INTRODUCTION
Plant-derived compounds have an undeniable role in the treatment of various diseases, including cancer. Several approved antitumor drugs have been extracted from plants, and several other compounds are in cancer clinical trials (Tayarani-Najaran and Emami, 2011). Many studies have showed that plant species are being used for cancer treatment or prevention of cancer development, especially in developing countries (Fouché et al., 2008; Ochwang’I et al., 2014). Cycads are plants belonging to class Gymnospermae, order Cycadales. The seeds of cycads in many parts of the world are used in the form of flour to produce many edible products (Nair and Van-Staden, 2012). Dioon genus is one of the cycads that belongs to the family Zamiaceae and is represented in Egypt by only two species: D. spinulosum Dyer ex. Eichler and D. edule Lindl (Hamdy et al., 2007). D. spinulosum Dyer ex. Eichleris is native to Mexico and South Africa and superficially resembles a palm (Johnson and Wilson, 1990). Being a member of cycads, Dioon genus has biflavonoids as secondary metabolites (Harborne, 1977). Amentoflavone-type biflavonoids, such as amentoflavone, bilobetin, sequoiaflavone, isoginkgetin, sciadopitysin, 7,4′,7″,4‴-tetra-O-methyl amentoflavone, and diooflavone (amentoflavone hexamethyl ether), were previously reported (Dossaji et al., 1975). It also has C-glycosylflavones, which were detected by Thin Layer Chromatography (TLC) (Carson and Wallace, 1972). On the other hand, hinokiflavone and its derivatives is absent from the whole family (Meurer and Stevenson, 1994).
Dioon spinulosum has many reported bioactivities, especially cytotoxic activities. It is traditionally used in Mexico for the treatment of ulcers (Mena-Rejon et al., 2009), gastric cancer, and gastrointestinal disorders (Alonso-Castro et al., 2011). The methanolic extract of D. spinulosum petioles showed good in vitro cytotoxic activity on Hep-2 cell line (IC50 = 20 µg/ml) among four tested cell lines: nasopharynx carcinoma (KB), laryngeal carcinoma (Hep-2), cervix adenocarcinoma (HeLa), and cervix squamous carcinoma cells (SiHa) (Mena-Rejon et al., 2009). In search for a potential treatment for estrogen-dependent tumors, a screening of five cycads including D. spinulosum as inhibitors of the cytochrome P-450 aromatase was carried out, and the ethyl acetate-derived fraction from D. spinulosum Dyer showed 97% aromatase enzyme inhibition (Kowalska et al., 1995). The total extract was used in the previous studies without chromatographic separation of the secondary metabolites in search for the bioactive principles in D. spinulosum.
In this study, the evaluation of the cytotoxic activity of total extract and fractions against different cancer cell lines, including endocervix carcinoma (HeLa) and breast cancer (MCF7) cell lines, was carried out, followed by chromatographic isolation in order to identify the chemical constituents of the active fractions using Gas Chromatography-Mass Spectrometry (GC–MS) analysis and column chromatography.
EXPERIMENTAL STUDY
General experimental procedures
Genetic profiling of D. spinulosum Dyer was conducted in the National Research Center, Dokki, Giza, using Inter simple Sequence Repeat (ISSR) and Start Codon Translation (SCoT) markers.
GC–MS analysis of unsaponifiable matter (USM) was carried out using Agilent’s gas–liquid chromatography, series 6,890, fitted with a flame ionization detector (FID). 1H and 13C Nuclear Magnetic Resonance (NMR) were carried out on 400 MHz and 100 MHz, respectively, and were recorded on Bruker Avance III 400 MHz (Bruker AG, Fällanden, Switzerland) with AEON Nitrogen-Free Magnet and BBFO Smart Probe. Data acquisition and processing were carried out using Topspin 3.1 software.
For chromatography, silica gel 60 for CC precoated TLC plates (silica gel: 60 F254) (Merck Darmstadt, Germany), Sephadex LH-20 (Pharmacia Uppsala, Sweden), and 1% P-anisaldehyde’s reagent were used. The chemicals for cytotoxicity assay were dimethyl sulfoxide, RPMI-1640 medium, trypan blue, fetal bovine serum, penicillin/streptomycin antibiotic, trypsin-EDTA (Sigma Aldrich), and Tris buffer (AppliChem). All chemicals and reagents used in this study were of analytical grade.
Plant material
Dioon spinulosum Dyer ex. Eichler leaves were collected in the years from 2015 to 2016, from the Orman Garden in Cairo, Egypt. The plant was authenticated by Dr. Reem Samir, Professor of Botany, Faculty of Sciences, Cairo University, Egypt. The voucher specimen was deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University. The leaves were dried in an oven at a temperature not exceeding 40°C, pulverized, and kept for further study.
Extraction and fractionation
Dioon spinulosum leaves (1 kg) were powdered and extracted by cold maceration with 70% ethanol until exhaustion. The solvent was evaporated under reduced pressure at 45°C to yield 50 g residue. Around 45 g of the residue was suspended in 100 ml of distilled water and extracted successively with n-hexane, dichloromethane (DCM), and ethyl acetate, and then n-butanol was saturated with water. Different fractions were evaporated separately under decreased pressure to yield 6, 10, 6, and 5 g residues, respectively.
Genetic profiling
The bulked DNA extraction was carried out using DNeasy Plant Mini Kit (QIAGEN) as mentioned by Rachmayanti et al. (2006), followed by polymerase chain reaction for SCoT and ISSR analysis, and gel electrophoresis methods were carried out as mentioned by Collard and Mackill (2009).
Chromatographic isolation
Preparation of USM
Preparation of the USM for Gas Liquid Chromatography (GLC) analysis was conducted as that mentioned in the Egyptian Pharmacopoeia (2005). The hexane extract (6 g) of D. spinulosum Dyer ex. leaves was refluxed with 80 ml alcoholic potassium hydroxide (10%) for 24 h, then concentrated, diluted with water, and extracted with diethyl ether, until removal of the unsaponifiable matter to yield 5.5 g USM.
GC–MS analysis of USM
This analysis was carried out by using Agilent’s gas–liquid chromatography, series 6,890, fitted with FID detector. Identification of the components was carried out by comparing their retention times, retention indices, and mass spectral fragmentation patterns with those of the available references and/or published data (Adams, 2007), and identification was carried out through MS libraries (NIST, Wiley and PubChem).
Chromatographic separation of USM
A weighed amount of unsaponifiable matter (3 g) was subjected to fractionation by silica gel column (150 g, 5 × 70 cm). Elution was initiated with 100% n-hexane and the polarity gradually increased with EtOAc in 10% stepwise increment to 100%. Fractions (25 ml each) were collected, concentrated, and monitored by TLC. Similar fractions were pooled together. The subfractions (18–30) eluted with 20%–30% EtOAc in n-hexane were pooled and dried to yield 700 mg fraction. It was further purified on Sephadex LH-20 column (2 × 75 cm) and isocratic elution DCM-MeOH (95:5) to yield compound 1 (200 mg).
Investigation of DCM fraction
DCM extract of D. spinulosum leaves (8 g) was chromatographed on a silica gel column (5 × 75 cm) using DCMMeOH with a 5% increment, collecting 25 ml fractions that were monitored by TLC. Similar fractions were pooled together to give three main subfractions. Fraction A (0.25 g, eluted with 5% MeOH in DCM) was rechromatographed on a silica gel column (1 × 60 cm) eluted isocratically with DCM-MeOH (95:5) to yield compound 2 (8 mg). Fraction B (0.5 g, 25–39, eluted with 10%–15% MeOH in DCM) was rechromatographed on a silica gel column (1 × 70 cm) eluted with 100% DCM; then the polarity increased with 1% MeOH to yield compound 3 (5 mg). Fraction C (2 g, eluted with 20% MeOH in DCM) was rechromatographed over Sephadex LH-20 (2 × 60 cm) eluted with DCM-MeOH (80:20) to yield compound 4 (300 mg).
Investigation of EtOAc fraction
The EtOAc extract of D. spinulosum Dyer ex. leaves (5 g) was chromatographed on a polyamide column (5 × 70 g) eluted with 100% H2O and the polarity gradually decreased with MeOH in 20% stepwise increments to 100% MeOH. Fractions (25 ml each) were collected and monitored by TLC. The subfraction eluted with 20% MeOH was rechromatographed over Sephadex LH-20 column (2 × 75 cm), eluted with 20% H2O in MeOH to yield compound 5 (10 mg).
In-vitro cytotoxic activity
D. spinulosum Dyer ex. leaves, total ethanolic extract, and its four fractions were evaluated for cytotoxicity using the sulforhodamine B (SRB) assay in MCF7 cell lines (human breast adenocarcinoma) and HeLa (human endocervix carcinoma). The cell lines used in this study were obtained from the American Type Culture Collection (ATCC, Minnesota, MN) and were maintained by serial subculture at the National Cancer Institute in Cairo, Egypt. The study was carried out according to the SRB assessment (Vichai and Kirtikara, 2006). The relationship between the surviving fraction and the concentration of the drug is designed to reach the survival curve of each tumor cell line after the defined compound. The IC50 (dosage of the sample which reduces survival to 50%) was calculated for each extract and cell line.
 | Table 1. The distribution of monomorphic and polymorphic bands generated by the SCoT markers. [Click here to view] |
 | Table 2. The distribution of monomorphic and polymorphic bands generated by the ISSR markers. [Click here to view] |
RESULTS
DNA analysis
Genetic diversity revealed by SCoT markers
Five SCoT polymorphic markers were screened to access the level of genetic diversity and similarity between two Dioon species which grow in Egypt. They yielded 31 scorable bands as shown in Table 1. The bands ranged from 275 to 2,360 bp and the band numbers varied from two to seven in each primer. The total polymorphism was estimated to be 45.16%. The distribution of monomorphic (common) and polymorphic bands generated by the five SCoT markers showed 14 unique markers, both positive and negative markers; two unique bands were obtained from D. spinulosum with primer SCoT2; one of them was positive and the other was negative with a molecular size of 370 and 630, respectively. Three positive unique bands were obtained from D. spinulosum with primer SCoT4 with a molecular size of 730, 930, and 1,500 bp. Four unique bands were obtained from D. spinulosum with primer SCoT6; two of them were positive with a molecular size of 600 and 760 bp, and the others were negative with a molecular size of 700 and 2,360, respectively. Five unique bands were obtained from D. spinulosum with primer SCoT8; two of them were positive with a molecular size of 275 and 530 and the others were negative with a molecular size of 300, 800, and 1,000 bp, respectively.
Genetic diversity revealed by ISSR markers
In the case of ISSR markers, five ISSR polymorphic markers were screened to access the level of genetic diversity and similarity between two Dioon species which grow in Egypt. They yielded 30 scorable bands. The bands ranged from 240 to 1,860 bp and the band numbers varied from three to eight in each primer. The total polymorphism was estimated to be 50% as shown in Table 2. The distribution of monomorphic and polymorphic bands produced by the five ISSR markers revealed that primer 14A did not produce any unique marker with Dioon species. Primer 44B showed one negative unique band with D. spinulosum with molecular size 860 bp. Primer HB-9 showed three positive unique bands with D. spinulosum with a molecular size of 780 and 560 bp and one negative band with a molecular size of 400 bp. Primer HB-12 produced two positive unique bands with D. spinulosum with a molecular size of 670 and 980, respectively. Primer HB-14 produced one positive unique band with a molecular size of 240 bp and two negative unique bands with a molecular size of 1,500 and 1,860 bp, respectively.
SCoT and ISSR markers gave a total of 61 scorable bands. The SCoT markers yielded 31 scorable bands and they ranged from 275 to 2,360 bp and the band numbers varied from 2 to 7 in each primer and showed 14 unique bands, and the total polymorphism was estimated to be 45.16%. The ISSR markers yielded 30 scorable bands, these bands ranged from 240 to 1,860 bp, and the band numbers varied from 3 to 8 in each primer; the total polymorphism was estimated to be 33.33%. The proportion of polymorphism indicates that the examined cultivars exhibit a moderate level of polymorphism.
Column chromatography
The phytochemical study of D. spinulosum plant resulted in the isolation of five compounds; the structures of the isolated compounds were elucidated using 1H-NMR and DEPT-Q experiments, in addition to the comparison with published data. The isolated compounds were identified as β-sitosterol (1) (Nyigo et al., 2016), 7,7ʺ,4ʹ,4ʺʹ-O-tetra-methyl amentoflavone (2) (Moawad and Amir, 2016), sciadopitysin (3) (Wollenweber et al., 1998), amentoflavone (4) (Lee et al., 2011), and aromadendrin (5) (Chunhakant and Chaicharoenpong, 2019). Compounds 1 and 5 were being reported for the first time in D. spinulosum; the structure of the isolated compounds is shown in Figure 1, and 1H NMR data of isolated flavonoids were compiled in Table 3.
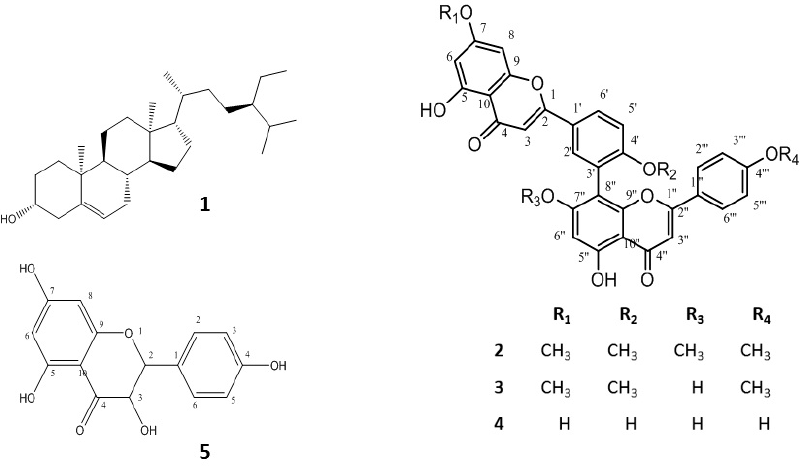 | Figure 1. Compounds isolated from D. spinulosum leaves. [Click here to view] |
GLC analysis
The unsaponifiable matters of D. spinulosum Dyer ex. were dominated by a large amount of hydrocarbons, 18 compounds, and represent 75.02%, including 1-butylhexyl benzene 4.42%, 1-propylheptyl benzene 3.63%, 1-ethyloctyl benzene 2.49%, 1-methylnonyl benzene 2.54%, 1-propyloctyl benzene 7.25%, 1-ethylnonyl benzene 5.8%, 1-methyldecyl benzene 7.18, 1-butyloctyl benzene 4.54%, 1-propylnonyl benzene 6.02%, 1-ethyldecyl benzene 4.34%, 1-methylundecyl benzene 5.94%, 1-pentyloctyl benzene 5.98%, 1-butylnonyl benzene 4.17%, 1-propyldecyl benzene 4.13%, 1-ethylundecyl benzene 2.30%, 1-methyldodecyl benzene 3.51%, heneicosane 0.2%, and pentatriacontane 0.6%. Sterols, 6 compounds, represent 12.21%, including β-sitosterol 3.32%, stigmastan-3,5-diene 2.44%, pregnan-20-one,3-(acetyloxy)-5,6:16,17diepoxy 2.79%, androstane-11,17-dione,3-[(trimethylsilyl) oxy]-17-[o-(phenylmethyl)oxime] 3.62%, stigmastan-6,22-dien,3,5-dedihydro 0.02%, and 4,6-cholestadienol 0.02%. Terpenes were represented by 3 compounds (12.53%), squalene 3.32%, nerolidol 5.13%, and cis-Farnesol 4.08% as shown in Table 4.
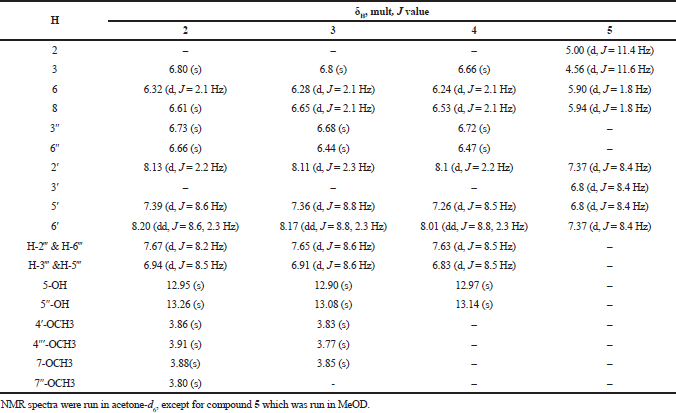 | Table 3. 1H NMR data of isolated flavonoids (compounds 2–5) at 400 MHz. [Click here to view] |
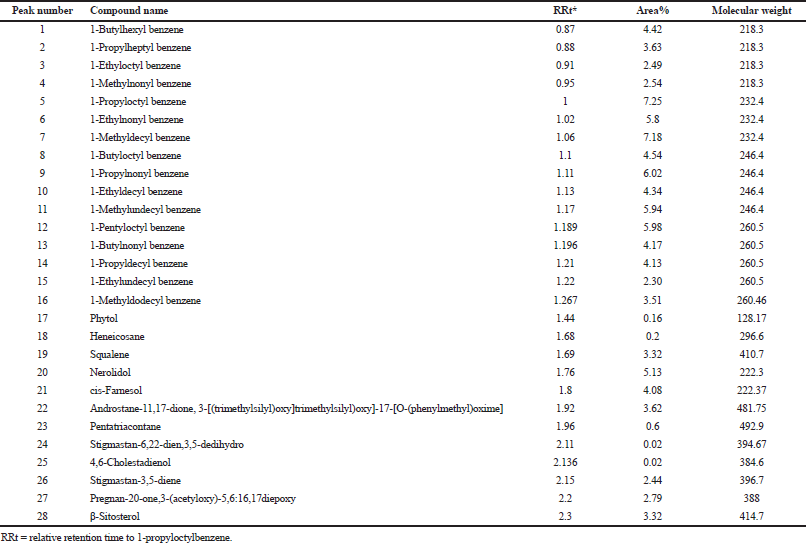 | Table 4. Identified compounds in GC/MS analysis of the unsaponifiable matter of D. spinulosum leaves. [Click here to view] |
Cytotoxic activity
This study reported the in-vitro cytotoxicity evaluations of the D. spinulosum extracts by sulforhodamine B assay against MCF-7 and HeLa cell lines; D. spinulosum showed good cytotoxic activity against HeLa cell line, as the total ethanolic extract, n-hexane, DCM, ethyl acetate, and n-butanol fractions showed IC50 of 21.8, 21.1, 24.5, 26.5, and 21.4 µg/ml, respectively. The total ethanolic extract, EtOAc fraction of D. spinulosum, and aromadendrin (separated from EtOAc fraction) exhibited good cytotoxic activity against MCF7 cell line (IC50 of 22.5, 18, and 12.5 µg/ml, resp.), as shown in Table 5 and Figure 2.
 | Table 5. Cytotoxic potential of different extracts of D. spinulosum leaves against endocervix carcinoma (HeLa) and breast cancer (MCF7) cell lines. [Click here to view] |
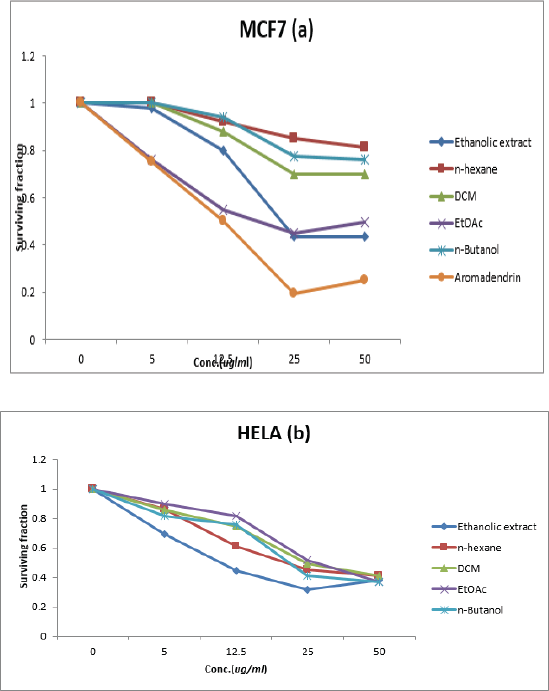 | Figure 2. Cytotoxic effect of D. spinulosum leaf extracts against breast cancer (MCF-7) cell line (a) and human endocervix carcinoma (HeLa) cell lines (b). [Click here to view] |
DISCUSSION
Dioon genus can be differentiated from other cycad genera by the lack of midribs in their leaflets. There is a close similarity between Dioon species in morphological characters. Molecular markers have a higher precision in genetic differentiation and diversity studies (Satya et al., 2015). Of the various DNA marker systems, the effectiveness of the SCoT markers for identification of the species and assessing its genetic diversity (Collard and Mackill, 2009) is superior to other DNA marker systems, like Random Amplified Polymorphic DNA (RAPD) and ISSR for higher polymorphism (Gorji et al., 2011). The DNA analysis of two Dioon species, D. edule and D. spinulosum, showed a total polymorphism of 33.33% using the ISSR markers and a total polymorphism of 45.16% using the SCoT markers, which indicates the genetic diversity of the two species.
Chromatographic analysis and isolation of major active constituents revealed that D. spinulosum leaves contain many secondary metabolites with previously reported anticancer activity.
Amentoflavone is one of the biflavonoids existing in all cycads and is considered as a chemical marker in these plants. It is a major compound in D. spinulosum leaves, as previously reported by Meurer and Stevenson (1994). Amentoflavone showed potent cytotoxicity against a variety of human cancer cell lines, i.e., glioblastoma (Ibrahim et al., 2006), melanoma (Guruvayoorappan and Kuttan, 2007), cervical carcinoma (Lee et al., 2011), breast and endocervix (Lee et al., 2012), erythroleukemia and nasopharyngeal carcinoma (Li et al., 2014), and lung adenocarcinoma (Jung et al., 2017).
β-Sitosterol has a history of anticancer activity against many human cancer cell lines: larynx carcinoma (Matos et al., 2006), breast carcinoma (Awad et al., 2007), and colon carcinoma (Baskar et al., 2010). Furthermore, 7,7″,4′,4‴-O-tetra-methyl amentoflavone showed good cytotoxic activity against lung adenocarcinoma cell line (PC-9) (Li et al., 2014). Generally, biflavonoids isolated from many plants reported good cytotoxic activity, for example, biflavonoids from Selaginella delicatula (Lin et al., 2000) and Anacardium occidentale L. (Konan et al., 2012).
Aromadendrin has reported cytotoxic activity against breast carcinoma (BT474), lung bronchus carcinoma, liver carcinoma, gastric carcinoma, colon adenocarcinoma (Chunhakant and Chaicharoenpong, 2019), and ovarian carcinoma (Kwak et al., 2009).
GC–MS analysis of the USM revealed the presence of other sterols and terpenes that may contribute to the cytotoxic activity of D. spinulosum. A large number of hydrocarbons (18 compounds identified in GC–MS, representing 75.02% of the unsaponifiable matter, may be attributed to foliar epicuticular wax predominant in cycads (Osborne et al., 1993).
From the identified secondary metabolites and their previously documented cytotoxic activity, we can report that the presence of biflavonoids, β-sitosterol, and aromadendrin contributed to the cytotoxic properties of D. spinulosum leaves against breast and endocervix carcinoma cell lines. Thus, the plant under investigation offers a potential herbal product for adjunctive therapy in cancer patients.
CONCLUSION
DNA fingerprinting of D. spinulosum Dyer ex. was determined by SCoT 4, SCoT 6, SCoT 8, and HB 9 since they generated the largest number of bands with large molecular sizes. The phytochemical examination resulted in the isolation of five compounds from D. spinulosum, including one sterol, three biflavones, and one flavanonol. β-Sitosterol and aromadendrin were obtained for the first time from this plant. The assessment of in-vitro cytotoxic activities of the ethanolic extract and the different fractions against endocervix carcinoma (HeLa) cell line and breast cancer (MCF7) cell line showed good cytotoxic activity, and aromadendrin (separated from EtOAc fraction) is responsible for the good effect against MCF7 cell line (IC50= 20 µg/ml).
CONFLICT OF INTEREST
All the authors declare that they have no conflicts of interest for this work.
FUNDING
None.
REFERENCES
Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Carol Stream, IL, 2007.
Alonso-Castro AJ, Villarreal ML, Salazar OLA, Gomez SM, Dominguez F, Garcia CA. Mexican medicinal plants used for cancer treatment: pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol, 2011; 133:945–72. CrossRef
Awad A, Chinnam M, Fink C, Bradford P. β-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine, 2007; 14:747–54. CrossRef
Baskar AA, Ignacimuthu S, Paulraj GM, AL Numair KS. Chemo preventive potential of β-sitosterol in experimental colon cancer model an in vitro and in vivo study. BMC Complement Altern Med, 2010; 10:24 CrossRef
Carson J, Wallace W. The detection of C-glycosylflavones in Dioon spinulosum. Phytochemistry, 1972; 11:842–43; doi:10.1016/0031-9422(72)80063-3 CrossRef
Chunhakant S, Chaicharoenpong C. Anti-tyrosinase antioxidant and cytotoxic activities of phytochemical constituents from Manilkara zapota L. Bark. Molecules, 2019; 24:2798. CrossRef
Collard BC, Mackill DJ. Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Rep, 2009; 27:86–93. CrossRef
Dossaji SF, Mabry TJ, Bell EA. Biflavanoids of the cycadales. Biochem Syst, 1975; 2:171–5. CrossRef
Egyptian Pharmacopoeia. Cenral Administration of Pharmaceutical Affairs (CAPA). Ministry of Health and Population, Cairo, Egypt, 2005.
Fouché G, Cragg G, Pillay P, Kolesnikova N, Maharaj V, Senabe J. In vitro anticancer screening of South African plants. J Ethnopharmacol, 2008; 119:455–61. CrossRef
Gorji AM, Poczai P, Polgar Z, Taller J. Efficiency of arbitrarily amplified dominant markers (SCoT, ISSR and RAPD) for diagnostic fingerprinting in tetraploid potato. Am J Potato Res, 2011; 88:226–37. CrossRef
Guruvayoorappan C, Kuttan G. Effect of amentoflavone on the inhibition of pulmonary metastasis induced by B16F-10 melanoma cells in C57BL/6 mice. Integr Cancer Ther, 2007; 6:185–97. CrossRef
Hamdy RS, El-Ghani MA, Youssef T, El Sayed M. The floristic composition of some historical botanical gardens in the metropolitan of Cairo Egypt. Afr J Agric Res, 2007; 2:610–48.
Harborne J. Flavonoids and the evolution of the Angiosperms. Biochem Syst Ecol, 1977; 5(1):7–22; doi: 10.1016/0305-1978(77)90013-8 CrossRef
Ibrahim NA, Gengaihiand S, Nazif N. Chemical composition and biological activity of Taxodium distichum L. Rich leaves growing in Egypt. JASMR, 2006; 1:53–62.
Johnson LAS, Wilson KL. 1990. Zamiaceae. In: Kramer KU, Green PS. Pteridophytes and gymnosperms. Verlag: Springer, Berlin, Germany, pp 371–7, 1990. CrossRef
Jung YJ, Lee EH, Lee CG, Rhee KJ, Jung WS, Choi Y, Pan CH, Kang K. AKR1B10 inhibitory Selaginella tamariscina extract and amentoflavone decrease the growth of A549 human lung cancer cells in vitro and in vivo. J Ethnopharmacol, 2017; 202:78–84. CrossRef
Konan NA, Lincopan N, Díaz IEC, de Fátima Jacysyn J, Tiba MMT, Amarante Mendes JGP, et al. Cytotoxicity of cashew flavonoids towards malignant cell lines. Exp Toxicol Pathol 2012;64:435–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21106357 CrossRef
Kowalska MT, Itzhak Y, Puett D. Presence of aromatase inhibitors in cycads. J Ethnopharmacol, 1995; 47:113–16; doi: 10.1016/0378-8741(95)01259-G. CrossRef
Kwak JH, Kang MW, Roh JH, Choi SU, Zee OP. Cytotoxic phenolic compounds from Chionanthus retusus. Arch Pharm Res, 2009; 32:1681–7. CrossRef
Lee EJ, Shin SY, Lee JY, Lee SJ, Kim JK, Yoon DY, Woo ER, Kim YM. Cytotoxic activities of amentoflavone against human breast and cervical cancers are mediated by increasing of PTEN expression levels due to peroxisome proliferator activated receptor γ activation. Bull Korean Chem Soc 2012; 33:2219–23. CrossRef
Lee S, Kim H, Kang JW, Kim JH, Lee DH, Kim MS, Yang Y, Woo ER, Kim YM, Hong J. The biflavonoid amentoflavone induces apoptosis via suppressing E7 expression cell cycle arrest at sub G1 phase and mitochondria emanated intrinsic pathways in human cervical cancer cells. J Med Food, 2011; 14:808–16. CrossRef
Li S, Zhao M, Li Y, Sui Y, Yao H, Huang L, Lin X. Preparative isolation of six anti tumour biflavonoids from Selaginella doederleinii Hieron by high speed counter current chromatography. Phytochemical Anal, 2014; 25:127–33. CrossRef
Lin L-C, Kuo Y-C, Chou C-J. Cytotoxic Biflavonoids from Selaginella delicatula. J Nat Prod 2000; 63:627–30. Available from: http://pubs.acs.org/doi/abs/10.1021/np990538m CrossRef
Matos M, Leite L, Brustolim D, De Siqueira J, Carollo C, Hellmann A, Pereira N, Da Silva D. Antineoplastic activity of selected constituents of Duguetia glabriuscula. Fitoterapia, 2006; 77:227–9. CrossRef
Mena-Rejon G, Caamal Fuentes E, Cantillo Ciau Z, Cedillo Rivera R, Flores Guido J, Moo Puc R. In vitro cytotoxic activity of nine plants used in Mayan traditional medicine. J Ethnopharmacol, 2008; 121:462–5. CrossRef
Meurer GB, Stevenson DW. The biflavones of the cycadales revisited: biflavones in Stangeria eriopus, Chigua restrepoi and 32 other species of Cycadales. Biochem Syst, 1994; 22:595–603. CrossRef
Moawad A, Amir D. Ginkgetin or isoginkgetin: the dimethylamentoflavone of Dioon edule Lindl. leaves. Eur J Med Plant, 2016; 16:1–7. CrossRef
Nair JJ, Van Staden J. Isolation and quantification of the toxic methylazoxymethanol glycoside macrozamin in selected South African cycad species. S Afr J Bot, 2012; 82:108–12. CrossRef
Nyigo VA, Peter X, Mabiki F, Malebo HM, Mdegela RH, Fouche G. Isolation and identification of euphol and β-sitosterol from the dichloromethane extracts of Synaenium glaucescens. J Phytopharmacol, 2016; 5:100–4.
Ochwang’I DO, Kimwele CN, Oduma JA, Gathumbi PK, Mbaria JM, Kiama SG. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J Ethnopharmacol, 2014; 151:1040–55. CrossRef
Osborne R, Salatino A, Salatino MLF, Sekiya CM, Torres MV. Alkanes of foliar epicuticular waxes from five cycad genera in the zamiaceae. Phytochemistry, 1993; 33:607–9; doi: 10.1016/0031-9422(93)85456-2 CrossRef
Rachmayanti Y, Leinemann L, Gailing O, Finkeldey R. Extraction, amplification and characterization of wood DNA from Dipterocarpaceae. Plant Mol Biol Rep, 2006; 24:45–55. CrossRef
Satya P, Karan M, Jana S, Mitra S, Sharma A, Karmakar PG, Ray DP. Start codon targeted (SCoT) polymorphism reveals genetic diversity in wild and domesticated populations of Ramie (Boehmeria Nivea L. Gaudich.), a premium textile fiber producing species. Meta Gene, 2015; 3:62–70. CrossRef
Tayarani-Najaran Z, Emami SA. Cytotoxic plants potential uses in prevention and treatment of cancer. In: Özdemir Ö (ed.). Current cancer treatment novel beyond conventional approaches. IntechOpen, London, UK, 2011. CrossRef
Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc, 2006; 1:1112–6. CrossRef
Wollenweber E, Krau L, Mues R. External accumulation of biflavonoids on gymnosperm leaves. Z Naturforsch C, 1998; 53:946–50. CrossRef