INTRODUCTION
Chromenes have recently gained the attention of many researchers due to their various applications. Chromene derivatives have shown different remarkable biological activities against various targets. 4-Substituted-4H-chromenes have shown significant anticancer activity (Aridoss et al., 2012). Also, 4-substituted-4H-chromenes have anticoagulant activity (Bonsignore et al., 1993) and are used as regulators of the potassium cation channel (Jin et al., 2004). 2-Amino-6-bromo-4-(nitromethyl)-4H-chromene-3-carbonitrile (Ia) and 2-amino-6-bromo-4-(1-nitroethyl)-4H-chromene-3-carbonitrile (Ib) have afforded good cytotoxic activity with IC50<4 µg/ml and they have activity four times more than the standard drug Etoposide (Zonouzi et al., 2013).
 | [Click here to view] |
In addition, 4H-chromene derivatives have shown spasmolytic, diuretic, anticoagulant, and antianaphylactic activities (Ghorbani-Vaghei et al., 2011). 4H-Chromene derivatives bind to the Bcl-2 protein and initiate apoptosis in cancer cells. The Bcl-2 protein improves neoplastic cell proliferation by preventing normal cell turnover. Increasing Bcl-2 gene expressions are present in many types of human cancers and can result in cancer cell resistance to chemotherapy and radiotherapy. Therefore, Bcl-2 protein-binding compounds are promising compounds as anticancer agents (Ghorbani-Vaghei et al., 2011).
Aminochromene derivatives have also shown antihypertensive and anti-ischemic behavior (Ghorbani-Vaghei et al., 2011).
Chromenes are also used as food additives, cosmetic agents, and potent biodegradable agrochemical (Subbareddy et al., 2017). They are used as antifungal, anti-HIV, antimalarial, antibacterial, antioxidant, and anti-influenza virus agents (Subbareddy et al., 2017). The chromene derivative MX58151 has been used in the treatment of drug-resistant cancers (Fig. 1) (Subbareddy et al., 2017). In addition, chromene derivative EPC2407 is used in phase I/II clinical trials as a vascular disrupting anti-tumoral drug for the treatment of advanced solid tumors (Fig. 1) (Subbareddy et al., 2017). Chromene derivative HA14-1 is used as an inhibitor of acute myeloid leukemia. Ethyl 2-amino-4-(1H-indol-3-yl)-4H-chromene-3-carboxylate II is used as an anti-human immunodeficiency virus reverse transcriptase (anti-HIV-1 RT) (Fig. 1) (Subbareddy et al., 2017). N-(4-Chlorophenyl)-8-methoxy-2-methyl-4-(2-methyl-1H-indol-3-yl)-4H-chromene-3-carboxamide III has high antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Micrococcus luteus, Escherichia coli, Klebsiella pneumonia, and Pseudomonas aeruginosa. Compound III has a minimum inhibitory concentration in the range of 9.3–18.7 mg/ml (Subbareddy et al., 2017).
The pyranopyrimidines have also shown various pharmacological activities, e.g., antibacterial activity, antifungal activity, antigenotoxic activity, antiplatelet activity, antithrombotic activity, and analgesic and anti-inflammatory activity (Chaker et al., 2017).
All the aforementioned biological activities and our previous work (El-Gazzar et al., 2008; Fayed and Yousif, 2019; Fayed et al., 2019a, 2019b; Nemr et al., 2019; Soliman et al., 2014; Yousif et al., 2017; 2018, Yousif et al., 2019,; 2019a; 2019b; 2019c; 2020; 2021) directed us to prepare novel chromene derivatives and measure the cytotoxic activity of the prepared compounds.
4-H-Chromene derivative (I) has been synthesized from aromatic aldehyde, malononitrile, and phenol derivatives in a one-pot three-component reaction (El-Maghraby et al., 2014).
 | [Click here to view] |
Experimental section
The apparatus used was as in a previously reported study (Yousif et al., 2019b). Compound 1 (diarylidene cyclohexanone) was prepared according to previously known literature (Kumar et al., 2011).
2-Amino-8-(2-chlorobenzylidene)-4-(2-chlorophenyl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 2
A mixture of diarylidene cyclohexanone (0.01 mmol.), malononitrile (0.01), and 5-ml triethylamine in 50 ml absolute ethanol was refluxed for 8 hours. Then, the reaction mixture was cooled and filtered. The precipitate was crystalized from ethanol. Yield: 95%; m.p. 244–246°C; IR (KBr) cm−1, ν: 2,215 (CN), 3,210 (NH2); 1H NMR (DMSO) δ/ppm: 1.74 (t, 2H, J =7.1 Hz, CH2), 2.04 (t, 2H, J =7.1 Hz, CH2), 2.10 (m, 2H, CH2), 2.46 (brs, 2H, NH2), 3.91 (s, 1H, CHAr), 5.23 (s, 1H, CH=), 7.27–7.51 (m, 8 H, Ar). 13C NMR (DMSO) δ/ppm: 22.19, 26.97, 27.06 (3CH2), 39.38 (CH), 115.8, 119.8, 120.6, 127.3, 128.5, 129.24, 129.27, 129.7, 129.9, 130.9, 131.4, 131.5 (12 aromatic C=), 132.8 (CN), 133.3, 135.2, 135.4 (3 C=), 141.27 (=C-O), 160.6 (=CNH2). MS (m/z): 409.3 (M+, 23%). Anal. calcd. for C30H22Cl2N2O2: C, 70.18; H, 4.32; N, 5.46; Found: C, 70.43; H, 4.50; N, 5.67.
N-(8-(2-chlorobenzylidene)-4-(2-chlorophenyl)-3-cyano-5,6,7,8-tetrahydro-4H-chromen-2-yl)benzamide 3
A mixture of compound 2 (0.01 mol) and benzoyl chloride (0.01 mol) in 50-ml pyridine was refluxed for 4 hours. The reaction mixture was cooled and filtered. The precipitate crystalized from ethanol. Yield: 50%; m.p. 184°C–186°C; IR (KBr) cm−1, ν: 1,660 (C=O), 2,215 (CN), 3,210 (NH); 1H NMR (DMSO) δ/ppm: 1.51 (t, 2H, J =7.1 Hz, CH2), 1.84 (t, 2H, J =7.1 Hz, CH2), 1.93 (m, 2H, CH2), 2.34 (brs, 1H, NH), 4.10 (s, 1H, CHAr), 4.41 (s, 1H, CH=), 7.12–7.40 (m, 13H, Ar). 13C NMR (DMSO) δ/ppm: 21.0, 24.92, 26.02 (3CH2), 40.20 (CH), 110.10, 116.20, 118.1,118.4, 118.40, 124.80, 125.3, 126.1, 126.48, 127.8, 128.15, 129.57, 129.60, 129.91, 130.30, 130.9, 131.32, 131.40 (18 aromatic C=), 131.6 (CN), 132.1, 133.2, 134.1 (3 aromatic C=), 141.27 (=C-O), 160.6 (=CNH), 165.23 (C=O). MS (m/z): 513.4 (M+, 17%). Anal. calcd. for C30H22Cl2N2O2: C, 70.18; H, 4.32; N, 5.46; Found: C, 70.43; H, 4.50; N, 5,67.
9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-phenyl-3,5,6,7,8,9-hexahydro-4H-chromeno[2,3-d]pyrimidin-4-one 4
A mixture of compound 3 (0.01) and 30-ml acetic anhydride was refluxed for 12 hours. The reaction mixture was cooled and filtered. The precipitated filtered crystallized from ethanol. Yield: 56%; m.p. 270–272°C; IR (KBr) cm−1, ν: 1,675 (C=O), 1,620 (C=N); 1H NMR (DMSO) δ/ppm: 1.21 (t, 2H, J =7.1 Hz, CH2), 1.64 (t, 2H, J =7.1 Hz, CH2), 1.73 (m, 2H, CH2), 2.51 (brs, 1H, NH), 4.10 (s, 1H, CHAr), 4.73 (s, 1H, CH=), 7.21–7.35 (m, 13H, Ar). MS (m/z): 513.4 (M+, 29%). Anal. calcd. for C30H22Cl2N2O2: C, 70.18; H, 4.32; N, 5.46; Found: C, 70.20; H, 4.42; N, 5.49.
9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-methyl-3,5,6,7,8,9-hexahydro-4H-chromeno[2,3-d]pyrimidin-4-one 5
A mixture of compound 2 (0.01 mol) and 30-ml acetic anhydride was refluxed for 10 hours. The reaction mixture was cooled and filtered. The precipitate crystalized from ethanol. Yield: 55%; m.p. 260°C–262°C; IR (KBr) cm−1, ν: 1,655 (C=O), 1,630 (C=N); 1H NMR (CDCl3) δ/ppm: 1.32 (t, 2H, J =7.1 Hz, CH2), 1.51 (t, 2H, J =7.1 Hz, CH2), 1.62 (m, 2H, CH2), 1.70 (s, 3H, CH3), 2.91 (brs, 1H, NH), 3.80 (s,1H, CHAr), 4.91 (s, 1H, CH=), 7.32–7.45 (m, 8H, Ar); MS (m/z): 451.3 (M+, 31%). Anal. calcd. for C25H20Cl2N2O2: C, 66.53; H, 4.47; N, 6.21; Found: C, 66.63; H, 4.50; N, 6.29.
9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-3,5,6,7,8,9-hexahydro-4H-chromeno[2,3-d]pyrimidin-4-one 6
A mixture of compound 2 (0.01 mol) and 30-ml formic acid was refluxed for 10 hours. The reaction mixture was cooled and filtered. The precipitate crystalized from ethanol. Yield: 60%; m.p. 224°C–226°C; IR (KBr) cm−1, ν: 1,675 (C=O), 1,615 (C=N); 1H NMR (CDCl3) δ/ppm: 1.41 (t, 2H, J =7.1 Hz, CH2), 1.62 (t, 2H, J =7.1 Hz, CH2), 1.75 (m, 2H, CH2), 3.81(s, 1H, CHAr), 4.42 (brs, 1H, NH), 5.23 (s, 1H, CH=), 7.13–7.25 (m, 8H, Ar), 8.43 (s, 1H, NCH); 13C NMR (DMSO) δ/ppm: 23.6, 23.9, 25.9 (3 CH2), 32.4 (CHAr), 124.0, 124.9 (2 C=), 126.1, 126.8, 126.9, 127.4, 127.9, 128.3, 129.4, 130.1, 135.1, 137.2, 138.3, 139.8 (12 Ar C), 140.1, 146.9, 147.9, 148.2(4 C=)150.4 (C=N), 162.3 (C=O). MS (m/z): 437.3 (M+, 41%). Anal. calcd. for C24H18Cl2N2O2: C, 65.92; H, 4.15; N, 6.41; Found: C, 66.03; H, 4.20; N, 6.49.
 | Figure 1. Chemical structures of biological active chromenes. [Click here to view] |
General procedure for the preparation of compounds 7a–c
A mixture of compounds 4–6 (0.01 mol), 30-ml phosphorus oxychloride, and 2 g phosphorous pentachloride was refluxed for 6 hours. Then, the reaction mixture was cooled and filtered. The precipitate was filtered and crystalized from ethanol to give compound 7a–c.
4-chloro-9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-phenyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 7a
Yield: 50%; m.p. 100°C–102°C; IR (KBr) cm−1, ν: 1,635 (C=N); 1H NMR (CDCl3) δ/ppm: 1.34 (t, 2H, J =7.1 Hz, CH2), 1.71 (t, 2H, J =7.1 Hz, CH2), 1.78 (m, 2H, CH2), 4.20 (brs, 1H, NH), 3.95 (s, 1H, CHAr), 5.12 (s, 1H, CH=), 7.21–7.35 (m, 13H, Ar). MS (m/z): 531.8 (M+, 17%). Anal. calcd. for C30H21Cl3N2O: C, 67.75; H, 3.98; N, 5.27; Found: C, 67.80; H, 4.01; N, 5.31.
4-chloro-9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-methyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 7b
Yield: 55%; m.p. 140°C–142°C; IR (KBr) cm−1, ν: 1,615 (C=N); 1H NMR (CDCl3) δ/ppm: 1.16 (t, 2H, J =7.1 Hz, CH2), 1.45 (t, 2H, J =7.1 Hz, CH2), 1.73 (m, 2H, CH2), 1.70 (s, 3H, CH3), 4.01 (s, 1H, CHAr), 5.81 (brs, 1H, NH), 5.31 (s, 1H, CH=), 7.21–7.35 (m, 8H, Ar); MS (m/z): 469.7 (M+, 31%). Anal. calcd. for C25H19Cl3N2O: C, 63.92; H, 4.08; N, 5.96; Found: C, 64.02; H, 4.15; N, 6.02.
4-chloro-9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 7c
Yield: 60%; m.p. 120°C–122°C; IR (KBr) cm−1, ν: 1,628 (C=N); 1H NMR (CDCl3) δ/ppm: 1.20 (t, 2H, J =7.1 Hz, CH2), 1.71 (t, 2H, J =7.1 Hz, CH2), 1.95 (m, 2H, CH2), 3.82 (brs, 1H, NH), 4.21 (s, 1H, CHAr), 5.43 (s, 1H, CH=), 7.21–7.45 (m, 8H, Ar), 8.19 (s, 1H, NCH); 13C NMR (DMSO) δ/ppm: 23.4, 23.6, 24.1 (3 CH2), 35.2 (CHAr), 123.0, 125.1 (2 C=), 127.2, 127.3, 127.4, 127.9, 128.1, 128.3, 129.9, 131.2, 134.3, 136.1, 137.1, 140.8, 145.6 (13 Ar C), 147.0, 147.1, 147.9, 148.2 (4 C=), 151.4 (C=N). MS (m/z): 455.7 (M+, 35%). Anal. calcd. for C24H17Cl3N2O: C, 63.25; H, 3.76; N, 6.15; Found: C, 63.30; H, 3.85; N, 6.21.
General procedure for the preparation of compounds 8a–c
A mixture of compounds 7a–c (0.01 mol), 1-ml hydrazine hydrate in 30-ml dioxane was refluxed for 4 hours. Then, the reaction mixture evaporated under reduced pressure. The residue was crystallized from ethanol to give compounds 8a–c.
9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-4-hydrazinyl-2-phenyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 8a
Yield: 60%; m.p. 224°C–226°C; IR (KBr) cm−1, ν: 1,610 (C=N); 1H NMR (CDCl3) δ/ppm: 1.20 (t, 2H, J =7.1 Hz, CH2), 1.51 (t, 2H, J =7.1 Hz, CH2), 1.78 (m, 2H, CH2), 5.10 (brs, 3H, NH,NH2), 3.71 (s, 1H, CHAr), 5.53 (s, 1H, CH=), 7.17–7.35 (m, 13H, Ar). MS (m/z): 527.4 (M+, 20%). Anal. calcd. for C30H24Cl2N4O: C, 68.32; H, 4.59; N, 10.62; Found: C, 68.42; H, 4.69; N, 10.74.
9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-4-hydrazinyl-2-methyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 8b
Yield: 55%; m.p. 170°C–172°C; IR (KBr) cm−1, ν: 1,615 (C=N); 1H NMR (CDCl3) δ/ppm: 1.23 (t, 2H, J =7.1 Hz, CH2), 1.35 (t, 2H, J =7.1 Hz, CH2), 1.53 (m, 2H, CH2), 1.80 (s, 3H, CH3), 4.30 (s, 1H, CHAr), 5.84 (s, 1H, CH=), 7.10–7.43 (m, 8H, Ar), 8.51 (brs, 3H, NH, NH2); MS (m/z): 465.3(M+, 38%). Anal. calcd. for C25H22Cl2N4O: C, 64.52; H, 4.77; N, 12.04; Found: C, 64.61; H, 4.87; N, 12.21.
9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-4-hydrazinyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 8c
Yield: 60%; m.p. 164°C–166°C; IR (KBr) cm−1, ν: 1,627 (C=N); 1H NMR (CDCl3) δ/ppm: 1.31 (t, 2H, J =7.1 Hz, CH2), 1.54 (t, 2H, J =7.1 Hz, CH2), 1.78 (m, 2H, CH2), 4.10 (s, 1H, CHAr), 4.32 (brs, 3H, NH, NH2), 5.81 (s, 1H, CH=), 7.12–7.41 (m, 8H, Ar), 7.81 (s, 1H, NCH); 13C NMR (DMSO) δ/ppm: 22.1, 23.4, 25.2 (3 CH2), 36.1 (CHAr), 124.1, 126.2 (2 C=), 127.5, 127.7, 127.8, 128.0, 128.2, 128.5, 128.9, 131.9, 134.1, 136.2, 137.2, 141.5, 146.4 (13 Ar C), 147.3, 147.7, 147.9, 148.1 (4 C=), 152.4 (C=N). MS (m/z): 451.3 (M+, 29%). Anal. calcd. for C24H20Cl2N4O: C, 63.87; H, 4.47; N, 12.41; Found: C, 63.95; H, 4.56; N, 12.59.
General procedure for the preparation of compounds 9a–d
A mixture of compounds 8a,b (0.01 mol), 40-ml ethanol, 5-ml distilled water, 1-ml acetic acid, and glucose or xylose (0.01 mol) was refluxed for 6 hours. The reaction mixture evaporated under reduced pressure. The residue crystallized from ethanol to give compounds 9a–d.
5-(2-(9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-phenyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidin-4-yl) hydrazono)pentane-1,2,3,4-tetraol 9a
Yield: 60%; m.p. 170°C–172°C; IR (KBr) cm−1, ν: 1,624 (C=N), 3,210 (NH), 3,345 (OH); 1H NMR (DMSO) δ/ppm: 1.06 (t, 2H, J=7.1 Hz, CH2), 1.23 (brs, 4H, 4OH), 2.62 (t, 2H, J=7.1 Hz, CH2), 2.91 (m, 2H, CH2), 3.06 (d, 1H, J=7.0 Hz CHO), 3.40 (q, 1H, J =7.0 Hz, CHO), 3.52 (t, 1H, J =7.0 Hz, CHO), 3.90 (s, 1H, CHAr), 4.18 (d, 2H, J =7.0 Hz, CH2OH), 4.48 (brs, 1H, NH), 6.14 (s, 1H, CH=), 7.17 (d, 1H, J=6.2 Hz, NCH=), 7.25–7.34 (m, 13H, Ar). Anal. calcd. for C35H32Cl2N4O5: C, 63.74; H, 4.89; N, 8.49; Found: C, 63.90; H, 4.97; N, 8.70.
6-(2-(9-(2-Chlorobenzylidene)-5-(2-chlorophenyl)-2-phenyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidin-4-yl) hydrazono)hexane-1,2,3,4,5-pentaol 9b
Yield: 65%; m.p. 130°C–132°C; IR (KBr) cm−1, ν: 1,635 (C=N), 3,140 (NH), 3,310 (OH); 1H NMR (DMSO) δ/ppm: 1.02 (t, 2H, J=7.1 Hz, CH2), 1.13 (brs, 5H, 5OH), 2.82 (t, 2H, J=7.1 Hz, CH2), 2.84 (m, 2H, CH2), 3.02 (d, 1H, J=7.0 Hz, CHO), 3.33 (q, 1H, J =7.0 Hz, CHO), 3.49 (t, 1H, J =7.0 Hz, CHO′), 3.49 (t, 1H, J =7.0 Hz, CHO), 3.82 (s, 1H, CHAr), 4.20 (d, 2H, J =7.0 Hz, CH2OH), 4.50 (brs, 1H, NH), 6.10 (s, 1H, CH=), 7.20 (d, 1H, J=6.2 Hz, NCH=), 7.30–7.36 (m, 13H, Ar). Anal. calcd. for C36H34Cl2N4O6: C, 62.70; H, 4.97; N, 8.12; Found: C, 62.89; H, 5.10; N, 8.21.
5-(2-(9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-methyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidin-4-yl) hydrazono)pentane-1,2,3,4-tetraol 9c
Yield: 70%; m.p. 220°C–222°C; IR (KBr) cm−1, ν: 1,642 (C=N), 3,240 (NH), 3,325 (OH); 1H NMR (DMSO) δ/ppm: 1.12 (t, 2H, J=7.1 Hz, CH2), 1.54 (brs, 4H, 4OH), 2.21 (s, 3H, CH3), 2.31 (t, 2H, J=7.1 Hz, CH2), 2.51 (m, 2H, CH2), 3.12 (t, 1H, J=7.0 Hz, CHO), 3.40 (t, 1H, J =7.0 Hz, CHO), 3.52 (q, 1H, J =7.0 Hz, CHO), 3.82 (s, 1H, CHAr), 4.18 (d, 2H, J =7.0 Hz, CH2OH), 4.78 (brs, 1H, NH), 6.14 (s, 1H, CH=), 7.17 (d, 1H, J=6.2 Hz, NCH=), 7.25–7.34 (m, 8H, Ar). 13C NMR (DMSO) δ/ppm: 21.2, 22.1, 24.2 (3 CH2), 37.1 (CHAr), 38.1 (CH3), 70.1, 71.3, 73.2 (3 CHOH), 75.1 (CH2OH), 123.1, 124.2 (2 C=), 126.5, 127.1, 127.5, 128.1, 128.5, 128.7, 128.9, 130.9, 131.1, 134.2, 136.2, 140.5, 145.4 (13 Ar C), 146.3, 146.7, 147.1, 148.2 (4 C=), 152.3, 155.2 (2 C=N). Anal. calcd. for C30H29Cl2N4O5: C, 60.41; H, 4.90; N, 9.39; Found: C, 60.47; H, 5.10; N, 9.50.
6-(2-(9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-methyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidin-4-yl) hydrazono)hexane-1,2,3,4,5-pentaol 9d
Yield: 75%; m.p. 194°C–196°C; IR (KBr) cm−1, ν: 1,617 (C=N), 3,140 (NH), 3,442 (OH); 1H NMR (DMSO) δ/ppm: 1.02 (t, 2H, J=7.1 Hz, CH2), 1.64 (brs, 5H, 5 OH), 2.31 (s, 3H, CH3), 2.40 (t, 2H, J=7.1 Hz, CH2), 2.57 (m, 2H, CH2), 3.02 (t, 1H, J=7.0 Hz, CHO), 3.30 (t, 2H, J =7.0 Hz, 2CHO), 3.68 (q, 1H, J =7.0 Hz, CHO), 4.12 (s, 1H, CHAr), 4.22 (d, 2H, J =7.0 Hz, CH2OH), 4.61 (brs, 1H, NH), 6.01 (s, 1H, CH=), 7.09 (d, 1H, J=6.2 Hz, NCH=), 7.16–7.49 (m, 8H, Ar). Anal. calcd. for C31H32Cl2N4O6: C, 59.34; H, 5.14; N, 8.93; Found: C, 59.45; H, 5.20; N, 9.05.
General procedure for the preparation of compounds 10a–d
A mixture of compounds 9a–d (0.01 mol) and 10-ml acetic anhydride was refluxed for 20 hours. Then, the reaction mixture was poured into water and the solid formed filtered, dried, and crystallized from ethanol to give compounds 10a–d.
1-(2-Acetyl-8-(2-chlorobenzylidene)-12-(2-chlorophenyl)-5-phenyl-2,3,8,10,11,12-hexahydro-9H-chromeno[3,2-e] [1,2,4]triazolo[4,3-c]pyrimidin-3-yl)butane-1,2,3,4-tetrayl tetraacetate 10a
Yield: 60%; m.p. 130°C–132°C; IR (KBr) cm−1, ν: 1,644 (C=N), 1,744 (C=O); 1H NMR (CDCl3) δ/ppm: 1.12, 1.68 (2s, 12H, 4CH3), 2.01 (s, 9H, 3CH3CO), 2.23 (t, 2H, J=7.1 Hz, CH2), 2.35 (t, 2H, J=7.1 Hz, CH2), 2.58 (m, 2H, CH2), 4.28 (s, 1 H, CHAr), 4.97, 5.08, 5.14 (3 d, 3H, J=7 Hz, 3CHO), 5.27 (s, 1H, CH=), 5.95 (d, 1H, J=7 Hz, CHN), 6.10 (m, 1H, CHO), 7.19–7.47 (m, 13H, Ar). Anal. calcd. for C45H42Cl2N4O10: C, 62.14; H, 4.87; N, 6.44; Found: C, 62.30; H, 4.96; N, 6.60.
1-(2-Acetyl-8-(2-chlorobenzylidene)-12-(2-chlorophenyl)-5-phenyl-2,3,8,10,11,12-hexahydro-9H-chromeno[3,2-e][1,2,4] triazolo[4,3-c]pyrimidin-3-yl)pentane-1,2,3,4,5-pentayl pentaacetate 10b
Yield: 65%; m.p. 105°C–107°C; IR (KBr) cm−1, ν: 1,631 (C=N), 1,734 (C=O); 1H NMR (CDCl3) δ/ppm: 1.41, 1.72 (2s, 12H, 4CH3CO), 2.12 (s, 9H, 2CH3CO), 2.31 (t, 2H, J=7.1 Hz, CH2), 2.41 (t, 2H, J=7.1 Hz, CH2), 2.49 (m, 2H, CH2), 4.31 (s, 1 H, CHAr), 4.51, 5.19, 5.20 (3 d, 4H, J=7 Hz, 4CHO), 5.29 (m, 1H, CHOAc), 5.31 (d, 2H, CH2OAc), 5.35 (s, 1H, CH=), 5.83 (d, 1H, J=7 Hz, CHN), 7.19–7.47 (m, 13H, Ar). Anal. calcd. for C48H46Cl2N4O12: C, 61.21; H, 4.92; N, 5.95; Found: C, 61.36; H, 5.0; N, 6.10.
1-(2-Acetyl-8-(2-chlorobenzylidene)-12-(2-chlorophenyl)-5-methyl-2,3,8,10,11,12-hexahydro-9H-chromeno[3,2-e] [1,2,4]triazolo[4,3-c]pyrimidin-3-yl)butane-1,2,3,4-tetrayl tetraacetate 10c
Yield: 70%; m.p. 152°C–154°C; IR (KBr) cm−1, ν: 1,614 (C=N), 1,746 (C=O); 1H NMR (CDCl3) δ/ppm: 1.22, 1.35 (2s, 12H, 4CH3CO), 2.01 (s, 3H, CH3CO), 2.10 (s, 3H, CH3C=N), 2.13 (t, 2H, J=7.1 Hz, CH2), 2.23 (t, 2H, J=7.1 Hz, CH2), 2.41 (m, 2H, CH2), 4.14 (s, 1 H, CHAr), 4.86, 5.13 (2 d, 3H, J=7 Hz, 2CHO), 5.19 (s, 1H, CH=), 5.81 (d, 1H, J=7 Hz, CHN), 6.10 (m, 1H, CHO), 7.13–7.32 (m, 8H, Ar). 13C NMR (DMSO) δ/ppm: 20.1, 21.4, 25.7 (3 CH2), 36.7 (CHAr), 38.2, 38.3, 38.6, 39.3, 39.7, 50.9 (6 CH3), 60.1, 60.9, 73.6 (3 CHOAc), 74.3 (CH2OH), 121.1, 122.4 (2 C=), 124.8, 126.2, 126.7, 127.2, 127.7, 128.2, 128.1, 130.1, 130.9, 134.7, 135.1, 139.4, 145.9 (13 Ar C), 146.1, 146.4, 147.3, 148.1 (4 C=), 151.3, 154.1 (2 C=N), 161.2 (C=O). Anal. calcd. for C40H40Cl2N4O10: C, 59.48; H, 4.99; N, 6.94; Found: C, 59.60; H, 5.10; N, 7.16.
1-(2-Acetyl-8-(2-chlorobenzylidene)-12-(2-chlorophenyl)-5-methyl-2,3,8,10,11,12-hexahydro-9H-chromeno[3,2-e][1,2,4] triazolo[4,3-c]pyrimidin-3-yl)pentane-1,2,3,4,5-pentayl pentaacetate 10d
Yield: 75%; m.p. 120°C–122°C; IR (KBr) cm−1, ν: 1,632 (C=N), 1,748 (C=O); 1H NMR (CDCl3) δ/ppm: 1.11, 1.28 (2s, 12H, 4CH3CO), 2.05 (s, 3H, 2CH3CO), 2.12 (s, 3H, CH3C=N), 2.14 (t, 2H, J=7.1 Hz, CH2), 2.35 (t, 2H, J=7.1 Hz, CH2), 2.48 (m, 2H, CH2), 4.30 (s, 1 H, CHAr), 4.39 (d, 2H, CH2OAc), 4.86, 5.13, 5.24 (3 d, 3H, J=7 Hz, 2CHO), 5.28 (s, 1H, CH=), 5.62 (d, 1H, J=7 Hz, CHN), 6.10 (m, 1H, CHOAc), 7.24–7.46 (m, 8H, Ar). Anal. calcd. for C43H44Cl2N4O12: C, 58.71; H, 5.04; N, 6.37; Found: C, 58.90; H, 5.19; N, 6.50.
Cytotoxic activity
The cytotoxic activity was carried out based on a previously reported procedure (Yousif et al., 2019c).
RESULTS AND DISCUSSION
Diarylidene cyclohexanone 1 reacts with malononitrile in triethylamine to produce 2-Amino-8-(2-chlorobenzylidene)-4-(2-chlorophenyl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 2. Compound 2 has been previously reported (Wang et al., 2004a; Jin et al., 2005; Wang et al., 2004b; Kumar et al., 2011). The method of preparation of compound 2 was a modified method, by using triethylamine as a weak base instead of sodium methoxide in a solvent-free reaction. The proposed structure is in agreement with spectral data. The IR of compound 2 shows the absorption band for CN group and NH2 group and shows the disappearance of carbonyl group absorption band. Mass spectroscopy for compound 2 shows a molecular ion peak at m/z 409.
2-amino-8-(2-chlorobenzylidene)-4-(2-chlorophenyl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 2 reacts with benzoyl chloride to afford N-(8-(2-chlorobenzylidene)-4-(2-chlorophenyl)-3-cyano-5,6,7,8-tetrahydro-4H-chromen-2-yl) benzamide 3. Compound 3 is heated under reflux in acetic anhydride to give 9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-phenyl-3,5,6,7,8,9-hexahydro-4H-chromeno[2,3-d] pyrimidin-4-one 4. Also, 2-amino-8-(2-chlorobenzylidene)-4-(2-chlorophenyl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 2 is heated under reflux in acetic anhydride to give 9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-methyl-3,5,6,7,8,9-hexahydro-4H-chromeno[2,3-d]pyrimidin-4-one 5. Compound 2 reacts with formic acid to afford 9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-3,5,6,7,8,9-hexahydro-4H-chromeno[2,3-d]pyrimidin-4-one 6. The spectral data of compounds 3–6 are compatible with the proposed structure. The IR spectrum of compound 3 shows the absorption band for carbonyl group. The 13C NMR of compound 3 shows a characteristic signal for carbonyl group at δ 165.23 ppm. The IR of compound 4 shows the disappearance of the absorption band for cyano group (CN). The mass spectrum for compound 4 shows a molecular ion peak at m/z 513. The IR spectrum of compounds 5,6 shows the disappearance of the absorption band of cyano functional group. The mass spectrum of compound 5 shows a molecular ion peak at m/z 451. The 13C NMR of compound 6 shows a signal at δ 162.3 ppm characteristic for carbonyl group.
Chlorination of compounds 4–6 using phosphorous pentachloride and phosphorus oxychloride affords 4-chloro-9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-phenyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 7a, 4-chloro-9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-methyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 7b, and 4-chloro-9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 7c respectively. Also, compounds 7a–c react with hydrazine hydrate to give 9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-4-hydrazinyl-2-phenyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 8a, 9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-4-hydrazinyl-2-methyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 8b, and 9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-4-hydrazinyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidine 8c, respectively. The structures of compounds 7a–c and 8a–c were elucidated from 1H NMR, IR, and mass spectral data. The IR of compounds 7a–c shows the disappearance of the absorption band of carbonyl function group. The 13C NMR of compound 7c shows the disappearance of signal for carbonyl group. Also, the IR of compounds 8a–c shows the appearance of the absorption band of NH, NH2 groups. The mass spectrum of compound 8a shows a molecular ion peak at m/z 527.
 | [Click here to view] |
Compounds 8a–b react with xylose and glucose to afford 5-(2-(9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-phenyl-6,7,8,9-tetrahydro-5 H -chromeno[2,3- d ]pyrimidin-4-yl)hydrazono) pentane-1,2,3,4-tetraol 9a, 6-(2-(9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-phenyl-6,7,8,9-tetrahydro-5 H -chromeno[2,3- d ]pyrimidin-4-yl)hydrazono) hexane-1,2,3,4,5-pentaol 9b, 5-(2-(9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-methyl-6,7,8,9-tetrahydro-5 H -chromeno[2,3- d ]pyrimidin-4-yl)hydrazono) pentane-1,2,3,4-tetraol 9c, and 6-(2-(9-(2-chlorobenzylidene)-5-(2-chlorophenyl)-2-methyl-6,7,8,9-tetrahydro-5H-chromeno[2,3-d]pyrimidin-4-yl)hydrazono)hexane-1,2,3,4,5-pentaol 9d, respectively. In addition, compounds 9a–d were acetylated using acetic anhydride to afford acetylated sugar derivatives 10a–d. The spectral data of compounds 9a–d and 10a– d are compatible with the proposed structure. The IR spectrum of compounds 9a–d shows the absorption band for hydroxyl group. Also, the IR of compounds 10a–d shows the absorption band for carbonyl group and disappearance of absorption band for hydroxyl group, indicating acetylation of hydroxyl groups of compounds 9a–d. The 13C NMR of compound 10c shows a signal at δ 161.2 ppm indicating carbonyl function group.
Cytotoxic activity
The cytotoxic activity of the new synthesized compounds was carried out against three different cancer cell lines, namely adenocarcinomic human alveolar basal epithelial cells A-549, human epithelial colorectal adenocarcinoma cells CaCo-2, and human colorectal adenocarcinoma cell line HT-29, using (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Yousif et al., 2019c). The results are presented in Table 1 as cytotoxic activity of the synthesized compounds at 100 μM on the three cell lines. The results show that compounds 9b,d and 10b,d have moderate cytotoxic activity toward A-549 cell lines when compared to doxorubicin as the reference drug. Compounds 3–6, 7b, and 8a–b have a weak cytotoxic activity toward A-549 cell lines. Compound 2 has high cytotoxic activity toward CaCo-2 cell lines when compared to doxorubicin as the reference drug. Compounds 5, 6, 9b, and 10b have a weak cytotoxic activity toward CaCo-2 cell lines. Compound 2 shows high cytotoxic activity toward HT-29 cell lines. Compounds 3, 5, 6, 7a–b, 8a–b, 9b,d, and 10b,d show a weak cytotoxic activity toward HT-29 cell lines.
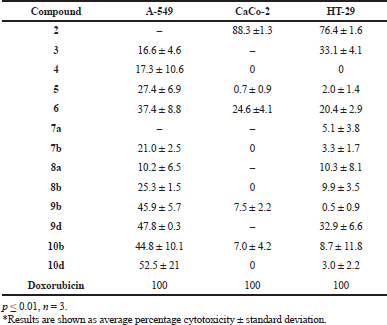 | Table 1. Percentage cytotoxicity of compounds on human tumor cancer cell lines at 100 µM. [Click here to view] |
From the aforementioned biological activity, we can deduce the structural activity relationship. The presence of the amino group at position 2 and the cyano group at position 3 in compound 2 increases the cytotoxic activity toward CaCo-2 and HT-29 cell lines. The presence of the hydrazine group linked to glucose in compounds 9b,d makes the cytotoxic activity moderate toward A-540 cell lines. The presence of the triazolo ring linked to acetylated glucose in compound 10b,d makes the cytotoxic activity moderate toward A-549 cell lines. The disappearance of the amino group in compound 3 and the presence of the pyrimidine ring linked to chromene afford a weak cytotoxic activity toward A-549 cell lines. The presence of the pyrimidine ring linked to chromene and chlorine atom at position 4 in compound 7b makes cytotoxic activity weak toward A-549 cell lines. Also, the presence of the pyrimidine ring linked to chromene and hydrazine function group at position 4 in compound 8a,b makes the cytotoxic activity weak toward A-549 cell lines. The presence of the pyrimidine ring linked to chromene in compounds 5,6 makes the cytotoxic activity toward CaCo-2 cell lines weak. Also, the presence of the pyrimidine ring linked to the chromene and hyrazino function group and linked to glucose in compound 9b makes cytotoxic activity weak towards CaCo-2 cell lines. In addition, the presence of the pyrimidine ring and triazolo ring linked to chromene and acetylated glucose in compound 10b makes the cytotoxic activity weak toward CaCo-2 cell lines.
CONCLUSION
Novel compounds derived from chromene have been synthesized and structurally elucidated using mass spectroscopy, infrared, and nuclear magnetic resonance spectroscopy. Screening of most of the synthesized compounds against A-549, CaCo-2, and HT-29 cell lines has been made.
ACKNOWLEDGMENTS
The authors acknowledge the National Research Centre for providing the chemicals needed for this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING
The authors received funding for this paper from National Research centre internal project number 12010104.
REFERENCES
Aridoss G, Zhou B, Hermanson DL, Bleeker NP, Xing C. Structure–Activity Relationship (SAR) study of ethyl 2-amino-6-(3,5-dimethoxyphenyl)-4-(2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (CXL017) and the potential of the lead against multidrug resistance in cancer treatment. J Med Chem, 2012; 55(11):5566–81; https://doi.org/10.1021/jm300515q
Bonsignore L, Loy G, Secci D, Calignano A. Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives. Eur J Med Chem, 1993; 28(6):517–20. https://doi.org/10.1016/0223-5234(93)90020-F
Chaker A, Najahi E, Nepveu F, Chabchoub F. Microwave-assisted synthesis of chromeno[2,3-d]pyrimidinone derivatives. Arab J Chem, 2017; 10:S3040–7; https://doi.org/10.1016/j.arabjc.2013.11.045
El-Gazzar ABA, El-Enany MM, Mahmoud MN. Synthesis, analgesic, anti-inflammatory, and antimicrobial activity of some novel pyrimido[4,5-b]quinolin-4-ones. Bioorg Med Chem, 2008; 16:3261–73. CrossRef
El-Maghraby AM. Green chemistry: new synthesis of substituted chromenes and benzochromenes via three-component reaction utilizing Rochelle salt as novel green catalyst. Org Chem Int, 2014; 2014:1–6; https://doi.org/10.1155/2014/715091
Fayed AA, Bahashwan SA, Yousif MNM, Yousif NM, Gad FA. Synthesis and anti-inflammatory activity of some new pyrimidinothienocinnoline derivatives. Russ J Gen Chem, 2019a; 89(9):1887–95. CrossRef
Fayed AA, Bahashwan SA, Yousif MNM, El Shafey HM, Amr AE, Yousif NM, and Shadid KA, Synthesis and Antiproliferative Activity of Some Newly Synthesized Pyrazolopyridine Derivatives. Russ J Gen Chem, 2019; 89(6):1209–17. CrossRef
Fayed AA, Yousif MNM, Abdelgawad TT, Amr AE, Yousif NM, Gad FA. Synthesis and antimicrobial activity of novel polycyclic thienopyridazine derivatives. Chem Heterocycl Compds, 2019b; 55(8):773–8. CrossRef
Ghorbani-Vaghei R, Toghraei-Semiromi Z, Karimi-Nami R. one-pot synthesis of 4H-chromene and dihydropyrano[3,2-c] chromene derivatives in hydroalcoholic media. J Braz Chem Soc, 2011; 22(5):905–9. CrossRef
Jin T, Liu L, Zhao Y, Li T. Clean, One-pot synthesis of 4H-pyran derivatives catalyzed by hexadecyltrimethyl ammonium bromide in aqueous media. Synth Commun, 2005; 35(14):1859–63; https://doi.org/10.1081/SCC-200064898
Jin TS, Wang AQ, Wang X, Zhang JS, Li TS. A clean one-pot synthesis of tetrahydrobenzo[b]pyran derivatives catalyzed by hexadecyltrimethyl ammonium bromide in aqueous media. Synlett, 2004; 5:871–3; https://doi.org/10.1055/s-2004-820025
Kumar RR, Perumal S, Menéndez JC, Yogeeswar P, Sriram D. Antimycobacterial activity of novel 1,2,4-oxadiazole-pyranopyridine/chromene hybrids generated by chemoselective 1,3-dipolar cycloadditions of nitrile oxides. Bioorg Med Chem, 2011; 19:3444–50; https://doi.org/10.1016/j.bmc.2011.04.033
Nemr MTM, Yousif MNM, Barciszewski J. Interaction of small molecules with polynucleotide repeats, and frameshift site RNA. Arch Pharm, 2019; 352(80):1–7. CrossRef
Soliman HA, Yousif MNM, Said MM, Hassan NA, Ali MM, Awad HM, Abdel-Megeid FME. Synthesis of novel 1,6-naphthyridines, pyrano[3,2-c]pyridines and pyrido[4,3-d] pyrimidines derived from 2,2,6,6-tetramethylpiperidin-4-one for in vitro anticancer and antioxidant evaluation. Der Pharma Chemica, 2014; 6(3):394–410.
Subbareddy CV, Subbareddy, Sumathi S. One-pot three-component protocol for the synthesis of indolyl-4H-chromene-3-carboxamides as antioxidant and antibacterial agents. New J Chem, 2017; 41:9388–96; https://doi.org/10.1039/c7nj00980a
Wang X, Shi D, Du Y, Zhou Y, Tu S. Synthesis of 2-aminopyran derivatives and 3-arylpropionitrile derivatives catalyzed by KF/Al2O3. Synth Commun, 2004a; 34(8):1425–32; https://doi.org/10.1081/SCC-120030692
Wang X, Shi D, Tu S, Yao C. Synthesis and crystal structures of 2-amino-3-cyano-4-(2-chlorophenyl)-1,4-dihydro-2H-pyrano[3,2-h]quinoline and 2-amino-3-cyano-4-(2-chlorophenyl)-8-(2-chlorobenzylidene)-1,4,5,6,7,8-hexahydrobenzo[b]pyran. J Chem Crystallogr, 2004b; 34(2):159–65. CrossRef
Yousif MNM, El-Gazzar ABA, El-Enany MM. Synthesis and biological evaluation of pyrido(2,3-d)pyrimidines. Mini Rev Org Chem, 2021; 18. CrossRef
Yousif MNM, El-Sayed WA, Abbas HS, Awad HM, Yousif NM. Anticancer activity of new substituted pyrimidines, their thioglycosides and thiazolopyrimidine derivatives. J Appl Pharm Sci, 2017; 7(11): 21–32.
Yousif MNM, Fayed AA, Yousif NM. Reactions of triacetonamine part II, synthesis of novel pyrazolo(4,3-c)pyridine derivatives, and 2-(Piperidin-4-ylidene)hydrazinecarbothioamide derivatives derived from 2,2,6,6-tetramethyl-piperidin-4-one for in vitro anticancer evaluation. Der Pharma Chem, 2018; 10(8):105–9.
Yousif MNM, Fayed AA, Yousif NM. Synthesis and reactions of N-Hydroxy-triacetonamine derivatives. Egypt J Chem, 2019a; 62(8):1759–66.
Yousif MNM, Hussein HAR, Yousif NM, El-Manawaty MA, El-Sayed WA. Synthesis and cytotoxic activity of novel 2-phenylindole linked imidazolothiazole, thiazolo-s-triazine and imidazolyl-sugar Systems. J Appl Pharm Sci, 2019b; 9(1):6–14. CrossRef
Yousif MNM, Nassar IF, Yousif NM, Awad HM, El-Sayed WA. Synthesis and anticancer activity of new substituted piperidone compounds and their derived pyrimidine, thiazole and triazole glycoside derivatives. Russ J Gen Chem, 2019c; 89(8):1673–82. CrossRef
Yousif MNM, Soliman HA, Said MM, Hassan NA, Abdel-Megeid FME. Synthesis and biological activity of triacetonamine. Russ J Gen Chem, 2020; 90(3):460–9; https://doi.org/10.1134/S1070363220030202
Zonouzi A, Mirzazadeh R, Safavi M, Ardestani SK, Emami S, Foroumadi A. 2-Amino-4-(nitroalkyl)-4H-chromene-3-carbonitriles as new cytotoxic agents. Iran J Pharm Res, 2013; 12(4):679–85.