INTRODUCTION
Respiratory tract viral diseases have continued to be a global health threat since they are among the most encountered human infections with high rates of morbidity and mortality. These diseases are contagious and transmitted mainly by contact droplets, aerosols, and hands or objects touching (Lee and Treanor, 2016; Richard et al., 2020). “Flu,” a viral respiratory disease, is caused by a highly pathogenic organism “influenza virus”. This virus is highly mutagenic and remains an alarming threat to both human and animal health worldwide. It infects many hosts, including poultry, birds, swine, and mammals (Yoo et al., 2018). The capacity of influenza virus to cause seasonal or sometimes global outbreak (Neumann et al., 2009) is attributed mainly to the ability of the virus to mutate its genome in several regions “antigenic drift and/or shift” combined with re-assortment and generation of modified or new virus capable of invading various host (Shao et al., 2017). In addition, governance, socioeconomic, and environmental factors have been shown to play a role (Chawla et al., 2009). Despite its high mutagenicity, influenza virus invades mainly the respiratory tract, affecting both the upper and lower airways (Lee and Treanor, 2016).
Curing influenza infections has been a challenge since only a few drugs have been approved to eradicate the disease. Furthermore, the emergence of high resistance rate has called upon withholding two anti-influenza drugs, namely, amantadine and rimantadine. Although oseltamivir, peramivir, and zanamivir are still used for influenza infections, the fear of reemergence of new resistant strains has tackled their prescription (FDA, 2018). The literature revealed independent attempts to develop either new anti-influenza drugs or vaccines; nonetheless, all have gone unsuccessful due to rapid emergence of resistance, escaping host immunity, genetic diversity of the virus, and high rate of mutation and, thus, reassortment that can generate new traits giving the virus abilities of infecting various host (Lyons and Lauring, 2018).
Medicinal plants have always been an important source for therapeutic agents or leads for curing infectious diseases and serve as an alternative therapy for local community. The environmental factors could affect the nature of the plants’ secondary metabolites, and consequently, various biological activities might evolve (Li et al., 2020). In this context, a variety of medicinal plants have been screened against influenza virus, and the data revealed promising results. However, not a single therapeutic agent has reached the market yet (Chantrill et al., 1952). Sudan flora is rich in herbs which are used traditionally for malaria, inflammation, jaundice, HIV infections, respiratory tract infections, and other microbial diseases (Chothani and Vaghasiya, 2011; El-Tahir et al., 1999; Fisher, 1990; Gebreegziabher, 2014; Hamid et al., 1999; Heinrich et al., 2009; Saeed, 2006; Suliman et al., 2014; von Maydell, 1986). In the current study, five Sudanese medicinal plants, namely, Balanites aegyptiaca, Cordia africana, Aristolochia bracteolate, Boscia senegalensis, and Leptadenia arborea, were selected for screening against influenza virus based on their folkloric use for the treatment of infectious diseases and known safety since long time.
METHODOLOGY
Cell culture
Madin–Darby canine kidney (MDCK, American Type Culture Collection) cells were maintained in minimum essential medium (Invitrogen) containing 2 mM l-glutamine, 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 U/ml penicillin and incubated at 37°C and 5% CO2.
Viruses
Influenza virus strain A/WSN/33(H1N1) was prepared and stored as described previously (Brauer and Chen, 2015). It was grown in 10-day-old embryonated chicken eggs at 36 °C for 48 hours. The infected allantoic fluids were collected 48 hours after egg inoculation and stored at −80°C.
Preparation of plants’ extracts
Medicinal plants were collected from healers and authenticated by the taxonomist Mr. Yahia Mohammed, Herbarium Unit, Medicinal and Aromatic Plants Research Institute, Sudan (code numbers shown in Table 1). The parts collected were cleaned, dried, powdered, and weighed. About 1 g of each plant material was extracted for 24 hours at room temperature in glass bottle using water or 70% methanol with continuous shaking, followed by heating at 85°C for 10 minutes. After cooling at room temperature, the extracts were filtered by Whatman filter paper, and the solvents were evaporated. The obtained powders were stored at 4°C. The powders were dissolved in reverse osmosis (RO) water at the time of cytotoxicity assays.
 | Table 1. Medicinal plants and parts used. [Click here to view] |
Cytotoxicity and in vitro antiviral activity
The effects of plant extracts on cell proliferation and viability were evaluated using water-soluble tetrazolium-1 reagent (WST-1, Dojindo) as described previously (Chen et al., 2017). The overnight MDCK cells plated in 96-well plate were treated with serial two-fold dilutions of the prepared plant extract in the medium and incubated at 37°C for 24 hours together with negative control (untreated cells). About 10 μl of the WST-1 solution was added to each well including the negative control and incubated for 4 hours, and then, the absorbance of each well was measured at 450 and 630 nm using a microplate reader (Life Sciences-Tecan). The mean values of triplicate experiments were obtained, and the percentage cell viability was determined for both the water and methanol extracts according to the negative control measurement. The 50% cytotoxic concentrations (CC50) were calculated from the plot of percent cell viability (y-axis) against the concentration of the plant extracts (x-axis).
In vitro antiviral activity
The cells were seeded into 96-well plate and treated with a preincubated (37°C for 1 hour) mixture of serial two-fold dilutions of the prepared plant extract and virus inocula of 100 median tissue culture infective dose (TCID50). The treated cells were incubated for 1 hour, washed with medium, and cultured for 3 days. The wells containing cells and virus only served as the negative controls. WST-1 reagent was added to all wells as described above, and the 50% effective concentrations (EC50) for water and methanol extracts were calculated in the same manner as CC50. The ratio of the CC50 to the EC50 (selective index) was calculated for all extracts.
Hemagglutination inhibition (HI) assay
The lowest concentrations of plant extracts that inhibit hemagglutination of chicken red blood cells induced by influenza virus strain A/WSN/33(H1N1) were determined as described previously (Pedersen, 2014). The serial two-fold dilutions of methanol plant extract (25 μl) were added to each well of 96-well microtiter plates containing an equal volume of virus suspension (64 HA unit) and incubated for 1 hour at 4°C. The prepared chicken erythrocytes, 0.5% (v/v) (50 μl), in phosphate-buffered saline (−) was added and then incubated for 30 minutes at room temperature for hemagglutination. Red blood cells (RBCs) without virus served as the negative control, whereas RBCs with virus only served as the positive control. The concentrations of plant extracts marked as (−) were defined as those with inhibitory effects on viruses, which prevent hemagglutination (prevent the attachment of virus to RBCs and appear as small red pellet in the center of a well, as in negative control), whereas the concentrations marked as (+) were defined as those without inhibitory effect on viruses and allow the hemagglutination of RBCs, which appear as a lattice structure (suspended RBCs make the whole well appear red as in positive control).
RESULTS AND DISCUSSION
Cytotoxicity and antiviral effect of plants’ extracts
The cytotoxicity of the plants’ extracts was assessed against MDCK cells. WST-1 assay was used to determine CC50 value which was found to be 80 mg/ml. There was no significant cytotoxicity observed at concentrations up to 40 mg/ml for all extracts. This finding might support the claim that medicinal plants used folklorically for long time are safe. The anti-influenza activity of the plants’ extracts was evaluated using virus inocula of 100 (TCID50). The EC50 was calculated using WST-1 assay. Zanamivir was used as a control in a concentration range of 3–100 nM, and it was safe to the cells within the concentrations used (Fig. 6). The EC50 was 12.5 nM.
Aristolochia bracteolata
Aristolochia bracteolata is a popular medicinal herb in most of the African, Asian, and South American countries. It is used traditionally for the treatment of infectious diseases as well as other diseases (Heinrich et al., 2009). As shown in Figure 1, the EC50 of the plant against influenza virus was found to be 2.5 mg/ml for methanol extract and 10 mg/ml for water extract with selectivity indices of 32 and 8, respectively (Table 2). Being the most active in terms of EC50, the methanolic extract was tested for the inhibition of RBC hemagglutination induced by viruses, and it was found to be active at 2.5 mg/ml (Table 3). These findings together with the known antibacterial effect of AB (Heinrich et al., 2009; Negi, 2003; Suliman et al., 2014) might explain its traditional use for respiratory tract infections.
Boscia senegalensis
Boscia senegalensis is a medicinal plant grown in Sudan and locally known as Mikheit. The plant was used as a food by western Sudanese population during the starvation period, 1984–1985 (Fisher, 1990). BS is a promising medicinal plant due to its folkloric use for the treatment of cough and nutritional value (Doka and Yagi, 2009; Salih et al., 1991). The activity of BS against influenza virus was comparable to AB, where CC50 was 80 mg/ml, EC50 was 2.5 and 10 mg/ml, and selectivity indices were 32 and 8 for methanol and water extracts, respectively (Fig. 2 and Table 2). Similar to AB, the methanolic extract of BS at 2.5 mg/ml inhibited RBC hemagglutination induced by influenza virus (Table 3). It appears that the most active compounds in both AB and BS plants are relatively less polar since the methanol extracts were more active than the aqueous one. However, bioactivity-guided fractionation with less polar solvents is necessary to confirm this observation.
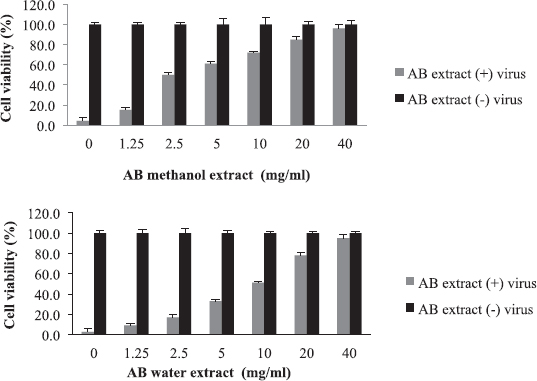 | Figure 1. Cytotoxicity and anti-influenza effect of A. bracteolata (AB) extracts. The EC50 value was 2.5 mg/ml for methanol extract and 10 mg/ml for water extract. [Click here to view] |
Leptadenia arborea
Leptadenia arborea is used in the folkloric medicine for the treatment of cough, diarrhea, dandruff, and jaundice (Doka and Yagi, 2009; Issa et al., 2018). As shown in Figure 3, LA demonstrated less potency against influenza virus when compared to AB and BS. The EC50 of the methanolic extract was two-fold less than that of the aqueous extract (EC50 = 10 and 20 mg/ml, respectively). The corresponding selectivity indices were 8 for the organic extract and 4 for the aqueous one (Table 2). Regarding HI assay, LA was found less active than AB and BS, where it inhibited RBC hemagglutination induced by influenza virus at a concentration of 10 mg/ml (Table 3).
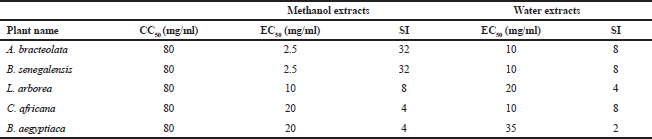 | Table 2. The selectivity index (SI) of different plants extracts. [Click here to view] |
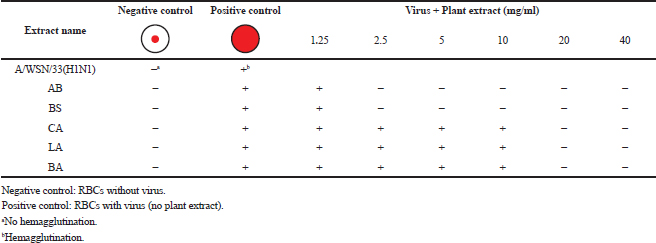 | Table 3. Inhibitory effects of plants' methanol extracts on RBC hemagglutination. [Click here to view] |
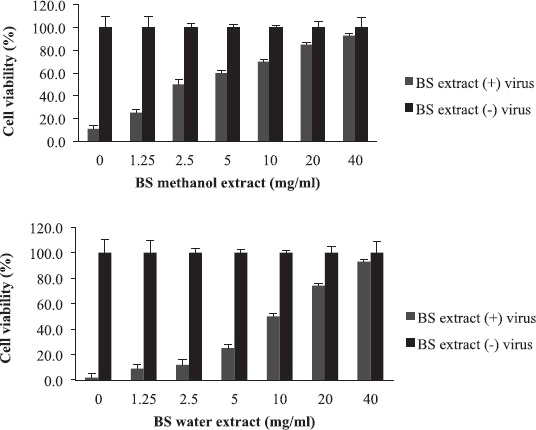 | Figure 2. The activity of B. senegalensis (BS) extracts against influenza virus: The EC50 value was 2.5 mg/ml for methanol extract and 10 mg/ml for water extract. [Click here to view] |
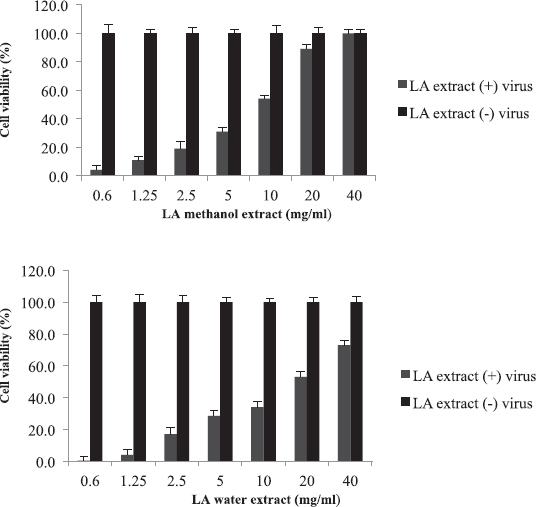 | Figure 3. Cytotoxicity and anti-influenza effect of L. arborea (LA) extracts: The EC50 value was 10 mg/ml for methanol extract and 20 mg/ml for water extract. [Click here to view] |
Cordia africana
Cordia africana (Sudan teak) is widely distributed in whole Africa and the Middle East. Its fruit is edible (can be eaten raw or cooked) and used locally as medicine. The antimicrobial and antioxidant properties of the plant have been documented in the literature (Isa et al., 2016). CA is a potential source of antiviral compounds as it is traditionally employed for the treatment of respiratory tract infections and jaundice (Gebreegziabher, 2014). Contrary to AB, BS, and LA, the water extract has shown a higher antiviral activity (EC50 = 10 mg/ml) compared to the organic extract (EC50 = 20 mg/ml) (Fig. 4). The SI for water extract was higher than methanol extract, which were 8 and 4, respectively (Table 2). The aqueous extract of CA inhibited RBC hemagglutination induced by influenza virus at 20 mg/ml (Table 3). The resultant anti-influenza activity of CA, its multipurpose use (Teklay et al., 2013), supported by the traditional use of the plant against respiratory tract infections and jaundice, make it very important herb as a potential source of antiviral compounds.
Balanites aegyptiaca
Balanites aegyptiaca, the desert dates, is a known medicinal plant in Sudan and other countries (Sarker et al., 2000). BA has several medical traditional uses; it is almost used for all microbial infections including viral ones (Chothani and Vaghasiya, 2011; Issa et al., 2018). In the current study, BA showed the weakest activity against influenza viruses compared to the other tested plants. As shown in table 2, the EC50 of the plant against influenza virus was found to be 20 mg/ml for methanol extract and 35 mg/ml for water extract with selectivity indices of 4 and 2, respectively, being the least active medicinal herb tested in this study. The concentration at which BA inhibited virus-induced RBC hemagglutination was 20 mg/ml (Table 3). It is worth noting that this plant is culturally acceptable and sold in local food shops due to its nutritional value, medicinal use, and other local uses. We believe that such a weak antiviral activity could be attributed to the potential mutation acquired through long term exposure of the plant to different human viruses
There is a discrepancy between the usefulness of purified compounds, standardized extract, and whole plant extracts that have been used for the treatment of various disorders. However, the use of whole medicinal plants for the treatment of respiratory tract infections is widely accepted by the local communities due to their own experience about their efficacy and safety for long time. The potential bioactive compounds could be isolated from medicinal plants and subjected to the drug discovery approach, hit-to-lead exploration, for the identification of promising molecules.
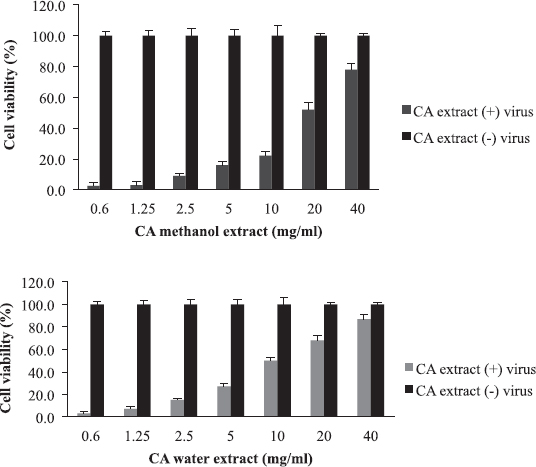 | Figure 4. The antiviral activity and cytotoxicity of C. africana (CA) extracts: The EC50 value was 20 mg/ml for methanol extract and 10 mg/ml for water extract. [Click here to view] |
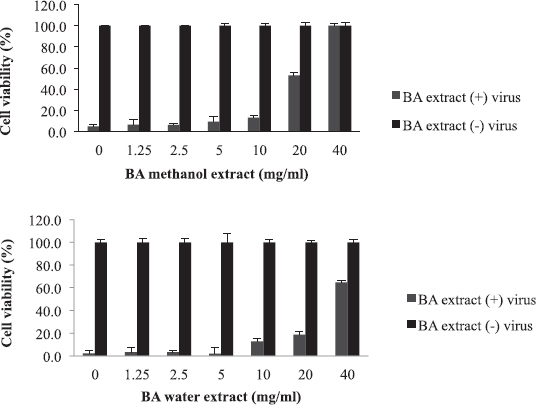 | Figure 5. The activity of B. aegyptiaca (BA) extracts against influenza virus: The EC50 value was 20 mg/ml for methanol extract and 35 mg/ml for water extract. [Click here to view] |
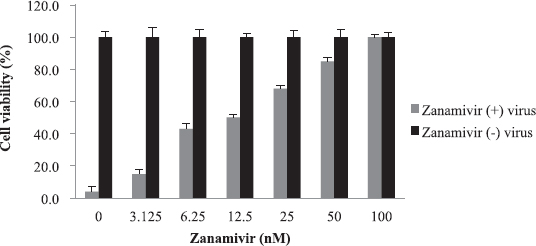 | Figure 6. Cytotoxicity and anti-influenza activity of zanamivir: No cytotoxicity seen up to 100 nM. The EC50 was 12.5 nM. [Click here to view] |
CONCLUSION
The most safe and potent plants against influenza virus strain A/WSN/33(H1N1) were proved to be AB and BS, where the EC50 were 2.5 and 10 mg/ml and selectivity indices were 32 and 8 for the organic and aqueous extracts, respectively. The least potent aqueous extract was that of BA with an EC50 of 35 mg/ml, whereas the least active methanolic extracts (with an EC50 of 20 mg/ml) were associated with CA and BA. All plants inhibited the virus-induced hemagglutination of chicken RBCs at concentrations equal to their EC50 values. The proven anti-influenza activity of the tested plants could rationalize their efficacy against some respiratory tract infections. Future studies should concentrate on the in vivo efficacy and toxicity of the tested plants to open the way toward standardized herbal therapy for influenza virus.
CONFLICT OF INTEREST
Authors declare that they do not have any conflicts of interest.
FINANCIAL SUPPORT
None.
REFERENCES
Albino Rats. Master Thesis. Faculty of Veterinary Medicine, University of Khartoum, Khartoum, Sudan.
Brauer R, Chen P. Influenza virus propagation in embryonated chicken eggs. J Vis Exp, 2015; 97:52421. CrossRef
Chantrill BH, Coulthard CE, Dickinson L, Inkley GW, Morris W, Pyle AH. The action of plant extracts on a bacteriophage of Pseudomonas pyocyanea and on influenza A virus. J Gen Microbiol, 1952; 6(1–2):74–84. CrossRef
Chawla R, Sharma RK, Madaan D, Dubey N, Arora R, Goel R, Singh S, Kaushik V, Singh PK, Chabbra V, Bhardwaj JR. Mitigation approaches to combat the flu pandemic. J Glob Infect Dis, 2009; 1(2):117–30. CrossRef
Chen M, Aoki-Utsubo C, Kameoka M, Deng L, Terada Y, Kamitani W, Sato K, Koyanagi Y, Hijikata M, Shindo K, Noda T, Kohara M, Hotta H. Broad-spectrum antiviral agents: secreted phospholipase A2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane. Sci Rep, 2017; 7(1):15931. CrossRef
Chothani DL, Vaghasiya HU. A review on Balanites aegyptiaca Del (desert date): phytochemical constituents, traditional uses, and pharmacological activity. Pharmacogn Rev, 2011; 5(9):55–62. CrossRef
Doka IG, Yagi SM. Ethnobotanical survey of medicinal plants in west Kordofan (western Sudan). Ethnobot Leaflets, 2009; 13:1409–16.
El-Tahir A1, Satti GM, Khalid SA. Antiplasmodial activity of selected Sudanese medicinal plants with emphasis on Acacia nilotica. Phytother Res, 1999; 13(6):474–8. CrossRef
FDA. Influenza (Flu) antiviral drugs and related information. U. S. Food and Drug Administration, Silver Spring, MD, 2018. Available via https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information (Accessed 23 October 2019).
Fisher HJ. Famine that Kills: Darfur, Sudan, 1984–1985. By Alexander De Waal. Oxford: Clarendon Press, 1989. Cambridge University Press. J Afr Hist, 1990; 31(3):519–20. CrossRef
Gebreegziabher STB. Cordia africana (Lam.) fruit and its uses. PhD Thesis, ISSN 1503-1667. Department of Chemistry, Biotechnology and Food Science, Norwegian University of Life Sciences, Ås, Norway, 2014.
Hamid OA, Wahab ME, Abdu ZZ, Idris SM. Balanites aegyptiaca extracts for treatment of HIV/AIDS and leukemia. Patent No. WO/2001/049306. International Application No.: PCT/SD1999/000002. 1999.
Heinrich M, Chan J, Wanke S, Neinhuis C, Simmonds MS. Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2--a global assessment based on bibliographic sources. J Ethnopharmacol, 2009; 125(1):108–44. CrossRef
Isa AI, Saleh MIA, Abubakar A, Dzoyem JP, Adebayo SA, Musa I, Sani UF, Daru PA. Evaluation of anti-in-ammatory, antibacterial and cytotoxic activities of Cordia africana leaf and stem bark extracts. J Pure Appl Sci, 2016; 9(1):228–35. CrossRef
Issa TO, Mohamed YS, Yagi S, Ahmed RH, Najeeb TM, Makhawi AM, Khider TO. Ethnobotanical investigation on medicinal plants in Algoz area (South Kordofan), Sudan J Ethnobiol Ethnomed, 2018; 14(1):31. CrossRef
Lee FE-H, Treanor JJ. Textbook of respiratory medicine. 6th Edition, Chap 32, Elsevier Saunders, Philadelphia, PA, 2016.
Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Bioch, 2020; 148:80–9. CrossRef
Lyons DM, Lauring AS. Mutation and epistasis in influenza virus evolution. Viruses, 2018; 10(8):407. CrossRef
Negi PS, Anantharamakrishnan C, Jayaprakasha GK. Antibacterial activity of Aristolochia bracteolata roots extracts. J Med Food, 2003; 6(4):401–3. CrossRef
Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature, 2009; 459:931–9. CrossRef
Pedersen JC. Hemagglutination-inhibition assay for influenza virus subtype identification and the detection and quantitation of serum antibodies to influenza virus. In: Spackman E (ed.). Animal influenza virus. Methods Mol Biol, (Methods and Protocols), Humana Press, New York, NY, pp 1161:11–25, 2014.
Richard M, van den Brand JMA, Bestebroer TM, Lexmond P, de Meulder D, Fouchier RAM, Lowen AC, Herfst S. Influenza a viruses are transmitted via the air from the nasal respiratory epithelium of ferrets. Nat Commun, 2020; 11:766. CrossRef
Saeed TA. Effect of Leptadenia arborea and Syzygium aromaticum on Albino rats. Thesis, The Khartoum University Press Publisher, 2006. Available via http://khartoumspace.uofk.edu/handle/123456789/877.
Salih OM, Nour AM, Harper DB. Chemical and nutritional composition of 2 famine food sources used in Sudan, mukheit (Boscia senegalensis) and maikah (Doberaroxburghi). J Sci Food Agric, 1991; 57(3):367–77. CrossRef
Sarker SD, Bartholomew B, Nash RJ. Alkaloids from Balanites aegyptiaca. Fitoterapia, 2000; 71(3):328–30. CrossRef
Shao W, Li X, Goraya MU, Wang S, Chen JL. Evolution of influenza a virus by mutation and re-assortment. Int J Mol Sci, 2017; 18(8):1650. CrossRef
Suliman MM, Timan IM, Khedr AI, Abd AlGadir H, Takeshita S, Shah MM, Ichinose Y, Maki T. Activity of Aristolochia bracteolata against moraxella catarrhalis. Int J Bacteriol, 2014; 2014:481686. CrossRef
Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed, 2013; 9(1):65. CrossRef
von Maydell HJ. Trees and shrubs of the sahel: their characteristics and uses. GTZ, Eschborn, Germany, 1986.
Yoo SJ, Kwon T, Lyoo YS. Challenges of influenza A viruses in humans and animals and current animal vaccines as an effective control measure. Clin Exp Vaccine Res, 2018; 7(1):1–15. CrossRef