INTRODUCTION
Mosquitoes are tiny flying insects of great importance as vectors to many pathogenic organisms, including the viruses causing dengue fever, yellow fever, chikungunya, West Nile, Japanese encephalitis, and Zika viruses, as well as protozoa, such as Plasmodium spp., and filarial nematodes, such as Wuchereria bancrofti, Brugia malayi, and Brugia timori (Du et al., 2019; Tolle, 2009) responsible for disease and many deaths worldwide (Franklinos et al., 2019; Mirzaian et al., 2010). Mosquitoes are considered to be temporary ectoparasites, and the female mosquitoes are obliged to feed on the blood of humans or animals to develop eggs (Killick-Kendrick, 1996). The blood-sucking behavior of the females is a major factor in the transmission of dangerous pathogens to humans (Chaves et al., 2010). Aedes aegypti (Linnaeus, 1762), known as “the yellow fever mosquito,” is a diurnal mosquito that is an important vector of the dengue virus causing dengue fever (Powell et al., 2018). Currently, dengue fever is a globally important disease, threatening people in developing countries, especially in tropical and subtropical regions (Kraemer et al., 2015). Aedes aegypti lives in proximity to human habitats (domestication) for taking human blood meals and is commonly found in cities, towns, and villages (Powell and Tabachnick, 2013). Therefore, this common domestic mosquito species can transmit and spread the dengue virus to people easily and quickly. The World Health Organization has reported that the incidence of dengue has increased dramatically worldwide over the past decade and estimates that as many as 390 million cases occur annually in more than 100 countries (World Health Organization, 2020).
Almost all breeding sites of A. aegypti are in and around houses, with the female mosquito laying eggs in a wide variety of natural and artificial water-holding containers, such as water tanks, plastic bottles, discarded vehicle tires, and flower vases (Ferede et al., 2018). Control of A. aegypti populations, to reduce the risk of dengue infection, focuses on the destruction of larvae and their breeding sites and includes environmental management, source reduction, larvicide, or biological control through the cooperation of people in each area (Roiz et al., 2018). This contrasts with practices used to control other types of mosquito, such as Culex, Mansonia, and Anopheles species, and the larvae of those are difficult to destroy since breeding sites are abundant and widespread in the natural environment, including rivers, marshes, ponds, and rice fields (Killick-Kendrick, 1996).
There are many ways to control the immature stages of Aedes mosquitoes, including the use of insecticides (Manjarres-Suarez and Olivero-Verbel, 2013) and releasing Gambusia mosquitofish into infested water containers as a biological control (Han et al., 2015). However, these popular methods are not always suitable because of certain obstacles, such as the development of resistance to insecticides that are used regularly (Marcombe et al., 2019). Temephos (commercial name Abate), the most popular product for mosquito larva control, is a non-systemic organophosphorus insecticide, which is relatively harmless to humans (Chaiphongpachara and Moolrat, 2017; George et al., 2015). Although this chemical has been highly effective in stopping the spread of dengue virus in many countries, there have been reports of insecticide resistance in mosquito larvae, including Argentina (Albrieu Llinás et al., 2010), Bolivia (Biber et al., 2006), Brazil (Pereira Lima et al., 2003), Cuba (Bisset et al., 2004), El Salvador (Lazcano et al., 2009), Peru, and Venezuela (Rodríguez et al., 2001). The use of larvivorous fish (Gambusia) is applicable in containers that are large enough for the fish to live in and survive, but this can be a limitation to their use for controlling the larvae of dengue vectors, and in many countries, this fish is not recommended for use because it is an exotic species that may affect native aquatic fauna if it escapes into the environment (Benelli et al., 2016).
Nowadays, plant-based larvicidal products targeting Aedes larvae in breeding sources and containers are gaining increased attention and have been accepted by communities due to their non-toxic effects in the local environment ( Chaiphongpachara et al., 2018; Ghosh et al., 2012). However, alternative products derived from plants for killing mosquito larvae in the water are still rare in the marketplace. Essential oils are natural products obtained from the material of a single plant species, including leaves, petals, stems, seeds, and roots (Butnariu and Sarac, 2018). They are popular and have many uses, including medicine for treating microbial skin diseases caused by Staphylococcus aureus, Staphylococcus epidermidis, and Propionibacterium acnes (Orchard and Vuuren, 2017) and in cosmetic products, such as creams and lotions (Sarkic and Stappen, 2018). Essential oils from some plants have been found to kill insects (Adorjan and Buchbauer, 2010; Campolo et al., 2018), and it is possible that, based on these, commercial products could be used to control the larval stage of dengue vectors. When considering alternative substances for insect vector control in communities, an important factor for success is that people can easily access and use them (Larson et al., 2017).
Therefore, this laboratory-based research screened seven commercially available herb essential oils reported to kill insects and larvae of some mosquito species for larvicidal activity against larvae of the dengue virus vector A. aegypti (L.). The oils were obtained from Cinnamomum cassia (Liu et al., 2014), Cinnamomum zeylanicum (Jeon et al., 2017), Cymbopogon flexuosus (Rahayu et al., 2018), Pimenta racemosa (Lee, 2006), Ocimum basilicum (Govindarajan et al., 2013), O. tenuiflorum (Kamaraj and Rahuman, 2010), and Zingiber officinale (Pushpanathan et al., 2008).
MATERIALS AND METHODS
Selection of commercial essential herb oils
Following a literature review for plants with insecticidal activity, seven commercial essential herb oils were selected, including C. cassia (cassia), C. zeylanicum (cinnamon), C. flexuosus (East Indian lemongrass), P. racemosa (bay), O. basilicum (sweet basil), O. tenuiflorum (holy basil), and Z. officinale (ginger). The essential oils were purchased from Chemipan Corporation Co., Ltd. (Bangkok, Thailand). The commercial oil products were cosmetic grade, and essential oil (100%) was obtained by steam distillation of herb leaves and packed in amber glass bottles. Detailed oil data and reports of the insecticidal properties of these plants are shown in Table 1. All experiments in this research were conducted from 2014 to 2015 in the laboratory of the College of Allied Health Sciences, Suan Sunandha Rajabhat University, Thailand.
Rearing of A. aegypti larvae
The second-stage larvae of A. aegypti were received from the Department of Medical Sciences, Ministry of Public Health, Bangkok, Thailand. Bright white plastic larval trays (length 14 × width 11 × depth 7 inches) containing water were used to nurture the larvae under laboratory conditions at 70%–80% relative humidity, 25°C–28°C, and 12:12 light:dark photoperiod. Ground dog biscuits were placed in the trays only once since the larvae only take 1–2 days to develop into late third-stage larvae, which was the stage required for the larvicidal bioassay.
 | Table 1. Detailed data of the seven commercial essential herb oils used in this experiment with brief literature reviews of their insecticidal efficacy. [Click here to view] |
Larvicidal bioassay
The larvicidal test used in this research was carried out according to the procedures of the World Health Organization for laboratory testing of mosquito larvicides (World Health Organization, 2005). For water preparation for testing, 1 ml of absolute methanol (solvent) was mixed with deionized water to dilute each concentration of the oils. The seven commercial essential herb oils were prepared in 250-ml beakers by serial dilution to 0.025, 0.050, 0.075, 0.100, 0.125, 0.150, and 0.175 ppm using deionized water. Following the WHO recommendations, the range of concentrations used was determined by first evaluating a wide range of concentrations until a narrow range was found, which yielded between 10% and 95% larval mortality (World Health Organization, 2005). A total of 25 late third-stage larvae were put into the beaker containing the prepared test herb oils. The mortality of mosquito larvae was recorded after 24 hours. Alive larvae were monitored for normal behavior and movement, whereas dead larvae exhibited no signs of movement. Four replicates per concentration were tested for each oil. A control treatment was also tested using deionized water mixed with 1 ml of methanol.
Statistical analyses
The mean larval mortality of A. aegypti larvae and standard error of the mean (SE) were calculated. SE was used to estimate the uncertainty due to random errors in the mean values of the data, which was calculated from the standard deviation (SD) by the square root of values in the dataset (Altman and Bland, 2005). A statistical comparison of larval mortality among different herb oils was performed using the analysis of variance, followed by the Duncan test in R software. A p-value < 0.05 was considered to be statistically significant. The probit analysis was used to calculate LC50 and LC90 (lethal concentration) values for toxicity and activity assessments. The median lethal concentration value is the lowest concentration that kills 50% of the tested mosquito larvae, whereas LC90 value is the lowest concentration that kills 90% of the tested larvae. In this study, the calculations of LC50 and LC90 values use a graphical method based on the Log concentration of essential oils on the X-axis and percentage of larval mortality on the Y-axis. The probit analysis calculated the slope of the probit mortality with the SE of the slope, Chi-squared values, and 95% confidence intervals of the upper and lower limits. The probit analysis operations were conducted by the LdP Line software (http://www.ehabsoft.com/ldpline/).
RESULT AND DISCUSSION
The results for the larvicidal activity of seven commercial essential oils against A. aegypti larvae at the concentrations ranging from 0.025 to 0.175 ppm evaluated after 24 hours of exposure are shown in Table 2. Mortality increased with an increase in the concentration of all seven essential oils, with no larval mortality found in the control group. Chi-squared values, which were p ˃ 0.05, showed that the models were consistent with the datasets (Table 2).
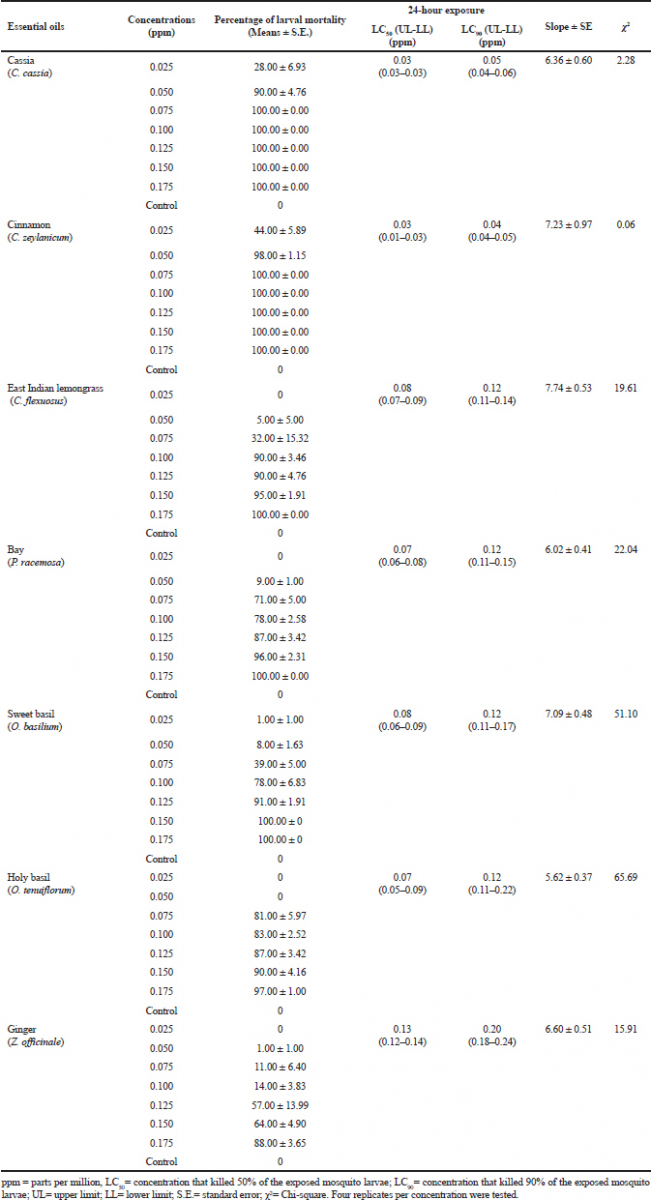 | Table 2. Probit analysis of larvicidal efficacy of seven essential oils against third-instar larvae of A. aegypti. [Click here to view] |
All seven commercial essential herb oils showed high toxicity to A. aegypti larvae (Table 2). Cinnamon essential oil had the highest larvicidal activity (LC50 = 0.03 ppm and LC90 = 0.04 ppm), followed by the essential oils of cassia (LC50 = 0.03 ppm and LC90 = 0.05 ppm), holy basil (LC50 = 0.07 ppm and LC90 = 0.12 ppm), bay (LC50 = 0.07 ppm and LC90 = 0.12 ppm), sweet basil (LC50 = 0.08 ppm and LC90 = 0.12 ppm), East Indian lemongrass (LC50 = 0.08 ppm and LC90 = 0.12 ppm), and ginger (LC50 = 0.13 ppm and LC90 = 0.20 ppm) (Table 2).
The statistical analysis ranked the essential oils as follows for efficacy: (cinnamon = cassia) > (holy basil = bay = sweet basil = East Indian lemongrass) > (ginger). The LC50 values are shown in Figure 1, whereas LC90 values are shown in Figure 2.
The results of this study revealed the efficacy against Aedes mosquito larvae of seven commercial pure essential oils, from cassia, cinnamon, East Indian lemongrass, bay, sweet basil, holy basil, and ginger. These products have the advantage of being easily accessible, relatively inexpensive, and environmental-friendly (Massebo et al., 2009). The previous studies have indicated that many essential oils have the potential to eliminate the larvae of A. aegypti (Cheng et al., 2003; Dias and Moraes, 2014). The seven essential oils were highly toxic to mosquito larvae (all with LC50 < 1 ppm or < 1 ml/l ) according to the criteria of Cheng et al. (2003), who stated that an LC50 value <50 ml/l equated to “highly active.” The larvicidal bioactivity of essential oils is mainly attributed to the major plant components but is also related to secondary substances, in which the former may work synergistically to enhance activity (Dias and Moraes, 2014).
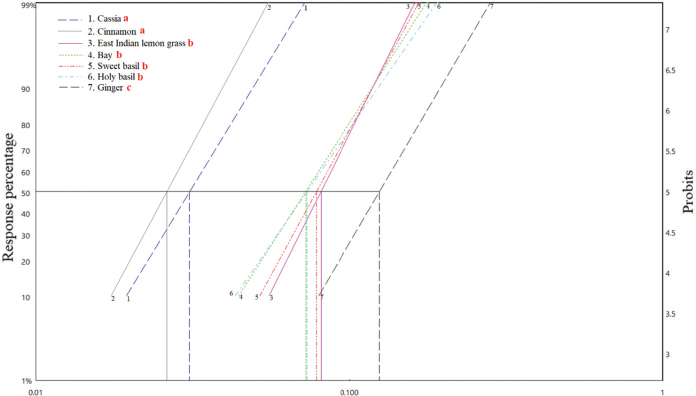 | Figure 1. Graph showing LC50 values of seven essential oils against A. aegypti larvae after 24-hour exposure. Statistically significant (p < 0.05) differences are indicated by different red lowercase letters after the names of the oils in the inset key, top left. [Click here to view] |
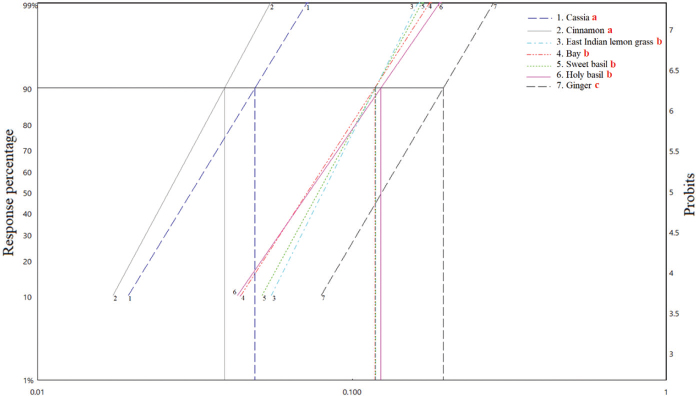 | Figure 2. Graph showing the LC90 values of seven essential oils against A. aegypti larvae after 24-hour exposure. Statistically significant (p < 0.05) differences are indicated by different red lowercase letters after the names of the oils in the inset key, top right. [Click here to view] |
The commercial cinnamon essential oil had the highest larvicidal activity, with a LC50 = 0.03 ppm and LC90 = 0.04 ppm. This result is consistent with the previous research, demonstrating that this oil could eliminate the larvae of A. aegypti (Luis, 2010), as well as control larvae of Culex tritaeniorhynchus and Anopheles subpictus (Govindarajan, 2011). Knauth et al. (2018) studied cinnamon essential oil (C. zeylanicum) and found that cinnamaldehyde (65%–80%) and eugenol (5%–10%) were the primary constituents. Cinnamaldehyde is an organic aromatic compound commonly found in cinnamon essential oil (Kaskatepe et al., 2016). Cheng et al. (2004) revealed that cinnamaldehyde had the effect of killing larvae of A. aegypti based on a laboratory experiment, whereas eugenol is a natural phenylpropanoid, formally derived from guaiacol, and is found in many aromatic and medicinal plants such as cinnamon, clove, and bay leaves (Carvalho et al., 2015). The previous research has studied the activity of eugenol derivatives against A. aegypti larvae and found that they were associated with the death of larvae (Barbosa et al., 2012).
Although pure cinnamon essential oil was the most effective, the other six essential oils (from cassia, holy basil, bay, sweet basil, East Indian lemongrass, and ginger) also exhibited strong effects against A. aegypti larvae, all with LC50 and LC90 values < 1 ppm. The cassia essential oil contains terpenoids as the major components (Zhang et al., 2019), which have reported toxicity to insects (Castilhos et al., 2018), whereas other essential oils have different major compounds including β-caryophyllene (38.90%) in holy basil (Sharma et al., 2016), eugenol (45.2%–52.7%) in the bay (Alitonou et al., 2012), linalool (44.18%) in sweet basil (Ismail, 2006), citral-a (33.1%) in East Indian lemongrass (Chowdhury et al., 2010), and zingiberene (23.69%) in ginger (Choudhari and Kareppa, 2013). All of these major compounds are toxic to insects (Tabari et al., 2017; Tak and Isman, 2016). These results are consistent with the previous research reporting the toxicity of cassia (Zhu et al., 2008), holy basil (Chokechaijaroenporn et al., 1994), bay (Leyva et al., 2009), sweet basil (Kumar et al., 2017), East Indian lemongrass (Cavalcanti et al., 2004), and ginger essential oils (Kalaivani et al., 2012) to mosquito larvae.
In this study, differences in the efficacy of the seven essential oils against mosquito larvae allow them to be placed into three groups according to their strength: group 1—cinnamon and cassia, group 2—holy basil, bay, sweet basil, and East Indian lemongrass, and group 3—ginger. This information could be important when selecting different essential plant oils to control larvae within a community. The differences in the efficacy of the different types of oil arise from several factors, primarily active components in the plants and the extraction method (Dias and Moraes, 2014). The larvicidal test used in this study has shown that the efficacy of commercial pure essential oils to kill mosquito larvae in water is very high compared to results from the previous research (Dias and Moraes, 2014). The high efficacy on mosquito larvae may be because essential oils, which are commercially available, are cosmetic grade pure oils which are not diluted or affected by solvents or other additives.
CONCLUSION
The results from this research are important from the public health perspective since they relate to a dengue vector (mosquito) that requires alternative organic substances for its control and elimination. It is clear that commercial essential oils of cassia, cinnamon, East Indian lemongrass, bay, sweet basil, holy basil, and ginger are highly effective at killing Aedes mosquito larvae. The important advantage of these oils is that they are easily accessible to the public and their use in the community could be promoted to aid control of A. aegypti larvae further.
ACKNOWLEDGMENTS
The authors would like to acknowledge the College of Allied Health Science, Suan Sunandha Rajabhat University, Thailand, for supporting research activities.
CONFLICT OF INTEREST
The authors declared that they have no conflict of interests.
REFERENCES
Adorjan B, Buchbauer G. Biological properties of essential oils: An updated review. Flavour Fragr J, 2010; 25:407–26. CrossRef
Albrieu Llinás G, Seccacini E, Gardenal CN, Licastro S. Current resistance status to temephos in Aedes aegypti from different regions of Argentina. Mem Inst Oswaldo Cruz, 2010; 105(1):113–6. CrossRef
Alitonou GA, Noudogbessi JP, Sessou P, Tonouhewa A, Avlessi F, Menut C, Sohounhloue DCK. Chemical composition and biological activities of essential oils of Pimenta racemosa (Mill.) J. W. Moore. from Benin. Int J Biosci, 2012; 2(9):1–12.
Altman DG, Bland JM. Standard deviations and standard errors. BMJ, 2005; 331:903. CrossRef
Barbosa JD, Silva VB, Alves PB, Gumina G, Santos RL, Sousa DP, Cavalcanti SC. Structure-activity relationships of eugenol derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag Sci, 2012; 68(11):1478–83. CrossRef
Benelli G, Jeffries CL, Walker T. Biological control of mosquito vectors: past, present, and future. Insects, 2016; 7(4):52. CrossRef
Biber PA, Dueñas JR, Almeida FL, Gardenal CN, Almirón WR. Laboratory evaluation of susceptibility of natural subpopulations of Aedes aegypti larvae to temephos. J Am Mosq Control Assoc, 2006; 22(3):408–11. CrossRef
Bisset JA, Magdalena Rodríguez M, Fernández D, Pérez O. Status of resistance to insecticides and resistance mechanisms in larvae from playa municipality collected during the intensive campaign against Aedes aegypti in Havana City, 2001–2002. Rev Cubana Med Trop, 2004; 56(1):61–6.
Butnariu M, Sarac I. Essential oils from plants. J Biotechnol Biomed Sci, 2018; 1(4):35–43. CrossRef
Campolo O, Giunti G, Russo A, Palmeri V, Zappalà L. Essential oils in stored product insect pest control. J Food Qual, 2018; 2018:6906105. CrossRef
Castilhos RV, Grützmacher AD, Coats JR. Acute toxicity and sublethal effects of terpenoids and essential oils on the predator Chrysoperla externa (Neuroptera: Chrysopidae). Neotrop Entomol, 2018; 47(2):311–7. CrossRef
Carvalho AA, Andrade LN, De Sousa ÉBV, De Sousa DP. Antitumor phenylpropanoids found in essential oils. Biomed Res Int, 2015; 2015:392674. CrossRef
Cavalcanti ESB, Morais SM de, Lima MAA, Santana EWP. Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem Inst Oswaldo Cruz, 2004; 99(5):541–4. CrossRef
Chaiphongpachara T, Moolrat L. Insecticide resistance of temephos on Aedes aegypti as dengue vector in Samut Songkhram, Thailand. Ann Trop Med Public Heal, 2016; 10(6):1439–42. CrossRef
Chaiphongpachara T, Sumchung K, Chansukh KK. Larvicidal and adult mosquito attractant activity of Auricularia auricula-judae mushroom extract on Aedes aegypti (L.) and Culex sitiens Wiedemann. J Appl Pharm Sci, 2018; 8(8):021–5. CrossRef
Chaves LF, Harrington LC, Keogh CL, Nguyen AM, Kitron UD. Blood feeding patterns of mosquitoes: Random or structured? Front Zool, 2010; 7(3):1–11. CrossRef
Cheng SS, Chang HT, Chang ST, Tsai KH, Chen WJ. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour Technol, 2003; 89(1):99–102. CrossRef
Cheng SS, Liu JY, Tsai KH, Chen WJ, Chang ST. Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J Agric Food Chem, 2004; 14(14):4395–400. CrossRef
Chil-Núñez I, Mendonça PM, Escalona-Arranz JC, Cortinhas LB, Dutok-Sánchez CM, de Carvalho Queiroz MM. Insecticidal effects of Ocimum sanctum var. cubensis essential oil on the diseases vector Chrysomya putoria. J Pharm Pharmacogn Res, 2018; 6(3):148–57.
Chokechaijaroenporn O, Bunyapraphatsara N, Kongchuensin S. Mosquito repellent activities of ocimum volatile oils. Phytomedicine, 1994; 1(2):135–9. CrossRef
Choudhari SS, Kareppa BM. Identification of bioactive compounds of Zingiber officinale roscoe rhizomes through gas chromatography and mass spectrometry. IJPRD, 2013; 5(8):16–20.
Chowdhury SR, Tandon PK, Chowdhury AR. Chemical composition of the essential oil of Cymbopogon flexuosus (steud) wats. growing in kumaon region. J Essent Oil-Bearing Plants, 2010; 13(5):588–93. CrossRef
Dias CN, Moraes DFC. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicides: review. Parasitol Res, 2014; 113(2):565–92. CrossRef
Du S, Liu Y, Liu J, Zhao J, Champagne C, Tong L, Zhang R, Zhang F, Qin CF, Ma P, Chen CH, Liang G, Liu Q, Shi PY, Cazelles B, Wang P, Tian H, Cheng G. Aedes mosquitoes acquire and transmit Zika virus by breeding in contaminated aquatic environments. Nat Commun, 2019; 10(1):13–24. CrossRef
Ferede G, Tiruneh M, Abate E, Kassa WJ, Wondimeneh Y, Damtie D, Tessema B. Distribution and larval breeding habitats of Aedes mosquito species in residential areas of northwest Ethiopia. Epidemiol Health, 2018; 40:e2018015. CrossRef
Franklinos LHV, Jones KE, Redding DW, Abubakar I. The effect of global change on mosquito-borne disease. Lancet Infect Dis, 2019; 19(9):e302–12.1. CrossRef
George L, Lenhart A, Toledo J, Lazaro A, Han WW, Velayudhan R, Runge Ranzinger S, Horstick O. Community-effectiveness of temephos for dengue vector control: a systematic literature review. PLoS Negl Trop Dis, 2015; 9(9):e0004006. CrossRef
Ghosh A, Chowdhury N, Chandra G. Plant extracts as potential mosquito larvicides. Indian J Med Res, 2012; 135(5):581–98.
Govindarajan M. Larvicidal and repellent properties of some essential oils against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera: Culicidae). Asian Pac J Trop Med, 2011; 4(2):106–11. CrossRef
Govindarajan M, Sivakumar R, Rajeswary M, Yogalakshmi K. Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.) against Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (Diptera: Culicidae). Exp Parasitol, 2013; 134(1):7–11. CrossRef
Hamada HM, Awad M, El-Hefny M, Moustafa MAM. Insecticidal activity of garlic (Allium sativum) and Ginger ( Zingiber officinale ) oils on the cotton leafworm, spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Afr Entomol, 2018; 26(1):84–94. CrossRef
Han WW, Lazaro A, Mccall PJ, George L, Runge-Ranzinger S, Toledo J, Velayudhan R, Horstick O. Efficacy and community effectiveness of larvivorous fish for dengue vector control. Trop Med Int Heal, 2015; 20(9):1239–56. CrossRef
Ismail M. Central properties and chemical composition of Ocimum basilicum essential oil. Pharm Biol, 2006; 44(8):619–26. CrossRef
Jeon YJ, Lee SG, Lee HS. Acaricidal and insecticidal activities of essential oils of Cinnamomum zeylanicum barks cultivated from France and India against Dermatophagoides spp., Tyrophagus putrescentiae and Ricania spp. Appl Biol Chem, 2017; 60:259–64. CrossRef
Kalaivani K, Senthil-Nathan S, Murugesan AG. Biological activity of selected Lamiaceae and Zingiberaceae plant essential oils against the dengue vector Aedes aegypti L. (Diptera: Culicidae). Parasitol Res, 2012; 110(3):1261–8. CrossRef
Kamaraj C, Rahuman AA. Larvicidal and adulticidal potential of medicinal plant extracts from south India against vectors. Asian Pac J Trop Med, 2011; 134(1):101–6.
Karamaouna F, Kimbaris A, Michaelakis Α, Papachristos D, Polissiou M, Papatsakona P, Tsora E. Insecticidal activity of plant essential oils against the vine mealybug, planococcus ficus. J Insect Scim, 2013; 13(142):1–13. CrossRef
Kaskatepe B, Kiymaci ME, Simsek D, Erol HB, Erdem SA. Comparison of the contents and antimicrobial activities of commercial and natural cinnamon oils. Indian J Pharm Sci, 2016;78(4):541–8. CrossRef
Killick-Kendrick R. Medical entomology for students. Trans R Soc Trop Med Hyg, 1996; 90(5):590. CrossRef
Kim JR, Haribalan P, Son BK, Ahn YJ. Fumigant toxicity of plant essential oils against Camptomyia corticalis (Diptera: Cecidomyiidae). J Econ Entomol, 2012; 105(4):1329–34. CrossRef
Knauth P, Lópe ZL, Hernández GJA, Sevilla MTE. Cinnamon essential oil: chemical composition and biological activities. In: Anaberta CM, Víctor M RG (eds.). Essential oils production, applications and health benefits. Nova Science Publishers, Hauppauge, New York, pp 215–44, 2018.
Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, Moore CG, Carvalho RG, Coelho GE, Van Bortel W, Hendrickx G, Schaffner F, Elyazar IR, Teng HJ, Brady OJ, Messina JP, Pigott DM, Scott TW, Smith DL, William Wint GR, Golding N, Hay SI. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife, 2015; 30:4:e08347. CrossRef
Kumar S, Warikoo R, Mishra M, Samal RR, Shrankhla Panmei K, Dagar VS, Sharma A. Impact of Ocimum Basilicum leaf essential oil on the survival and behaviour of an Indian strain of dengue vector, Aedes aegypti (L.). Vector Biol J, 2017; 2(2):1–6. CrossRef
Larson JL, Dale A, Held D, McGraw B, Richmond DS, Wickings K, Williamson RC. Optimizing pest management practices to conserve pollinators in turf landscapes: current practices and future research needs. J Integr Pest Manag, 2017; 8(1):1–10. CrossRef
Lazcano JAB, Rodríguez MM, San Martín JL, Romero JE, Montoya R. Assessing the insecticide resistance of an Aedes aegypti strain in El Salvador. Rev Panam Salud Publica, 2009; 26(3):229–34.
Lee EJ, Kim JR, Choi DR, Ahn YJ. Toxicity of cassia and cinnamon oil compounds and cinnamaldehyde-related compounds to Sitophilus oryzae (Coleoptera: Curculionidae). J Econ Entomol, 2008; 101(6):1960–6. CrossRef
Lee HS. Mosquito larvicidal activity of aromatic medicinal plant oils against Aedes aegypti and Culex pipiens pallens. J Am Mosq Control Assoc, 2006; 22(2):292–5. CrossRef
Leyva M, Marquetti Fernández M, Tacoronte J, Scull R, Tiomno Tiomnova O, Mesa A, Montada D. Actividad larvicida de aceites esenciales de plantas contra Aedes aegypti (L.) (Diptera: Culicidae). Rev Biomed, 2009; 20:5–13.
Leyva M, Tiomno O, Tacoronte JE, Carmen Marquetti del M, Montada D. Essential plant oils and insecticidal activity in Culex quinquefasciatus. In: Farzana P (ed.). Insecticides—pest engineering, InTech, Rijeka, Croatia, pp 221–38, 2012. CrossRef
Liu XC, Cheng J, Zhao NN, Liu ZL. Insecticidal activity of essential oil of Cinnamomum cassia and its main constituent, trans-cinnamaldehyde, against the booklice, Liposcelis bostrychophila. Trop J Pharm Res, 2014; 13 (10):1697–702. CrossRef
Luis S. Estudo químico e atividade larvicida frente a Aedes aegypti do óleo essencial das folhas de Cinnamomum zeylanicum Breyn (canela). Dissertation, Federal University of Maranhão, Sau Luis, Brazil, pp 13–63, 2010.
Madreseh-Ghahfarokhi S, Pirali Y, Dehghani-Samani A, Dehghani-Samani A. The insecticidal and repellent activity of ginger (Zingiber officinale) and eucalyptus (Eucalyptus globulus) essential oils against Culex theileri Theobald, 1903 (Diptera: Culicidae. Ann Parasitol. 2018; 64(4):351–60.
Manjarres-Suarez A, Olivero-Verbel J. Chemical control of Aedes aegypti: a historical perspective. Rev Costarric Salud Pública, 2013; 22:68–75.
Marcombe S, Fustec B, Cattel J, Chonephetsarath S, Thammavong P, Phommavanh N, David JP, Corbel V, Sutherland IW, Hertz JC, Brey PT. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl Trop Dis, 2019; 13(12):e0007852. CrossRef
Massebo F, Tadesse M, Bekele T, Balkew M, Gebre-Michael T. Evaluation on larvicidal effects of essential oils of some local plants against Anopheles arabiensis Patton and Aedes aegypti Linnaeus (Diptera, Culicidae) in Ethiopia. African J Biotechnol, 2009; 8(17):4183–8.
Mirzaian E, Durham MJ, Hess K, Goad JA. Mosquito-borne illnesses in travelers: a review of risk and prevention. Pharmacotherapy, 2010; 30(10): 1031–43. CrossRef
Orchard A, Van Vuuren S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid Based Complement Altern Med, 2017; 4517971. CrossRef
Pereira Lima JB, Pereira Da-Cunha M, Carneiro Da Silva R, Ribeiro Galardo AK, Da Silva Soares SD, Aparecida Braga I, Pimentel Ramos R, Valle D. Resistance of Aedes aegypti to organophosphates in several municipalities in the state of Rio de Janeiro and Espírito Santo, Brazil. Am J Trop Med Hyg, 2003; 68(3):329–33. CrossRef
Popović Z, Kostić M, Stanković S, Milanović S, SivÄev I, Kostić I, Kljajić P. Ecologically acceptable usage of derivatives of essential oil of sweet basil, ocimum basilicum, as antifeedants against larvae of the gypsy moth, lymantria dispar. J Insect Sci, 2013; 13:161. CrossRef
Powell JR, Gloria-Soria A, Kotsakiozi P. Recent history of Aedes aegypti: Vector genomics and epidemiology records. Bioscience, 2018; 68(11):854–60. CrossRef
Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti—a review. Mem Inst Oswaldo Cruz, 2013; 108(Suppl 1): 11–7. CrossRef
Pushpanathan T, Jebanesan A, Govindarajan M. The essential oil of Zingiber officinalis Linn (Zingiberaceae) as a mosquito larvicidal and repellent agent against the filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res. 2008; 102(6):1289–91. CrossRef
Rahayu R, Mairawita Jannatan R. Efficacy and residual activity of lemongrass essential oil (Cymbopogon flexuosus) against german cockroaches (Blattella germanica). J Entomol, 2018; 15(3):149–54. CrossRef
Rodríguez-González Á, Álvarez-García S, González-López Ó, da Silva F, Casquero PA. Insecticidal properties of Ocimum basilicum and Cymbopogon winterianus against Acanthoscelides obtectus, insect pest of the common bean (Phaseolus vulgaris, L.). Insects, 2019; 10(5):piiE151. CrossRef
Rodríguez MM, Bisset J, de Fernandez DM, Lauzán L, Soca A. Detection of insecticide resistance in aedes aegypti (diptera: culicidae) from cuba and Venezuela. J Med Entomol, 2001; 38(5):623–8. CrossRef
Roiz D, Wilson AL, Scott TW, Fonseca DM, Jourdain F, Müller P, Velayudhan R, Corbel V. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl Trop Dis, 2018; 12(12):e0006845. CrossRef
Sarkic A, Stappen I. Essential oils and their single compounds in cosmetics-a critical review. Cosmetics, 2018; 5(1):1–21. CrossRef
Silva SM, Cunha JPAR, da Carvalho SM, de Zandonadi CHS, Martins RC, Chang R. Ocimum basilicum essential oil combined with deltamethrin to improve the management of Spodoptera frugiperda. Ciência e Agrotecnologia, 2017; 41(6):665–75. CrossRef
Sharma V, Sharma A, Seth R. A study on antidermatophytic potential of Ocimum tenuiflorum essential oil and chemical composition evaluation. Int J PharmTech Res, 2016; 9(11):151–60.
Tabari MA, Youssefi MR, Esfandiari A, Benelli G. Toxicity of β-citronellol, geraniol and linalool from Pelargonium roseum essential oil against the West Nile and filariasis vector Culex pipiens (Diptera: Culicidae). Res Vet Sci, 2017; 114:36–40. CrossRef
Tak JH, Isman MB. Metabolism of citral, the major constituent of lemongrass oil, in the cabbage looper, Trichoplusia ni, and effects of enzyme inhibitors on toxicity and metabolism. Pestic Biochem Physiol, 2016; 133:20–5. CrossRef
Tolle MA. Mosquito-borne Diseases. Curr Probl Pediatr Adolesc Health Care, 2009; 45(3):393–407. CrossRef
World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization, Geneva, Switzerland, pp 1–18, 2005.
World Health Organization. Dengue and severe dengue, 2020 [ONLINE] Available via https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (Accessed 8 March 2020).
Zhang C, Fan L, Fan S, Wang J, Luo T, Tang Y, Chen Z, Yu L. Cinnamomum cassia Presl: a review of its traditional uses, phytochemistry, pharmacology and toxicology. Molecules, 2019; 24(19):3473. CrossRef
Zhu J, Zeng X, O’Neal M, Schultz G, Tucker B, Coats J, Bartholomay L, Xue R De. Mosquito larvicidal activity of botanical-based mosquito repellents. J Am Mosq Control Assoc, 2008; 24(1):161–8. CrossRef