INTRODUCTION
Cancer continues to be a major health concern in all over the world outpacing the cardiac diseases. It stands in the first position in mortality rate due to distinct factors. Even though many major advancements have been made in the management of cancer chemotherapy for some patients, there is a need for continued effort in the identification of efficacious novel anticancer agents with minimal side effects (Eckhardt, 2002; El-Azab et al., 2010). The contemporary approaches have been focused mainly on the design of ideal anticancer agents, which eradicate the cancer cells without involving the normal tissues. Inappropriately, none of the reported drugs meet this criterion.
Quinazolines are being an excellent reservoir of bioactive substances and have exhibited a wide spectrum of biological activities, especially antiproliferative (Cao et al., 2015; Govindaraj et al., 2009; Ho et al., 2002; Raffa et al., 2004; Rajveer et al., 2010). The stable nature of the quinazoline nucleus has encouraged the medicinal chemists to bring molecular modifications to this nucleus to synthesize new potential medicinal agents (Ghorab et al., 2016). It is evident from the literature that the quinazoline derivatives are effective as epidermal growth factor receptor (EGFR) inhibitors (Barbosa et al., 2014; Dissoki et al., 2007; Fernandes et al., 2007; Li et al., 2012; Mishani et al., 2004; Zhao et al., 2013; Zhang et al., 2014). The EGFR is a transmembrane glycoprotein, which belongs to the erbB family of closely related cell membrane receptors that include EGFR (erbB-1 or HER1), erbB-2 (HER2), erbB-3 (HER3), and erbB-4 (HER4). The expression and overexpression of EGFR are observed in many human solid tumors including breast, ovarian, non-small cell lung (NSCLC), colorectal, and head and neck cancers. In support of their important role in tumor biology, an EGFR activation may assist in tumor growth by increasing cell proliferation, motility, adhesion, invasive capacity, and blocking apoptosis. Due to their multidimensional role in the progression of cancer, EGFR is emerged as an attractive drug target for anticancer therapy (Seshacharyulu et al., 2012).
Recently, quinazolines were reported as versatile template molecules for the inhibition of a wide range of tyrosine kinases. Among these, EGFR is more widely studied target. Gefitinib, a small-molecule inhibitor from this class, was first to be approved for treating NSCLC refractory before chemotherapeutic intervention (Cohen et al., 2003). In view of the above observations and continuation of the research program on aiming to synthesize biologically active heterocycles for anticancer potentials (Singh et al., 2013), we report the design, synthesis, and anticancer activity evaluation of new 2, 4-disubstituted quinazolines.
MATERIALS AND METHODS
The synthesized compounds were checked for their melting point by an open glass capillary method and are uncorrected. The KBr pellet technique was adopted to record IR spectra using Shimadzu FT-IR 8400-S spectrophotometer. 1H Nuclear Magnetic Resonance (NMR) and 13C NMR spectra were recorded in AMX-400 NMR spectrophotometer at 400 MHz, in which Dimethyl sulfoxide DMSO-d6 was used as solvent and tetramethylsilane as an internal standard. The chemical shifts were expressed in δ ppm. Mass spectra were recorded in the Shimadzu LC-MS-2010A instrument by electrospray ionization technique.
Synthesis of 2-phenylquinazolin-4(3H)-one (2a)
An equimolar quantity of anthranilic acid (0.1 mol) and benzamide (0.1 mol) was taken in a glass mortar, mixed well, and then transferred into a round bottom flask and were heated at 130°C for 5 hours, a solvent-free reaction. The reaction progress was monitored by Thin Layer Chromatography (TLC). Then, the reaction mixture was poured on cold water and stirred. The solid obtained was filtered, washed with sodium bicarbonate solution (10%) to remove unreacted acid, dried, and recrystallized from ethanol to afford the compound (2a). A similar procedure was followed to synthesize 2b and 2c using pyridine-3-carboxamide and pyrazine-2-carboxamide, respectively.
2-phenylquinazolin-4(3H)-one (2a)
Yield: 58%. mp 136°C–138°C. IR (KBr) cm-1:1582 (C=N), 1,680 (C=O), 3,071 (C-H), 3,500 (N-H); 1H NMR (DMSO-d6, δ ppm): 7.26–8.33 (m, 5H, ArH), 8.1 (s, 1H, NH); 13C NMR (DMSO-d6, δ ppm): 120.9, 122.4, 126.1 (2), 127.4, 128.7, 128.8, 128.9 (2), 130.2, 133.5, 151.3, 159.0, (C=N), 161.0 (C=O); LC-MS m/z: 222.24 (M+).
2-(pyridin-3-yl)quinazolin-4(3H)-one (2b)
Yield: 69%. mp 142°C–143°C. IR (KBr) cm-1: 1,584 (C=N), 1,681 (C=O), 3,070 (C-H), 3,510 (N-H); 1H NMR (DMSO-d6, δ ppm): 7.62–8.04 (m, 4H, ArH), 8.2 (s, 1H, NH), 7.58–9.07 (m, 4H, ArH); 13C NMR (DMSO-d6, δ ppm): 120.9, 122.4, 123.9, 127.4, 128.0, 128.8, 133.5, 134.1, 147.1, 148.4, 151.3, 159.0, (C=N),161.0(C=O); LC-MS m/z: 223.23 (M+).
2-(pyrazin-2-yl) quinazolin-4(3H)-one (2c)
Yield: 77%. mp 149°C–150°C. IR (KBr) cm-1: 1,582 (C=N), 1,585 (C=N), 1,683 (C=O), 3,073 (C-H), 3512 (N-H); 1H NMR (DMSO-d6, δ ppm): 7.64–8.02 (m, 3H, ArH), 8.4 (s, 1H, NH), 8.76–9.34 (m, 2H, ArH); 13C NMR (DMSO-d6, δ ppm): 120.9, 122.4, 127.4, 128.8, 133.5, 141.2, 143.3, 144.3, 145.7, 147.1, 159.8, (C=N), 160.8 (C=O); LC-MS m/z: 224.22 (M+).
Synthesis of compounds N-(4-methoxyphenyl)-2-phenylquinazolin-4-amine (3a)
2-phenylquinazolin-4(3H)-one (0.01 mol), 4-methoxybenzenamine (0.01 mol), and zinc chloride (1.0 g) in a 15 ml of dimethyl formamide were taken in a round bottom flask and were refluxed at 150°C for 48 hours. The reaction progress was monitored by TLC. Then, the reaction mixture was cooled and poured on ice cubes. The solid obtained was collected by filtration, washed with dichloromethane, and recrystallized from ethanol to afford the compound 3a. A similar procedure was followed to synthesize 3b–f, 4a–c, 5a–c, and 6a–c using appropriate 2-substituted quinazolin-4(3H)-one with arylamide/aryl hydrazide/phenylhydrazine.
N-(4-methoxyphenyl)-2-phenylquinazolin-4-amine (3a)
Yield: 51%. mp 177°C–178°C. IR (KBr) cm-1: 1,649 (C=C), 1,483 (C=N), 3,340 (N-H); 1H NMR (DMSO-d6, δ ppm): 3.83 (s, 3H, OCH3), 9.86 (s, 1H, NH), 7.12–7.65 (m, 2H, ArH of methoxyphenyl), 7.78–8.26 (m, 4H, quinazoline), 7.56–8.58 (m, 5H, ArH); 13C NMR (DMSO-d6, δ ppm): 55.9, 115.1(2), 116.2, 117.3 (2), 120.1, 126.5, 127.5 (2), 128.8, 128.9, 129.3 (2), 130.7, 133.7, 135.4, 150.0, 150.7, 161.0, 170.0; LC-MS m/z: 327.38 (M+).
N-(4-methoxyphenyl)-2-(pyridin-3-yl)quinazolin-4-amine (3b)
Yield: 45%. mp 164°C–165°C; IR (KBr) cm-1: 1,649 (C=C), 1,483 (C=N), 3,336 (N-H); 1H NMR (DMSO-d6, δ ppm): 3.83 (s, 3H, OCH3), 9.96 (s, 1H, NH), 7.02–7.55 (m, 2H, ArH of methoxyphenyl), 7.58–8.16 (m, 4H, quinazoline), 7.57–9.24 (m, 4H, pyridine); 13C NMR (DMSO-d6, δ ppm): 55.9, 115.1 (2), 117.3 (2), 116.2, 120.1, 124.0, 126.5, 128.9, 133.0, 133.7, 134.1,135.4, 148.0, 149.1, 150,0, 150.7, 161.0, 170.0; LC-MS m/z: 328.37 (M+).
N-(4-methoxyphenyl)-2-(pyrazin-2-yl)quinazolin-4-amine (3c)
Yield: 57%. mp 159°C–160°C; IR (KBr) cm-1: 1,649 (C=C), 1,275 (C-O), 1,535 (C=N), 3,350 (N-H);1H NMR (DMSO-d6, δ ppm): 3.92 (s, 3H, OCH3), 10.24 (s, 1H, NH), 7.58–7.87 (m, 2H, ArH of methoxyphenyl), 7.68–8.26 (m, 4H, quinazoline), 7.45–7.48 (m, 3H, pyrazine); 13C NMR (DMSO-d6, δ ppm): 55.9, 115.1 (2), 116.2, 117.3 (2), 120.1, 126.5, 128.9, 133.7, 135.4, 141.2, 142.7, 144.1, 144.5, 150,0, 150.7, 161.0, 170.0; LC-MS m/z: 329.36 (M+).
N-(4-fluorophenyl)-2-phenylquinazolin-4-amine (3d)
Yield: 51%. mp 194°C–195°C; IR (KBr) cm-1: 1,644 (C=C), 1,276 (C-O), 1,483 (C=N), 3,333 (N-H); 1H NMR (DMSO-d6, δ ppm): 10.25 (s, 1H, NH), 7.41–7.46 (m, 2H, ArH of fluorophenyl), 7.68–8.36 (m, 4H, quinazoline), 7.61–8.35 (m, 5H, ArH); 13C NMR (DMSO-d6, δ ppm): 116.2, 116.3(2), 117.9(2), 120.1, 126.5, 127.5 (2), 128.8, 128.9, 129.3 (2), 130.7, 133.7, 138.7, 150.0, 152.9, 61.0, 170.0; LC-MS m/z: 315.38 (M+).
N-(4-fluorophenyl)-2-(pyridin-3-yl) quinazolin-4-amine (3e)
Yield: 58%. mp 209°C–210°C; IR (KBr) cm-1: 1,644 (C=C), 1,480 (C=N), 3,334 (N-H); 1H NMR (DMSO-d6, δ ppm): 9.92 (s, 1H, NH), 7.41–7.61 (m, 2H, ArH of fluorophenyl), 7.68–8.26 (m, 4H, quinazoline), 7.67–9.14 (m, 4H, pyridine); 13C NMR (DMSO-d6, δ ppm): 116.2, 116.3 (2), 117.9 (2), 120.1, 124.0, 126.5, 128.9, 133.0, 133.7, 134.1, 138.7, 148.0, 149.1, 150,0, 152.9, 161.0, 170.0; LC-MS m/z: 316.33 (M+).
N-(4-fluorophenyl)-2-(pyrazin-2-yl) quinazolin-4-amine (3f)
Yield: 59%. mp 181°C–182°C; IR (KBr) cm-1: 1,644 (C=C), 1,483 (C=N), 3,343 (N-H); 1H NMR (DMSO-d6, δ ppm): 9.96 (s, 1H, NH), 7.36–7.51 (m, 2H, ArH of fluorophenyl), 7.56–8.26 (m, 4H, quinazoline), 8.66–9.44 (m, 3H, pyrazine); 13C NMR (DMSO-d6, δ ppm): 116.2, 116.3 (2), 117.9(2), 120.1, 126.5, 128.9, 133.7, 138.7, 141.2, 142.7, 144.5, 144.7, 150.0, 152.9, 161.0, 170.0; LC-MS m/z: 317.32 (M+).
4-chloro-N’-(2-phenylquinazolin-4-yl) benzohydrazide (4a)
Yield: 60%. mp 189°C–190°C; IR (KBr) cm-1: 1,644 (C=C), 1,483 (C=N), 3,128 (N-H), 550 (C-Cl), 1,640 (C=O); 1H NMR (DMSO-d6, δ ppm): 9.91 (s, 1H, NH), 10.27 (d, 1H, CONH), 7.16–7.87 (m, 2H, ArH of chlorobenzene), 7.56–8.79 (m, 4H, quinazoline), 7.51–8.38 (m, 5H, phenyl); 13C NMR (DMSO-d6, δ ppm): 116.2, 120.1, 126.5, 127.5 (2), 128.8, 128.9 (3), 129.0(2), 129.3 (2), 130.7, 132.3, 133.7, 137.7, 150.0, 161.0, 164.9, 170.0; LC-MS m/z: 374.82 (M+).
4-chloro-N’-(2-(pyridin-3-yl) quinazolin-4-yl) benzohydrazide (4b)
Yield: 58%. mp 119°C–220°C; IR (KBr) cm-1: 1,644 (C=C), 1,483 (C=N), 3,353 (N-H), 550 (C-Cl), 1,640 (C=O); 1H NMR (DMSO-d6, δ ppm): 9.96 (s, 1H, NH), 8.0 (d, 1H, CONH), 7.66–7.96 (m, 2H, chlorobenzene), 7.59–8.26 (m, 4H, quinazoline), 7.26–8.69 (m, 4H, pyridyl); 13C NMR (DMSO-d6, δ ppm): 116.2, 120.1, 124.0, 126.5, 128.9 (3), 129.0 (2), 132.3, 133.0, 133.7, 134.1, 137.7, 148.0, 149.1, 150.0, 161.0, 164.9, 170.0; LC-MS m/z: 375.08 (M+).
4-chloro-N-(2-(pyrazin-2-yl) quinazolin-4-yl) benzohydrazide (4c)
Yield: 46%. mp 200°C–201°C; IR (KBr) cm-1: 1,644 (C=C), 1,483 (C=N), 3,353 (N-H), 550 (C-Cl), 1,640 (C=O); 1H NMR (DMSO-d6, δ ppm): 10.03 (s, 1H, NH), 8.0 (d, 1H, CONH), 7.57–7.87 (m, 2H, chlorobenzene), 7.68–8.26 (m, 4H, quinazoline), 8.86–9.24 (m, 3H, pyrazine); 13C NMR (DMSO-d6, δ ppm): 116.2, 120.1, 126.5, 128.9 (3), 129.0 (2), 132.3, 133.7, 137.7, 141.2, 142.7, 144.5, 144.7, 150,0, 161.0, 164.9, 170.0; LC-MS m/z: 376.08 (M+).
N-(2-phenylquinazolin-4-yl) isonicotinohydrazide (5a)
Yield: 59%. mp 237°C–238°C; IR (KBr) cm-1: 1,644 (C=C), 1,483 (C=N), 3,354 (N-H), 1,640 (C=O); 1H NMR (DMSO-d6, δ ppm): 10.21 (s, 1H, NH), 8.0 (d, 1H, CONH), 7.71–8.59 (m, 4H, pyridyl), 7.68–8.26 (m, 4H, quinazoline), 7.31–8.38 (m, 5H, ArH); 13C NMR (DMSO-d6, δ ppm): 116.2, 120.1, 122.8 (2), 126.5, 127.5 (2), 128.8, 128.9, 129.3 (2), 130.7, 133.7, 140.9, 149.8 (2), 150,0, 161.0, 164.9, 170.0; LC-MS m/z: 341.10 (M+).
N-(2-(pyridin-3-yl) quinazolin-4-yl) isonicotinohydrazide (5b)
Yield: 60%. mp 249°C–250°C; IR (KBr) cm-1: 1,644 (C=C), 1,483 (C=N), 3,354 (N-H), 1,640 (C=O); 1H NMR (DMSO-d6, δ ppm): 9.98 (s, 1H, NH), 8.0 (d, 1H, CONH), 7.26–8.49 (m, 4H, pyridyl), 7.68–8.36 (m, 4H, quinazoline); 13C NMR (DMSO-d6, δ ppm): 116.2, 120.1, 122.8 (2), 124.0, 126.5, 128.9, 133.0, 133.7, 134.1, 140.9, 148.0, 149.1, 149.8 (2), 150.0, 161.0, 164.9, 170.0; LC-MS m/z: 342.35 (M+).
N-(2-(pyrazin-2-yl) quinazolin-4-yl) isonicotinohydrazide (5c)
Yield: 58%. mp 264°C–265°C; IR (KBr) cm-1: 1,644 (C=C), 1,483 (C=N), 3,348 (N-H), 1,640 (C=O); 1H NMR (DMSO-d6, δ ppm): 9.98 (s, 1H, NH), 10.07 (d, 1H, CONH), 7.61–8.59 (m, 2H, pyridyl), 7.61–7.86 (m ,4H, quinazoline), 7.92–8.44 (m, 3H, pyrazine); 13C NMR (DMSO-d6, δ ppm): 116.2, 120.1, 122.8 (2), 126.5, 128.9, 133.7, 140.9, 141.2, 142.7, 144.5, 144.7, 149.8 (2), 150.0, 161.0, 164.9, 170.0; LC-MS m/z: 343.34 (M+).
2-phenyl-4-(2-phenylhydrazinyl) quinazoline (6a)
Yield: 53%. mp 311°C–312°C; IR (KBr) cm-1: 1,644 (C=C), 1,483 (C=N), 3,259 (N-H); 1H NMR (DMSO-d6, δ ppm): 10.21 (s, 1H, NH), 8.2 (d, 1H, NH), 6.99–7.47 (m, 5H, ArH), 7.58–8.26 (m, 4H, ArH of quinazoline), 7.31–8.38 (m, 5H, phenyl); 13C NMR (DMSO-d6, δ ppm): 113.2 (2), 116.2, 119.2, 120.1, 126.5, 127.5 (2), 128.8, 128.9, 129.3 (4), 130.7, 133.7, 150.0, 151.0, 161.0, 170.0; LC-MS m/z: 312.37 (M+).
4-(2-phenylhydrazinyl)-2-(pyridin-3-yl) quinazoline (6b)
Yield: 54%. mp 297°C–298°C; IR (KBr) cm-1: 1,646 (C=C), 1,484 (C=N), 3,359 (N-H); 1H NMR (DMSO-d6, δ ppm): 10.23 (s, 1H, NH), 8.0 (d, 1H, NH), 6.87–7.47 (m, 3H, ArH of phenyl), 7.59–8.26 (m, 4H, ArH of quinazoline), 7.36–8.49 (m, 4H, ArH, pyridine); 13C NMR (DMSO-d6, δ ppm): 113.2 (2), 116.2, 119.2, 120.1, 124.0, 126.5, 127.5 (2), 128.9, 129.3 (2), 133.0, 133.7, 134.1, 148.0, 149.1, 150.0, 161.0, 170.0; LC-MS m/z: 313.36 (M+).
4-(2-phenylhydrazinyl)-2-(pyrazin-2-yl) quinazoline (6c)
Yield 56%. mp 73°C–74°C; IR (KBr) cm-1: 1,644 (C=C), 1,483 (C=N), 3,333 (NH); 1H NMR (DMSO-d6, δ ppm): 9.97 (s, 1H, NH), 8.0 (d, 1H, NH), 6.97–7.36 (m, 3H, ArH of phenyl), 7.48–8.26 (m, 4H, ArH of quinazoline), 8.86–9.24 (m, 2H, ArH of pyrazine); 13C NMR (DMSO-d6, δ ppm): 113.2 (2), 116.2, 119.2, 120.1, 124.0, 126.5, 127.5 (2), 128.9, 129.3 (2), 133.0, 133.7, 134.1, 148.0, 149.1, 150.0, 161.0, 170.0; LC-MS m/z: 314.34 (M+).
Cytotoxicity assay
The cell viability of HT-29 (human adenocarcinoma), MDA-231 (breast cancer), and Ehrlich ascites carcinoma (EAC) cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Alley et al., 1988; Singh et al., 2013). The cells were placed at 3,000 per well in 96-well plates and were incubated for 24 hours in Dulbecco’s Modified Eagle medium contained with 10% fetal bovine serum in 5% CO2 at 37°C. After, the concentrations of 500, 250, 125, 62.5, 31.25, 16, 8, 4, 2, and 1 μg/ml of compounds were added to the wells and were cultured for 72 hours. Then, the MTT solution was added (10 μl/well) to the above, and the cells were again incubated further at 37°C for 4 hours. Cell lysates were added to the well (100 μl/well) and kept overnight incubation, and the absorbance value at 570 nm was detected employing ELISA reader. Each concentration was tested in triplicates. The % cytotoxicity was calculated using the following formula.
A graph of % cytotoxicity on the Y-axis and the concentration of test compound on the X-axis was extrapolated for the determination of cytotoxicity (IC50).
RESULTS AND DISCUSSION
Design strategy
It was proposed to develop 2, 4-disubstituted quinazolines as new structural lead with the hope of achieving potent chemotherapeutic agents in reducing the viability of the cancerous cells. The molecular modifications were made on the quinazoline nucleus primarily focused at the fourth position with the substitution of aryl moiety through amine or amide linkage and at the second position with aryl/heteroaryl substitutions that would improve the lipophilicity (Fig. 1).
 | Figure 1: Design strategy for the new quinazoline derivatives. [Click here to view] |
Chemistry
The title compounds 2, 4-disubstituted quinazoline derivatives (3a–f, 4a–i, 5a–c, and 6a–c) were conveniently synthesized from the corresponding key intermediates 2-phenylquinazolin-4(3H)-one (2a), 2-(pyridin-3-yl) quinazolin-4(3H)-one (2b) and 2-(pyrazin-2-yl)quinazolin-4(3H)-one (2c) as per synthetic methodology as shown in Figure 2. The intermediates 2a–c were synthesized from the anthranilic acid with an appropriate aryl/heteroaryl amide.
The physical characterization data of the synthesized compounds are shown in Table 1. The structures of all the synthesized compounds were characterized based on IR, 1HNMR, 13CNMR, and Liquid Chromatography-Mass Spectrometry (LCMS) data. The spectral data are consistent with the proposed structures. The IR spectrum of 2-phenylquinazolin-4(3H)-one (2a) exhibits characteristic carbonyl absorption band at 1,680 cm-1 and N-H at 3,350 cm-1. In the 1H NMR spectrum of the compound 2a, a singlet at 8.1 ppm integrates for a NH proton of the quinazoline ring, and the aromatic protons resonate as a complex multiplet between 7.26 and 8.33 ppm. These corresponding proton shifts indicate the formation of compound 2a. The IR spectrum of N-(4-methoxyphenyl)-2-(pyrazin-2-yl) quinazolin-4-amine (3a) exhibits characteristic C=N absorption band at 1,535 cm-1 and N-H at 3,350 cm-1. The 1HNMR spectrum of the 3a exhibits a singlet at 3.92 ppm integrates for the protons of OCH3 of the aromatic ring. The pyrazine ring protons appear as multiplet between 7.45 and 7.48 ppm and a singlet resonate at 10.24 ppm for NH proton. The aromatic protons appear as a complex multiplet between 7.58 and 7.87 ppm. These data indicate the formation of 3a. The IR spectrum of 4-chloro-N’-(2-phenylquinazolin-4-yl)benzohydrazide (4c) exhibits characteristic C=N absorption band at 1,483 cm-1 and N-H at 3,128 cm-1. In the 1HNMR spectrum of 4c, a singlet at 9.91 ppm integrates for a proton of NH attached to quinazoline. A singlet peak for NH adjacent to a carbonyl group appears at 10.27 ppm. The aromatic protons appear as a complex multiplet between 7.16 and 8.79 ppm. These data indicate the formation of 4c. The IR spectrum of N’-(2-(pyrazin-2-yl) quinazolin-4-yl)isonicotinohydrazide (5c) exhibits the characteristic C=N absorption band at 1,483 cm-1 and N-H at 3,348 cm-1. The 1HNMR spectrum exhibits a singlet peak at 9.98 ppm integrates for NH proton attached to the quinazoline ring. The protons of pyrazine ring appear between 7.43 and 8.69 ppm. A broad singlet peak for NH proton appears at 10.07 ppm. The aromatic protons appear as a complex multiplet between 7.61 and 8.44 ppm. The data indicate the formation of 5c. The IR spectrum of 2-phenyl-1-(2-phenylquinazolin-4-yl) hydrazine (6a) exhibits C=N absorption band at 1,483 cm-1 and N-H at 3,259 cm-1. The 1HNMR spectrum 6a exhibits the characteristic peaks at 8.2 and 10.21 ppm integrates for two protons of NH. The aromatic protons appear as a complex multiplet between 6.99 and 8.38 ppm. These data confirm the formation of 6a.
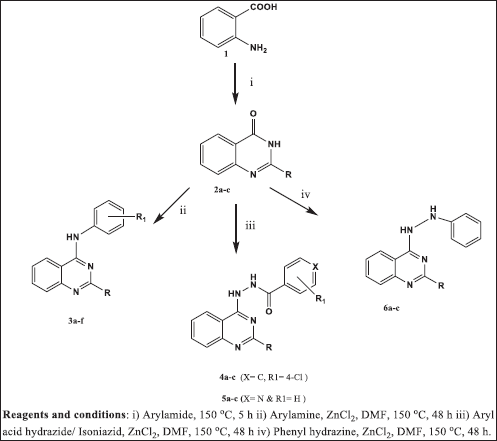 | Figure 2: Scheme of synthesis for the title compounds. [Click here to view] |
 | Table 1: In vitro cytotoxicity of the synthesized quinazolines. [Click here to view] |
Cytotoxicity evaluation
The anticancer activity of all the newly synthesized compounds was measured by microculture tetrazolium assay (MTT) assay against HT-29 (human adenocarcinoma), EAC, and MDA-231 (breast cancer) cells involving 5-fluorouracil as a positive control (1.23, 1.76, and 2.92 μM/ml, respectively). The cytotoxicity (IC50) details of the new compounds are shown in Table 1. The cytotoxicity (IC50) was analyzed by linear regression of the concentration-response curves of each compound. The synthesized compounds show better inhibitory activity toward HT-29 and MDA-231 cell lines, especially HT-29 cell lines. Compounds show an inhibitory action on HT-29, MDA-231, and EAC in the range of 5.33–746, 47.12–982.68, and 239.62–830 μM/ml, respectively. From the cytotoxicity activity of 3 series compound against HT-29 cell lines, it is evident that the role of phenyl/pyridyl ring as R substituent and 4-fluoro derivative as R1 is crucial for activity. Compound 3e is the most active in three series, which supports the above data. Compound 4 series had 4-chloro substitution at R1 having lesser activity than three series, except the compound 4c. Hence, chain linker difference might also influence the activity. However, controversy lies in five series that 5a, which had phenyl ring as R and hydrogen at R1 substitution with -NH-NH-C=O linker (same as that of four series), is highly potent among all synthesized compounds which m/z: show IC50 value of 5.33 μM/ml. This may due to some diverse interaction with the receptor pocket. In the case of MDA-231 cell lines, the cytotoxicity of compounds is slightly related to the presence of pyridyl, pyrazyl ring at R, and 4F derivative at R1 and –NH as a linker. However, linearity in a relationship is not as much in HT-29 cell lines.
CONCLUSION
In summary, 2, 4-disubstituted quinazolines were designed by substituting linkers (-NH-, -NHNH-, and -NHNHCO-) between the fourth position of the quinazoline moiety and aryl/heteroaryl ring system with the hope of achieving enhanced anticancer profiles. Fifteen 2, 4-designed compounds were synthesized in good yield and were evaluated for in vitro anticancer activity against HT-29, EAC, and MDA-231cell lines. Among the tested compounds, 5a has shown a significant anticancer activity against HT-29 cell lines. Among the linkers attached, compound 5a has -NHNHCO- group between fourth positions of the quinazoline moiety, and pyridinyl ring is emerged as a promising anticancer candidate. Exploring molecular modification and inhibitory activity of compound 5a on epidermal growth factor receptor would give a better insight into its anticancer potential.
ACKNOWLEDGMENTS
The authors are thankful to the Principal, JSS College of Pharmacy, Mysuru, India, for providing the necessary facilities and the Director, NMR Research Centre, Indian Institute of Science, Bangalore, for spectral data and Department of Pharmacology, HSK College of Pharmacy, Bagalkot, India, for cytotoxicity screening.
CONFLICT OF INTEREST
The authors declared that they have no conflict of interest.
FINANCIAL SUPPORT
None.
REFERENCES
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res, 1988; 48:589–601.
Barbosa ML, Lima LM, Tesch R, Sant Anna CM, Totzke F, Kubbutat MH, Schächtele C, Laufer SA, Barreiro EJ. Novel 2-chloro-4-anilino-quinazoline derivatives as EGFR and VEGFR-2 dual inhibitors. Eur J Med Chem, 2014; 71:1–14. CrossRef
Cao SL, Feng YP, Jiang YY, Liu SY, Ding GY, Li RT. Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocarbamate side chains. Bioorg Med Chem Lett, 2015; 15:1915–7. CrossRef
Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) tablets. Oncologist, 2003; 8:303–6. CrossRef
Dissoki S, Aviv Y, Laky D, Abourbeh G, Levitzki A, Mishani E. The effect of the [18F]-PEG group on tracer qualification of [4-(phenylamino)-quinazoline-6-YL]-amide moiety—an EGFR putative irreversible inhibitor. Appl Radiat Isot, 2007; 65:1140–51. CrossRef
Eckhardt S. Recent progress in the development of anticancer agents. Curr Med Chem, 2002; 2:419–39. CrossRef
El-Azab AS, Al-Omar MA, Abdel-Aziz AA, Abdel-Aziz NI, el-Sayed MA, Aleisa AM, Sayed-Ahmed MM, Abdel-Hamide SG. Design, synthesis, and biological evaluation of novel quinazoline derivatives as potential antitumor agents: Molecular docking study. Eur J Med Chem, 2010; 45:4188–98. CrossRef
Fernandes C, Oliveira C, Gano L, Bourkoula A, Pirmettis I, Santos I. Radioiodination of new EGFR inhibitors as potential SPECT agents for molecular imaging of breast cancer. Bioorg Med Chem, 2007; 15:3974–80. CrossRef
Ghorab MM, Alsaid MS, El-Gazzar MG, Higgins M, Dinkova-Kostova AT, Shahat AA. Synthesis and biological evaluation of novel 2- phenylquinazoline-4-amine derivatives: identification of 6-phenyl-8Hbenzo[g]quinazolino[4,3-]quinazolin-8-one as a highly potent inducer of NAD(P)H quinone oxidoreductase 1. J Enzyme Inhib Med Chem, 2016; 31:34–9. CrossRef
Govindaraj Y, Sathyamoorthy, Karthikeyan V, Melanaphuru V, Agrahari V, Gupta S, Nema RK. Synthesis and in vivo anticancer screening of 2-{[bis-(2-chloroethyl) amino] methyl} -6, 8-dinitro-1-(4-substituted ethyl)-1h-quinazolin-4-one derivatives. Academic J Cancer Res, 2009; 2:73–7.
Ho N, Harapanhalli RS, Dahman BA, Chen K, Wang K, Adelstein SJ, Kassis AI. Synthesis and biologic evaluation of a radioiodinated quinazolinone derivative for enzyme mediated insolubilization therapy. Bioconjug Chem, 2002; 3:357–64. CrossRef
Li S, Guo C, Sun X, Li Y, Zhao H, Zhan D, Lan M, Tang Y. Synthesis and biological evaluation of quinazoline and quinoline bearing 2,2,6,6-tetramethylpiperidine-N-oxyl as potential epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors and EPR bio-probe agents. Eur J Med Chem, 2012; 49:271–8. CrossRef
Mishani E, Abourbeh G, Rozen Y, Jacobson O, Laky D, Ben David I, Levitzki A, Shaul M. Novel carbon-11 labeled 4-dimethylamino-but-2-enoic acid [4-(phenylamino)-quinazoline-6-yl]-amides potential PET bioprobes for molecular imaging of EGFR-positive tumors. Nucl Med Biol, 2004; 31:469–76. CrossRef
Raffa D, Edler MC, Daidone G, Maggio B, Merikech M, Plescia S, Schillaci D, Bai R, Hamel E. Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2-styrylquinazolinones. Eur J Med Chem, 2004; 39:299–304. CrossRef
Rajveer C D, Kumaraswamy S, Sudharani S, Stephen R. Synthesis of some 6-bromo quinazolinone derivatives for their pharmacological activities. Int J Pharm Bio Sci, 2010; 1:1–10.
Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signalling pathway in cancer therapy. Expert Opin Ther Targets, 2012;16:15–31. CrossRef
Singh R, Pujar G, Purohit M, Chandrashekar VM. Synthesis, in vitro cytotoxicity, and antibacterial studies of new asymmetric bis-1,2,4-triazoles. Med Chem Res, 2013; 22:2163–73. CrossRef
Zhang Y, Huang YJ, Xiang HM, Wang PY, Hu DY, Xue W, Song BA, Yang S. Synthesis, and anticancer activities of 4-(4-substituted piperazin)-5,6,7-trialkoxy quinazoline derivatives. Eur J Med Chem, 2014; 78:23–34. CrossRef
Zhao F, Lin Z, Wang F, Zhao W, Dong X. Four-membered heterocycles-containing 4-anilino-quinazoline derivatives as epidermal growth factor receptor (EGFR) kinase inhibitors. Bioorg Med Chem Lett, 2013; 23:5385–8. CrossRef