INTRODUCTION
Amnesia is characterized by memory and learning impairment. Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases affecting the elderly, and to date, there is no treatment capable of reversing, stopping, or significantly slowing down the disease’s progression. Dementia represents a severe public health issue affecting nearly 55 million people worldwide (Prince et al., 2016). The number of AD patients is projected to escalate and targets 88 million by 2050 (Alzheimer’s Association, 2019). Oxidative stress in the brain is one of the pathophysiologic conditions for memory and learning impairment. Oxidative stress in the brain accelerates the production of reactive oxygen species (ROS) and impairs brain homeostasis. This leads to increased acetylcholinesterase (AChE) and biogenic amine dysregulation (Li et al., 2018). Moreover, in Alzheimer’s type of neurodegeneration, the declined acetylcholine level, especially in the hippocampus of the brain, is also one of the key factors for the cognitive deficit (Souza et al., 2020). The level of acetylcholine decreases when there is an increase in the AChE enzyme. It is well known that the increase in AChE is also caused by the generation of free radicals due to the accumulation of amyloid beta (Aβ) peptides (Madav et al., 2019). In AD brain, AChE works by initiating the conversion of Aβ peptides to a highly neurotoxic compound which is in the form of neurofibrils (Postu et al., 2019). The deposition of neurofibrils induces AD type of learning and memory impairment that is eventually mediated by cholinergic hypofunction. Scopolamine, a nonselective muscarinic receptor antagonist, acts as an amnesic agent by causing blockade in the cholinergic signaling. Thus, the memory and learning ability of an organism will be affected. Scopolamine is an experimental tool used to induce AD and associated amnesia in animal models. The exploratory behavior, memory, learning, locomotor activity, spontaneous alternation behavior, biochemical changes (oxidative stress and biogenic amine dysfunction), and the anxiety level in the animal model are affected through the administration of scopolamine (Ucel et al., 2020). The present study evaluated the neuropharmacological effect of Prunus domestica on scopolamine-induced memory impairment in mice. Among the various traditional herbs as nootropic agents, P. domestica fruit is presumed to ameliorate memory and learning in aged rats (Shukitt-Hale et al., 2009) and its efficacy in an AD model is explored in this investigation. The common name for P. domestica is European Plum. It is also categorized as stone fruit. The main phytoconstituents that are present in P. domestica are vitamin A, vitamin B complex, vitamin K, amino acids, and carbohydrates besides magnesium, zinc, pectin, hemicellulose, cellulose, lignins, boron, dietary fibers, sorbitol, linalool, potassium, benzoic and boric acids, selenium, fructose, benzaldehyde, malic acid, citric acid, ethyl nonanoate, ethyl cinnamate, chlorogenic acid, caffeic acid, coumaric acid, neochlorogenic acid, proanthocyanidin, and melanoidins (Jabeen and Aslam, 2011). Pharmacological activities of P. domestica include anti-inflammatory (Hooshmand et al., 2015), anxiolytic (Bouayed et al., 2007), obesity (Noratto et al., 2014), antibacterial and antifungal activity (Pimenta et al., 2012), and antioxidant (Sharma and Sisodia, 2012), which are used to treat hyperhomocysteinemia (Haddadi-Guemghar et al., 2017) and also reveal to improve learning and memory (Shahidi et al., 2013), and P. domestica fruits have various other health benefits (Igwe and Charlton, 2016).
METHODOLOGY
Drugs and chemicals
Scopolamine (scopolamine butyl bromide) 10 mg tablets were purchased from Alpro Pharmacy, Nilai, Malaysia, whereas chemicals such as ethanol, 1,1-diphenyl-2-picrylhydrazyl (DPPH), Folin–Ciocalteu reagent, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), potassium persulfate, Na2CO2, and Ethylenediaminetetraacetic acid (EDTA) were from Merck. Acetylthiocholine iodide and 5,5’-dithio-bis-(2-nitrobenzoic acid) (DTNB) were purchased from Alfa Aesar.
Plant material and preparation of fruit extract
The fruits of P. domestica were bought from a commercial fruit vendor and the fruits were authenticated by the Biodiversity Unit, University Putra Malaysia, Malaysia. The collected fruits (1.5 kg) were washed and cut into small pieces and homogenized with alcohol. The fruits were added portion by portion in a blender and blended together with a sufficient amount (700 ml) of double-distilled ethanol to enable it to homogenize coarsely. Once blended, the mixture was subjected to filtration and concentrated using a rotary vacuum evaporator. The concentrated extract was then labeled and refrigerated at 8°C–15°C.
Determination of total phenolic content
The polyphenol content of both extracts was determined by using the Folin–Ciocalteu methods with some modification by expressing it as mg of gallic acid equivalent (GAE) per g extract (Zilic et al., 2014). Briefly, an aliquot of 1 ml extract was mixed with 5 ml of distilled water. To this, 1 ml of 2N Folin–Ciocalteu reagent was added and shaken for 3 minutes. Upon shaking, 2 ml of 2% (w/v) of sodium carbonate solution was added and incubated for 3 hours at room temperature. Then, the absorbance of the mixture was measured using a UV-visible spectrophotometer (Shimadzu, Japan) at 760 nm against the solvent blank which was distilled water. The total phenolic content was expressed as mg of GAE per g extract.
Free radical scavenging ability on DPPH
The DPPH assay was measured according to the method reported in Bloism’s (1958) study, with some modifications. The stock solution was prepared by dissolving 3.94 mg of DPPH with 10 ml of ethanol, whereas the stock concentration of the test samples was prepared and diluted to get five concentrations of 200, 100, 50, 25, and 10 μg/ml, respectively. Then, 2 ml of different concentrations of the test solution (200, 100, 50, 25, and 10 μg/ml) was mixed with 1 ml of DPPH solution. 2 methanol with 2 ml DPPH solution was used as the control and ethanol as the blank (Haddadi-Guemghar et al., 2017). The absorbance of each sample was read at 517 nm by using a UV-visible spectrophotometer (Shimadzu, Japan) after 30 minutes of incubation at room temperature in dark. The scavenging capacity of the extract was compared to L-ascorbic acid and the percentage inhibition was calculated.
2,2′-azino-bis ABTS radical scavenging
The free radical scavenging assay was determined by the ABTS method. 7 mM ABTS stock solution was prepared by diluting 9 mM ABTS into water and ABTS•+ was prepared by reacting the stock solution with 2.45 mM potassium persulfate (which was kept in the dark for 16 hours at room temperature). Then, it was diluted in ethanol (1:89 v/v) and equilibrated to 30°C to give an absorbance at 734 nm (Re et al., 1999). 30 μl of the extract was added to 3 ml of fresh ABTS radical action and incubated at room temperature for 15 minutes. The absorbance was read at 734 nm using a UV-visible spectrophotometer (Shimadzu, Japan) (Re et al., 1999). The extract concentration providing 50% inhibition (IC50) was obtained by plotting inhibition percentage versus extract concentration. The lower IC50 value indicates high antioxidant activity. The unit of total antioxidant activity is defined as the concentration of L-ascorbic acid having equivalent antioxidant activity expressed as μg/ml sample extract.
Pharmacological evaluation
Dose selection
According to Organisation for Economic Co-operation and Development (OECD) guidelines, the acute toxicity study using P. domestica in both male and female Swiss albino mice exhibited nontoxic and shown the Median lethal dose (LD50) value of the P. domestica to be >2,000 mg/kg (Swaroop et al., 2015). Based on the reported study, two doses were selected. Considering 2000 mg/kg as non-toxic dose, 1/10th of the 2000 mg (200 mg/kg) and 1/5th of the 2000 mg (400 mg/kg) was administered for the experimental animals.
Experimental design
Grouping and induction of amnesia
A total of 24 Swiss male mice were selected in this study which weighed approximately 20–30 g each, aged 6–7 weeks old. They were kept in the vivarium of KPJ Healthcare University College, Kota Seriemas, Nilai, Negeri Sembilan, at a temperature of 2 ± 2 °C and 12-hours light/dark cycle. The animals were divided randomly into four groups, each consisting of six animals. The animals had water ad libitum and food pellets. They were allowed to acclimatize to the laboratory environment for a week before the experiments. Group I, Control, was administered with normal saline orally and Group II, Negative Control, was administered with scopolamine 1 mg/kg orally. Groups III and IV were treated with a low dose of ethanolic extract of P. domestica (EEPD) 200 mg/kg and a high dose of EEPD 400 mg/kg, respectively, and induced with amnesia. The experimental study and the drug (EEPD) treatment duration was 15 days; scopolamine 1 mg/kg body weight (Deng et al., 2019) was administered orally from the 8th day to the 14th day in Groups II, III, and IV. On the day of the behavioral test, the drug was administered 30 minutes prior to the test. The experimental protocol adhered to the ethical guidelines on animal experimentation; the protocol was approved by KPJUC ethics committee (Ref. No.KPJUC/RMC/BPH/EC/2017/107).
Behavioral study
Y-maze test
Y-maze with the specifications of 40 cm long, 13 cm high, and 3 cm wide was used. The arms were constructed in such a way that they are 120°C symmetrically disposed to each other. The floor was made up of dark opaque polyvinyl plastic. On the 15th day of the treatment, 1 hour before the test, EEPD was treated in the treatment group and all the mice were subject to the test. All the three arms of the maze were labeled as A, B, and C, respectively. The mice were placed at the end of the arm labeled “A” and one food pellet was placed at all the three arms in order to motivate the movement of the mice. The number of arm entries and their sequence over 8 minutes were recorded. The ability to alternate requires that the mice know which arm they have already visited. The series of arm entries, including possible returns into the same arm, was recorded. Alteration is defined as the successive entries into the three arms on overlapping triplet sets. The percentage of alteration is calculated as the ratio of actual alterations to possible alterations (Wahl et al., 2017).
Open-field test
The field for the test was conducted to measure the locomotor activity, explorative, cognitive, and anxiety levels of the mice. The mice were placed in a 40 cm × 50 cm × 60 cm open field which was painted across with yellow stripes and consisted of 16 quadratic blocks. At the beginning of the test, the animals were placed at the center of the field and the spontaneous ambulatory locomotion of each animal was observed for a duration of 5 minutes. During this period, the number of lines crossed, the number of head dipping, and the number of rearing were measured (Jayasingh Chellammal et al., 2019).
Traction test
The effect of scopolamine, as well as the extract of P. domestica treatment, on motor coordination of the mice was evaluated using the traction test. The experimental set-up was done using a horizontal bar of 12 mm in size, one 12-inch length fixed in two poles at a height of 40 cm. The height of the setup allows the animal sufficient time and space to land by losing muscle grip on its feet due to the righting reflex (Jänicke and Coper, 1996). The set-up was arranged on the tabletop and the animals were allowed to get a grip with their hind limbs on the horizontal bar. The total motivation time in seconds to hang onto the bar from the aversion to falling is considered as grip index. The motivation time is considered to be proportional to muscle grip strength and balance. Once done with the set-up, the mice were suspended on the static bar and the time taken (in seconds) for the mice to reestablish themselves and retain on the bar was recorded.
Biochemical studies
Determination of AChE
The brains were removed and rinsed with ice-cold normal saline before being dried and weighed. Then, the brain homogenates were prepared at a concentration of 10% (w/v) in an ice-cold medium [1:9 w/v of a 50 mM phosphate-buffered saline, pH 7.0] containing 0.1 mmol/l EDTA. The homogenates were then subjected to centrifugation at 4,000 rpm for 30 minutes at 4°C to prepare clear supernatants (10%). The assay was carried out by taking 0.1 mL of supernatant, added with 6 ml of sodium phosphate buffer (pH 8), 0.2 ml of acetylthiocholine iodide, and 02 ml of DTNB (Ellman reagent). The absorbance of the solution was recorded at 412 nm (Nanaware et al., 2017).
Statistical analysis
The data obtained were recorded and analyzed using one-way analysis of variance (ANOVA), followed by post hoc Tukey’s test in the GraphPad7 Prism software. The analyzed data were expressed as mean ± SEM and p < 0.05 was considered to be statistically significant.
RESULTS
Drug extract and percentage yield
The characteristic nature of the EEPD exhibited as dark brown, creamy, semisolid and percentage yield was noted as 13 compared to the original material.
Total phenolic content
The total phenolic content in the EEPD was 0.182 mg GAE/g. The result was obtained from a calibration curve (y = 0.003x + 1.105, R2 = 0.996) of gallic acid and was expressed as GAE.
DPPH scavenging capacity
The DPPH radical scavenging (%) activity is shown in Figure 1. P. domestica extract exerted the highest inhibition of 88.48% at 200 μg/ml. Ascorbic acid was used as the reference. The IC50 of the EEPD extract was 52.45 μg/ml, while that of the ascorbic acid was 75 μg/ml.
ABTS radical scavenging
The ABTS radical scavenging (%) activity is shown in Figure 2. EEPD exerted an inhibition of 85.33 at 200 μg/ml. The absorbance at 734 nm indicated R2 of 0.8826. IC50 value for EEPD was 23.77 μg/ml compared to that of ascorbic acid at 130 μg/ml.
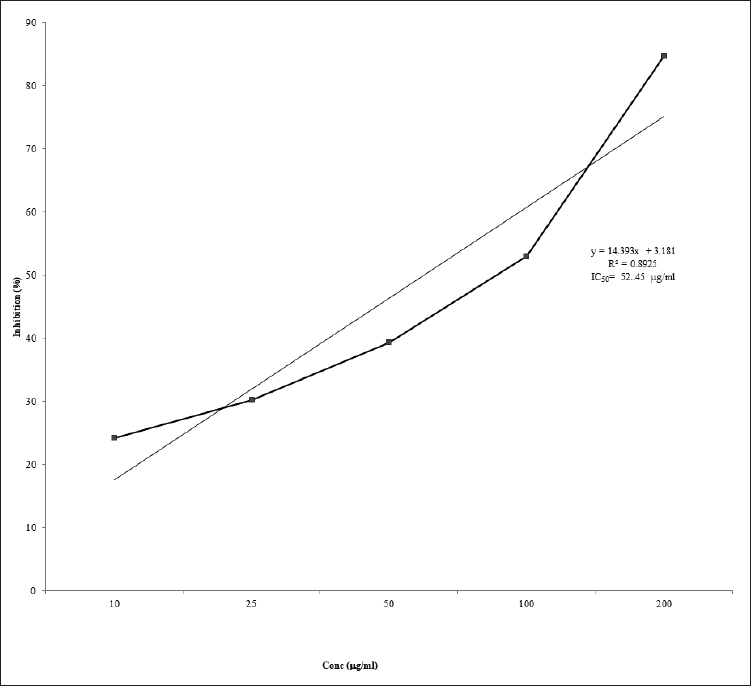 | Figure 1. Effect of P. domestica Ethanolic extract on DPPH scavenging. [Click here to view] |
Behavioral study
Open-field test
The treatment using EEPD (200 and 400 mg/kg) exhibited a significant (p < 0.001; p < 0.01) effect, respectively, on the head dips of open-field acquisition of memory compared to the negative control group (Group II). The results obtained indicated that the high dose of P. domestica (400 mg/kg) exhibited better exploratory behavior in head dipping (Table 1). The effect of EEPD on line crossings indicated that the administration of 200 and 400 mg/kg EEPD exhibited significant differences of p < 0.0001 and p < 0.01, respectively. In rearing, the result obtained indicated that the animal treated with 200 mg/kg exhibited a significant effect (p < 0.05) compared to the disease-induced group, while the high dose treated animals exhibited an improved behavioral performance (p < 0.001); also, a significant (p < 0.05) dose-dependent increase in rearing was noted between 200 and 400 mg/kg EEPD.
Y-maze test
The results obtained for the effect of EEPD on the percentage of spontaneous alternation in Y-maze are depicted in Table 2. The control group was compared with the negative control group (scopolamine-induced) and it was found that the induction of amnesia was significant. After the treatment, on comparing the negative group and the negative control group, both the low dose (200 mg/kg) and high dose (400 mg/kg) treated animals showed a significant (p < 0.001) improvement in memory and learning ability.
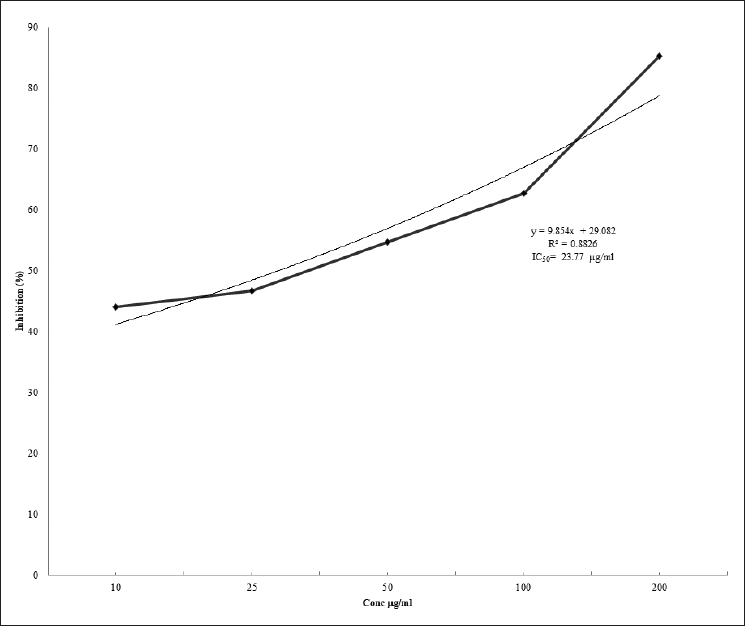 | Figure 2. Antioxidant effect P. domestica ethanolic extract on ABTS. [Click here to view] |
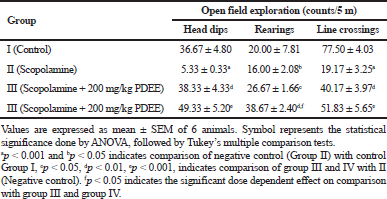 | Table 1. Effect of P. domestica open field exploration. [Click here to view] |
Traction test
In the traction test, the scopolamine-induced amnesiac group (negative control) exhibited loss of muscle coordination, which is considered as a sign of neurotoxicity in general. The animals in the negative control (p < 0.01) showed a significant reduction in retention time and downfall in a short duration of time when compared to the normal control group. The administration of EEPD has shown a significant effect on enhancing the motor coordination and balancing ability in mice; however, only the higher dose (EEPD 400 mg/kg) exhibited the neuroprotective effect. The animals after treatment with EEPD 400 mg/kg exhibited a significant (p < 0.001) difference in time, spending more time balancing when compared to the negative control group. The results are depicted in Table 2.
Biochemical study
Acetylcholinesterase enzyme
The effect of EEPD on AChE inhibitory activity is shown in Table 2 and the results indicate that the treated groups show a significant reduction in comparison to the negative control group. The negative control animals expressed a significantly (p < 0.001) higher level of AChE enzyme upon comparison with the control group after the induction of amnesia. In the treatment group, the enzyme levels have reduced as indicative of neuroprotection. The 200 mg/kg EEPD treated group exhibited the inhibition of AChE enzyme level to 11.27 ± 1.19 (μg/minute/mg protein) which was significantly (p < 0.05) lower when compared to the negative control group with the enzyme level, 17.23 ± 2.10 (μg/minute/mg protein). In the high dose (400 mg/kg EEPD) treated animals, the enzyme levels are significantly (p < 0.01) reduced to 9.01 ± 1.05 μg/minutes/mg protein and indicate the protective effect when compared to the negative control group.
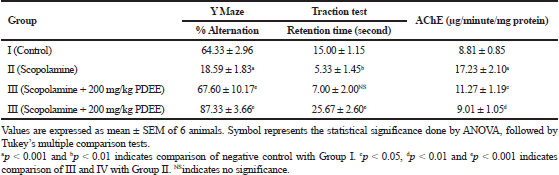 | Table 2. Effect of P. domestica Y Maze, traction test & AChE. [Click here to view] |
DISCUSSION
In the present investigation, our studies reveal the antioxidant and neuroprotective potential of P. domestica ethanolic extract. The results demonstrated that EEPD has the potential antioxidant properties and antiamnesiac effect on scopolamine-induced amnesia. This can be well correlated with improved behavioral activities and regulated cholinergic transmission through cholinesterase enzyme inhibition.
Oxidative stress is a major contributing factor for cognitive dysfunction and neurodegenerative disease which eventually leads to the progression of amnesia or disruption on memory and learning (Ngoupaye et al., 2017). The deterioration of the cholinergic neurotransmitter system is the pivotal target upon oxidative stress-induced neurodegeneration. The ROS or free radicals are responsible for the destruction of cholinergic producing cells in the basal forebrain area. ROS is the byproduct containing hydroxyl radical and is responsible for the deterioration of biomolecules, such as nucleic acid, protein, and lipids, which act as the main offender in inflammation-related diseases, especially in neurodegeneration (Wang et al., 2020).
It is well known that phytochemicals play a great therapeutic role in oxidative stress-induced neurodegenerative disease. Scopolamine induces amnesia and oxidative stress which eventually lead to Alzheimer’s type of dementia and it was studied with various phytochemicals for neuroprotection (Sawikr et al., 2017). In our investigation, the IC50 of P. domestica exhibited significant antioxidant and free radical scavenging activities that were comparable to references of ascorbic acid and the antioxidant potential is dose-dependent as the inhibition percentage increases dramatically with the increase of concentration. The highest concentration of the extract exhibited a high percentage of inhibition with a low number of absorbance. Ethanol extract from P. domestica exhibited good reducing power and showed a strong correlation in the antioxidant assay with the presence of phenolic compounds. The antioxidant activity exhibited by these plants is partly ascribed to the phenolic and flavonoids compounds. A previous study showed that free radical scavenging and antioxidant activity strongly correlated with aromatic, phenolic, and flavonoid contents due to redox activity (Cassidy et al., 2020).
Prunus domestica exhibits various pharmacological activities and it is reported to be used as a traditional medicine to enhance memory and learning efficiency in normal mice (Shahidi et al., 2013). In our study, we attempted to reveal the effect of P. domestica on habituation and behavioral memory with its influence on cholinergic neurotransmission. In neuroprotective evaluation, behavioral studies with Y-maze and open-field habituation memory exhibited promising results on cognitive improvement after scopolamine-induced amnesia. In the Y-maze test, the number of entries in each arm was observed and recorded as ABC, CAB, and BAC. There was a significant improvement, which indicates that there is a significant effect produced by the EEPD on treating memory and learning impairment. The open-field test was conducted followed by the Y-maze test. The purpose of this test was to measure the exploratory behavior of the mice in a new environment, anxiety level, and the level of cognition. In the open-field test, the study showed that the administration of scopolamine exhibited a significant effect on declining the memory and learning level in the mice and it was clearly observable in the line crossing test, rearing, and number of head dipping test. Open-field exploration and habituation memory are considered to be disturbed in age-related disease (Deacon et al., 2009) in animal models and studies suggest that phytoconstituents rich in polyphenols and flavonoids tend to improve (Ramirez et al., 2005). In our study, it is evident from the open-field test that the treatment with EEPD enhanced cognitive performance. In the traction test, the time taken for the mice to retain on the retort bar was recorded and this test was carried out to examine the changes in sensorimotor performance during aging (Jänicke and Coper, 1996). In this test, it was indicated that only the high dose group (EEPD 400 mg/kg) produced a significant effect toward amnesic mice, whereas the low dose group had no significant activity toward the amnesia induced by scopolamine, but an increase in the mean was observed in this group compared to the negative control group. This shows that EEPD is involved in neuroprotection and improves the sensorimotor performance in aging.
Administration of scopolamine for a prolonged period of time causes amnesia and this induction model is associated with Alzheimer’s model. Scopolamine is a nonselective muscarinic acetylcholine receptor which has the tendency to reduce the induction of long-term potentiation in the brain which relates to the decrease in memory and learning ability (Schon, 2005) and additionally the learning impairment is also known to be caused by oxidative stress, followed by a decreased acetylcholine level in the hippocampal region. The decrease of acetylcholine in the brain is due to the increase in the AChE enzyme (AChE) which plays a role in hydrolyzing the acetylcholine present in both cholinergic and noncholinergic neurons. The influence of oxidative stress is remarkable during neuronal stress which potentiates cognitive deterioration (Vanova et al., 2018). In our study, we evaluated the effect of EEPD on AChE activity and interpreted its neuroprotective activities. EEPD inhibited the enzyme significantly in the mice brain at two doses (200 and 400 mg/kg), which represent the neurocognitive improvement exerted by the P. domestica extract. The results of this research implicate the remarkable therapeutic involvement through AChE inhibition and oxidative stress diminution.
CONCLUSION
In conclusion, it is evident that P. domestica potentially exerts an antiamnesic effect through regulation of oxidative stress and AChE inhibition. This reveals that P. domestica could be therapeutically active for the treatment of Alzheimer’s type of dementia. Our study suggests further exploration with isolated phytoconstituents in relation to neuroinflammation and oxidative regulation with AChE inhibition in transgenic Alzheimer’s model values for an additional mechanistic impact.
ACKNOWLEDGMENTS
The authors would like to thank KPJ Healthcare University College for providing the facility for conducting the study.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
None.
REFERENCES
Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement, 2019; 15:321–87. CrossRef
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958; 181:1199–1200. CrossRef
Bouayed J, Rammal H, Dicko A, Younos C, Soulimani R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J Neurol Sci, 2007; 262:77–84. CrossRef
Cassidy L, Fernandez F, Johnson JB, Naiker M, Owoola AG, Broszczak DA. Oxidative stress in Alzheimer’s disease: a review on emergent natural polyphenolic therapeutics. Complement Ther Med, 2020; 49:102294. CrossRef
Deacon RMJ, Koros E, Bornemann KD, Rawlins JNP. Aged Tg2576 mice are impaired on social memory and open field habituation tests. Behav Brain Res, 2009; 197:466–8. CrossRef
Deng G, Wu C, Rong X, Li S, Ju Z, Wang Y, Ma C, Ding W, Guan H, Cheng X, Liu W, Wang C. Ameliorative effect of deoxyvasicine on scopolamine-induced cognitive dysfunction by restoration of cholinergic function in mice. Phytomedicine, 2019; 63:153007. CrossRef
Haddadi-Guemghar H, Tlili A, Dairou J, Paul JL, Madani K, Janel N. Effect of lyophilized prune extract on hyperhomocysteinemia in mice. Food Chem Toxicol, 2017; 103:183–7. CrossRef
Hooshmand S, Kumar A, Zhang JY, Johnson SA, Chai SC, Arjmandi BH. Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food Funct, 2015; 6:1719–25. CrossRef
Igwe EO, Charlton KE. A systematic review on the Health Effects of Plums (Prunus domestica and Prunus salicina). Phyther Res, 2016; 30(5):701–31. CrossRef
Jabeen Q, Aslam N. The pharmacological activities of prunes: the dried plums. J Med Plants Res, 2011; 5:1508–11.
Jänicke B, Coper, H. Tests in rodents for assessing sensorimotor performance during aging. Adv Psychol Study, 1996; 114:201–233. CrossRef
Jayasingh Chellammal HS, Veerachamy A, Ramachandran D, Gummadi SB, Manan MM, Yellu NR. Neuroprotective effects of 1`δ-1`-acetoxyeugenol acetate on Aβ(25-35) induced cognitive dysfunction in mice. Biomed Pharmacother, 2019; 109:1454–61. CrossRef
Li SP, Wang YW, Qi SL, Zhang YP, Deng G, Ding WZ, Ma C, Lin QY, Guan HD, Liu W, Cheng XM, Wang CH. Analogous β-carboline alkaloids harmaline and harmine ameliorate scopolamine-induced cognition dysfunction by attenuating acetylcholinesterase activity, oxidative stress, and inflammation in mice. Front Pharmacol, 2018; 9:346. CrossRef
Madav Y, Wairkar S, Prabhakar B. Recent therapeutic strategies targeting beta amyloid and tauopathies in Alzheimer’s disease. Brain Res Bull, 2019; 146:171–84. CrossRef
Nanaware S, Shelar M, Sinnathambi A, Mahadik KR, LohidasanS,. Neuroprotective effect of Indian propolis in β-amyloid induced memory deficit: impact on behavioral and biochemical parameters in rats. Biomed Pharmacother, 2017; 93:543–53. CrossRef
Ngoupaye GT, Pahaye DB, Ngondi J, Moto FCO, Bum EN. Gladiolus daleniilyophilisate reverses scopolamine-induced amnesia and reduces oxidative stress in rat brain. Biomed Pharmacother, 2017; 91:350–7. CrossRef
Noratto GD, Garcia-Mazcorro JF, Markel M, Martino HS, Minamoto Y, Steiner JM, Byrne D, Suchodolski JS, Mertens-Talcott SU. Carbohydrate-free peach (Prunus persica) and plum (Prunus domestica) juice affects fecal microbial ecology in an obese animal model. PLoS One, 2014; 9(7):e101723. CrossRef
Pimenta RS, Moreira da Silva JF, Buyer JS, Janisiewicz WJ. Endophytic Fungi from Plums (Prunus domestica) and their antifungal activity against Moniliniafructicola. J Food Prot, 2012; 75:1883–9. CrossRef
Postu PA, Sadiki FZ, El Idrissi M, Cioanca O, Trifan A, Hancianu M, Hritcu L. Pinushalepensis essential oil attenuates the toxic Alzheimer’s amyloid beta(1-42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed Pharmacother, 2019; 112:108673. CrossRef
Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer report 2016 improving healthcare for people living with dementia. Coverage, quality and costs now and in the future. Alzheimer’s Dis Int, 2016; 1-140. [ONLINE] Available in LSE Research Online: http://eprints.lse.ac.uk/67858/ September 2016 [Accessed 25 August 2020].
Ramirez M, Izquierdo I, Raseira M, Zuanazzi J, Barros D, Henriques A. Effect of lyophilised berries on memory, anxiety and locomotion in adult rats. Pharmacol Res, 2005; 52:457–62. CrossRef
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med, 1999; 26:1231–7. CrossRef
Sawikr Y, Yarla NS, Peluso I, Kamal MA, Aliev G, Bishayee A. Neuroinflammation in Alzheimer’s disease, in: advances in protein chemistry and structural biology. Adv Protein Chem Struct Biol, 2017; 108:33–57. CrossRef
Schon K. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci, 2005; 25:9112–23. CrossRef
Shahidi S, Setareye S, Mahmoodi M. Effect of Prunus domestica L. (mirabelle) on learning and memory in mice. Anc Sci Life, 2013; 32:139. CrossRef
Sharma G, Sisodia R. Accepted abstracts from the international brain injury association’s ninth world congress on brain injury. Brain Inj, 2012; 26:309–799. CrossRef
Shukitt-Hale B, Kalt W, Carey AN, Vinqvist-Tymchuk M, McDonald J, Joseph JA. Plum juice, but not dried plum powder, is effective in mitigating cognitive deficits in aged rats. Nutrition, 2009; 25:567–73. CrossRef
Souza SP, Roos AA, Gindri AL, Domingues VO, Ascari J, Guerra GP. Neuroprotective effect of red quinoa seeds extract on scopolamine-induced declarative memory deficits in mice: the role of acetylcholinesterase and oxidative stress. J Funct Foods, 2020; 69:103958. CrossRef
Swaroop A, Bagchi M, Kumar P, Preuss HG, Bagchi D. Safety and efficacy of a novel Prunus domestica extract (Sitoprin, CR002) on testosterone-induced benign prostatic hyperplasia (BPH) in male Wistar rats. Toxicol Mech Methods, 2015; 25:653–64. CrossRef
Ucel UI, Can OD, Demir U, Ulupinar E. Antiamnesic effects of tofisopam against scopolamine-induced cognitive impairments in rats. Pharmacol Biochem Behav, 2020; 190:172858. CrossRef
Vanova N, Pejchal J, Herman D, Dlabkova A, Jun D. Oxidative stress in organophosphate poisoning: role of standard antidotal therapy. J Appl Toxicol, 2018; 38:1058–70. CrossRef
Wahl D, Coogan S, Solon-Biet, S de Cabo R, Haran J, Raubenheimer D, Cogger V, Mattson M, Simpson S, Le Couteur D. Cognitive and behavioral evaluation of nutritional interventions in rodent models of brain aging and dementia. Clin Interv Aging Vol, 2017; 12:1419–28. CrossRef
Wang L, Pu Z, Li M, Wang K, Deng L, Chen W. Antioxidative and antiapoptosis: neuroprotective effects of dauricine in Alzheimer’s disease models. Life Sci, 2020; 243:117237. CrossRef
Zilic S, Basic Z, Hadzi V, Maksimovic V, Jankovic M, Filipovic M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int J Food Sci Technol, 2014; 49:1040–7. CrossRef