it appears that an increase in concentrations of B. monnieri supplementation did not translate to a corresponding increase in STM retention. The increase in STM retention of slp+ and slp− flies, in the mid and high concentrations of B. monnieri supplement, reached similar levels as indicated by their APR values not being significantly different (p > 0.05). There was a decrease in the APR values of the group slp−/Bm+ low in comparison to its non-sleep-deprived counterpart. Interaction between sleep deprivation and B. monnieri supplementation is not significant (p > 0.05) in the obtained STM retention rates.
Enhancement of STM retention of slp+/Bm+ and slp−/Bm+ treatment groups also followed a dose-dependent manner. Compared to the slp+/Bm+ group, a significant increase in STM retention in the slp−/Bm+ was only observed in the two highest concentrations. This result suggests that if little experiential information was formed in the first place during the learning phase of the sleep-deprived flies, then the recently learned behavior that can be retained and retrieved for use in future encounters the effect of B. monnieri supplement on the differential and increasing STM retention trends which were likely to be manifestations of (1) enhancement of B. monnieri supplement on memory phase following a dose-dependent manner and (2) the natural events outlining the extinction of the memory of the conditioned behavior.
Although we did not study the underlying mechanism, previous studies of B. monnieri have identified its effects on neural transmission. Saini et al. (2010) evaluated the neuroprotective effects of B. monnieri extract on colchicine-induced cognitive impaired rat models, where the altered enzyme activities of Na+/K+-ATPase was reported to be restored by B. monnieri. A study done by McPhee et al. (2016) showed that the administration of B. monnieri reduces neurological deficits together with increased Na+/K+-ATPase activity. In this study, the release of intracellular Ca2+ was glutamate and neurotrophin-dependent, in the postsynaptic cell through a number of downstream protein kinases that are crucial for synaptogenesis. They observed a significant increase in cAMP response element-binding protein activity in the hippocampus after B. monnieri consumption. A study done by Hosamani and Muralidhara (2009) found that B. monnieri inhibited dopamine depletion, which are found to be altered during sleep deprivation. Furthermore, B. monnieri prevents apoptosis in neurons from oxidative stress (Sukumaran, 2019). These studies together suggest that B. monnieri protects neurons from oxidative stress which is induced by sleep deprivation.
CONCLUSION
We found that B. monnieri treatment ameliorated learning and STM loss induced by sleep deprivation in Drosophila model. Supplementing fly food with B. monnieri not only enhanced the learning and STM retention deficits caused by sleep deprivation but also increased the learning and STM retention of these sleep-deprived flies to the same level of increase observed in non-sleep-deprived flies. The findings in this study might be applied to anticipated short-term sleep disturbances, such as temporary night shifts, staying up late for an exam, or jet lags. The inclusion of natural nootropic in a daily-based diet in humans may improve mental performance, without the risks of side effects. In dietary science, a proper combination of natural nootropics, known as nootropic stacking, synergistically enhances the efficacy of each active ingredient of the nootropic. In conclusion, this is an innovative study that may provide a new pharmacological treatment to aid in protecting learning deficits and memory loss in individuals with short-term sleep loss.
CONFLICT OF INTEREST
All the authors declare that they have no conflicts of interest for this work.
FUNDING
None.
REFERENCES
Abdul Manap AS, Vijayabalan S, Madhavan P, Chia YY, Arya A, Wong EH, Rizwan F, Bindal U, Koshy S. Bacopa monnieri, a neuroprotective lead in Alzheimer disease: a review on its properties, mechanisms of action, and preclinical and clinical studies. Drug Target Insights, 2019; 13:1177392819866412. CrossRef
Bai L, Sehgal A. Anaplastic lymphoma kinase acts in the Drosophila mushroom body to negatively regulate sleep. PLoS Genet, 2015; 11(11). CrossRef
Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res, 2003; 12(1):1–12. CrossRef
Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res, 2000; 14(3):174–9. CrossRef
Bhattacharya SK, Ghosal S. Anxiolytic activity of a standardized extract of Bacopa monniera: an experimental study. Phytomedicine, 1998; 5(2):77–82. CrossRef
Brondino N, De Silvestri A, Re S, Lanati N, Thiemann P, Verna A, Emanuele E, Politi P. A systematic review and meta-analysis of Ginkgo biloba in neuropsychiatric disorders: from ancient tradition to modern-day medicine. Evid Based Complement Alternat Med, 2013; 2013:915691. CrossRef
Chaudhari KS, Tiwari NR, Tiwari RR, Sharma RS. Neurocognitive effect of nootropic drug brahmi (Bacopa monnieri) in Alzheimer's disease. Ann Neurosci, 2017; 24(2):111–122. CrossRef
Dag U, Lei Z, Le JQ, Wong A, Bushey D, Keleman K. Neuronal reactivation during post-learning sleep consolidates long-term memory in Drosophila. Elife, 2019; 8:e42786. CrossRef
Deepak M, Amit A. The need for establishing identities of 'bacoside A and B', the putative major bioactive saponins of Indian medicinal plant Bacopa monnieri. Phytomedicine, 2004; 11(2–3):264–8. CrossRef
Dissel, S. Drosophila as a model to study the relationship between sleep, plasticity, and memory. Front Physiol, 2020; 11:533. CrossRef
Dubey T, Chinnathambi S. Brahmi (Bacopa monnieri): an ayurvedic herb against the Alzheimer's disease. Arch Biochem Biophys, 2019; 676:108153. CrossRef
Duncko R, Cornwell B, Cui L, Merikangas KR, Grillon C. Acute exposure to stress improves performance in trace eyeblink conditioning and spatial learning tasks in healthy men. Learn Mem, 2007; 14(5):329–35. CrossRef
Foley BR, Marjoram P, Nuzhdin SV. Basic reversal-learning capacity in flies suggests rudiments of complex cognition. PLoS One, 2017; 12(8):e0181749. CrossRef
Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S. Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann N Y Acad Sci, 2001; 928:168–75. CrossRef
Gregory AM, Sadeh A. Annual research review: sleep problems in childhood psychiatric disorders--a review of the latest science. J Child Psychol Psychiatry, 2015; 57(3):296–317. CrossRef
Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol, 2003; 54:199–228. CrossRef
Havekes R, Abel T. The tired hippocampus: the molecular impact of sleep deprivation on hippocampal function. Curr Opin Neurobiol, 2017; 44:13–9. CrossRef
Hosamani R, Muralidhara. Neuroprotective efficacy of Bacopa monnieri against rotenone induced oxidative stress and neurotoxicity in Drosophila melanogaster. Neurotoxicology, 2009; 30(6):977–85. CrossRef
Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in Drosophila melanogaster. Sleep, 2004; 27(4):628–39. CrossRef
Klumpers UM, Veltman DJ, van Tol MJ, Kloet RW, Boellaard R, Lammertsma AA, Hoogendijk WJ. Neurophysiological effects of sleep deprivation in healthy adults, a pilot study. PLoS One, 2015; 10(1):e0116906. CrossRef
LeVault KR, Tischkau SA, Brewer GJ. Circadian disruption reveals a correlation of an oxidative GSH/GSSG redox shift with learning and impaired memory in an Alzheimer's disease mouse model. J Alzheimers Dis, 2016; 49(2):301–16. CrossRef
Li X, Yu F, Guo A. Sleep deprivation specifically impairs short-term olfactory memory in Drosophila. Sleep. 2009; 32(11):1417–24. CrossRef
Mahato SB, Garai S, Chakravarty AK. Bacopasaponins E and F: two jujubogenin bisdesmosides from Bacopa monniera. Phytochemistry, 2000; 53(6):711–4. CrossRef
Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Curr Biol, 2005; 15(17):R700–13. CrossRef
McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol, 2005; 76(5):328–47. CrossRef
McPhee GM, Downey LA, Noble A, Stough C. Cognitive training and Bacopa monnieri: evidence for a combined intervention to alleviate age associated cognitive decline. Med Hypotheses, 2016; 95:71–6. CrossRef
Medina, PM, Cabaccan, JS, Asis, JL. Effect of natural and artificial sweeteners on the hemolymph glucose level (HGL) in Drosophila melanogaster. Int J Biosci, 2015; 7(1):119–31. CrossRef
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharmacol, 2016; 7:27–31. CrossRef
Rauf K, Subhan F, Abbas M, ul Haq I, Ali G, Ayaz M. Effect of acute and sub chronic use of Bacopa monnieri on dopamine and serotonin turnover in mice whole brain. Afr J Pharm Pharmacol, 2012; 6:2767–74.
Riemensperger T, Völler T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol, 2005; 15(21):1953–60. CrossRef
Saini N, Oelhafen S, Hua H, Georgiev O, Schaffner W, Büeler H. Extended lifespan of Drosophila parkin mutants through sequestration of redox-active metals and enhancement of anti-oxidative pathways. Neurobiol Dis, 2010; 40(1):82–92. CrossRef
Seugnet L, Suzuki Y, Stidd R, Shaw PJ. Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster. Genes Brain Behav, 2009; 8(4):377–89. CrossRef
Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science, 2000; 287:1834–7. CrossRef
Singh HK, Dhawan BN. Effect of Bacopa monniera Linn. (brahmi) extract on avoidance responses in rat. J Ethnopharmacol, 1982; 5(2):205–14. CrossRef
Singh A, Singh SK. Evaluation of antifertility potential of Brahmi in male mouse. Contraception, 2009; 79(1):71–9. CrossRef
Sireeratawong S, Jaijoy K, Khonsung P, Lertprasertsuk N, Ingkaninan K. Acute and chronic toxicities of Bacopa monnieri extract in sprague-dawley rats. BMC Complement Altern Med, 2016; 16:249. CrossRef
Sukumaran NP, Amalraj A, Gopi S. Neuropharmacological and cognitive effects of Bacopa monnieri (L.) Wettst - a review on its mechanistic aspects. Complement Ther Med, 2019; 44:68–82. CrossRef
Tang S, Guo A. Choice behavior of Drosophila facing contradictory visual cues. Science, 2001; 294(5546):1543–7. CrossRef
Thomas J, Overeem S, Dresler M, Kessels RPC, Claassen JAHR. Shift-work-related sleep disruption and the risk of decline in cognitive function: The CRUISE Study [published online ahead of print, 2020 Jun 8]. J Sleep Res, 2020; e13068. CrossRef
Tsuda L, Lim YM. Alzheimer's disease model system using Drosophila. Adv Exp Med Biol, 2018; 1076:25–40. CrossRef
Waser M, Lauritzen MJ, Fagerlund B, Osler M, Mortensen EL, Sørensen H, Jennum P. Sleep efficiency and neurophysiological patterns in middle-aged men are associated with cognitive change over their adult life course. J Sleep Res, 2019; 28:e12793. CrossRef
Yang H, Xie J, Mu W, Ruan X, Zhang J, Yao L, Diao Z, Wu M, Li Y, Ren W, Han J. Tea polyphenols protect learning and memory in sleep-deprived mice by promoting AMPA receptor internalization. Neuroreport, 2020; 31(12):857–64. CrossRef