INTRODUCTION
According to the global malaria programme, malaria is one of the diseases, which needs continuous monitoring and supervising, because it still has a high mortality rate (World Health Organization, 2018). The WHO has reported 228 million malaria cases worldwide, and 405 thousand died in 2018. Indonesia has been reported to have a high malaria prevalence, with more than 300 thousand incidents happened in 2017 (World Health Organization, 2019). The increasing resistance expects the high prevalence of malaria of the Plasmodium parasite to the administrated drugs (Ashley et al., 2014; Sibley, 2015). The development of new antimalarial drug agents is needed to overcome the resistance of Plasmodium parasite, so the number of the infection of malaria can be reduced.
The previous studies reported the biological activity of chalcone derivate compounds as antimalarial (Sharma et al., 2014; Syahri et al., 2017b; 2017c; Tadigoppula et al., 2013), antimicrobial (Chu et al., 2018; Khan et al., 2019), antioxidant (Wang et al., 2019), antidiabetic (Cai et al., 2017; Shin et al., 2018), anticancer (Castaño et al., 2019; Custodio et al., 2019; Muchtaridi et al., 2019), and anti-inflammatory (Ur-Rashid et al., 2019). Some functional groups such as hydroxyl, methoxy, and allyloxy in chalcone have been proved to increase the antimalarial activity (Syahri et al., 2017c) even though the activity was still categorized as low. The substitution of some amine groups into the chalcone ring could be proposed to increase the antimalarial activity of chalcone derivatives. Amine groups are expected to be the important molecule that could increase the antimalarial activity based on the fact that most of the antimalarial drugs is bearing nitrogen atoms (Fig. 1).
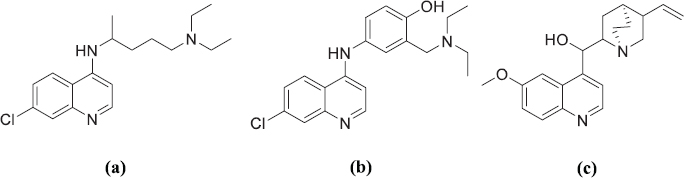 | Figure 1. Some commercial antimalarial drugs bearing amine in their structure: Chloroquine (a), Amodiaquine (b), and Quinine (c). [Click here to view] |
The Mannich reaction is one of the methods that can be conducted to add the amine groups to the chalcone derivatives. Some compound obtained from the Mannich reaction has been reported to have antimalarial (Funk et al., 2017; Wilhelm et al., 2015), anticytotoxic (Reddy et al., 2008; Yamali et al., 2016), anticancer, and antibacterial activities (Roman, 2015). In this work, we presented the synthesis of aminoalkylated chalcones through the Mannich reaction as well as the in vitro and in silico evaluation as antiplasmodial compounds.
MATERIALS AND METHODS
Materials
All of the materials used in synthesis were purchased from Sigma-Aldrich and Merck in analytical grade and used without further purification, i.e., 4-chloroacetophenone, vanillin, sodium hydroxide (NaOH), morpholine, piperidine, diethylamine, formaldehyde, ethanol, hexane, and ethyl acetate. Meanwhile, chloroform-d6 (CDCl3) and acetone_d6 were used in the nuclear magnetic resonance (NMR) spectroscopy analysis. The silica gel 60 GF254 was used for column chromatography, and the silica gel 60 F254 thin-layer chromatography (TLC) aluminum sheet was utilized in monitoring the reaction. The materials used in the in vitro antimalarial activity assay follow the previous work (Syahri et al., 2017c).
Instrumentation
Melting points of the prepared compounds were determined in an open capillary tube on Electrothermal 9100 (uncorrected). The molecular weight of the compounds was determined based on the MS from Shimadzu QP2010S. The 1H- and 13C-NMR spectra were recorded using tetramethylsilane as an internal standard on JEOL JNMECA (500 MHz).
General procedure for the synthesis of aminoalkylated chalcone derivates (4a–c)
The preparation of chalcone 3 has been reported before (Syahri et al., 2017c). The synthesis of aminoalkylated chalcone 4a–c was carried out through the Mannich reaction (Wilhelm et al., 2015) by dissolving chalcone 3 (10 mmol) in ethanol (75 ml) until homogenous solution obtained. To this solution, 10 mmol of formaldehyde solution (37%) and 10 mmol of secondary amines (morpholine for 4a, piperidine for 4b, and diethylamine for 4c) were added while stirring at room temperature. The mixture was then heated and refluxed for 20 hours or until no starting materials remain (monitored by TLC using hexane:ethyl acetate in 3:1 ratio). After the completion of the reaction, the solvent was evaporated under a reduced pressure rotary evaporator, and the solid product obtained was purified using column chromatography with hexane:ethyl acetate mixture (0%–50% gradient) as the eluent.
Antimalarial activity
An in vitro antimalarial activity assay was conducted against Plasmodium falciparum 3D7 strain (sensitive chloroquine) according to the method of Rieckmann et al. (1978) in 96-well microtiter plates with minor modifications as it was reported before (Syahri et al., 2017a). The antimalarial activity test was carried out by dissolving the test compounds in DMSO and then diluted the solution into serial concentration in the RPMI-1640 media to obtain a final concentration of 100, 10, 1, 0.1, and 0.01 μg/ml. To the test solution, a parasite suspension was added with a parasitemia level of ± 1% and a hematocrit of 5%. The culture was then incubated at 37°C for 48 hours. The culture was collected, and a thin layer of blood was prepared with 20% Giemsa stain. The percentage of parasitemia and also the percentage of the growth inhibition of P. falciparum were calculated by counting the number of infected erythrocytes for every 1,000 erythrocytes. The antimalarial activity (IC50 value) was determined by performing the statistical analysis using Probit log analysis based on the percentage inhibition data and the concentration of the test compound.
Molecular docking
Molecular docking was performed to dihydrofolate reductase–thymidylate synthase (PfDHFR-TS) protein with a code of 1J3I.pdb (2.33 Å) (Yuvaniyama et al., 2003). The docking procedure followed the standard protocol implemented from Discovery Studio® 3.1 software (Accelrys, San Diego). The ligands were prepared, and the energy was minimized first before the docking process. Hydrogen atoms were added to the protein structure before the docking process, and all of the ionizable amino acids (residues) were adjusted at pH 7.4 (default protonation). The ligands were allowed to flex, and the receptor was maintained rigid during the docking process. The docked conformer of ligand–receptor was set in a docking tolerance of 0.25 Å with a number of nonpolar or polar hotspots in the receptor, to start the conformer fitting, which were set at 500. Meanwhile, the conformations of the ligands produced from the docking process were fixed at 500 within the relative energy threshold of 20.
RESULTS AND DISCUSSION
Synthesis
The synthesis of chalcone 4a–c was carried out via the Claisen–Schmidt condensation reaction from vanillin and 4-chloroacetophenone, followed by the Mannich reaction to add secondary amine groups such as morpholine, piperidine, and diethylamine (Scheme 1). Vanillin is an aldehyde compound with hydroxyl (–OH) and methoxy (–OCH3) groups present in its structure, and it is abundantly available in nature. According to the previous studies, both hydroxyl and methoxy groups showed a positive effect on the increasing of antimalarial activity (Neto and Lavarda, 2014; Syahri et al., 2017c). The addition of chloro (–Cl) group is expected to increase the antimalarial activity as it is seen in chloroquine that has –Cl group in the structure. Based on the literature studies, compound 4a–c is a new compound that the structures have never been reported or published (in SciFinder), so its activity as an antimalarial has also not been reported.
The structure elucidation of all the prepared compounds was confirmed using MS to calculate the molecular weight and NMR spectrometers to determine the electronic (chemical) environment of each proton and carbon peaks. According to the 1H-NMR spectra, all of the synthesized chalcone compounds (4a–c) were afforded in trans (E) conformation as it can be seen from the coupling constant (J) of H-α (H-8) and H-β (H-7) with 15.5 Hz.
(E)-1-(4-chlorophenyl)-3-(4-hydroxy-3-methoxyphenyl)prop-2-en-1-one (3)
Yellow crystals, yield 60%, melting point 110°C–112°C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 7.98 (d, J = 8.50 Hz, 2H, H-11;15); 7.79 (d, J = 15.5 Hz, 1H, H-7); 7.72 (d, J = 15.57 Hz, 1H, H-8); 7.43 (d, J = 8.50 Hz, 2H, H-12;14); 7.36 (s, 1H, H-4); 7.22 (dd, J = 1,9; 8.4 Hz, 1H, H-1); 6.88 (d, J = 8.20 Hz, 1H, H-6); 3.74 (s, 3H, OCH3). 13C NMR (125 MHz, CDCl3) δ (ppm) 188.8 (C-9); 150.6 (C-3); 148.9 (C-2); 146.2 (C-7); 139.1 (C-13); 138.2 (C-10); 131.0 (C-11;15); 129.7 (C-12;14); 128.0 (C-5); 124.8 (C-6); 119.5 (C-8); 116.2 (C-1); 112.1 (C-4); 56.4 (C-17). MS (C16H13ClO3) [M]+: 288.
 | Scheme 1. Reagents and conditions of synthesis: (i) Sodium hydroxide (60%), ethanol, stir at RT overnight; (ii) Secondary amine (R), formaldehyde, ethanol, stir for 20 hours. [Click here to view] |
(E)-1-(4-chlorophenyl)-3-(4-hydroxy-3-methoxy-5-(morpholinomethyl)phenyl)-prop-2-en-1-one (4a)
Yellow crystals, yield 80%, melting point 151°C–152°C. 1H -NMR (500 MHz, acetone-d6) δ (ppm): 8.12 (d, J = 8.5 Hz, 2H, H-11;15); 7.74 (d, J = 15.5 Hz, 1H, H-7); 7.71 (d, J = 15.5 Hz, 1H, H-8); 7.56 (J = 8.5 Hz, 1H, H-12;14); 7.43 (s, 1H, H-4); 7.20 (s, 1H, H-6); 3.89 (s, 3H, H-17); 3.77 (s, 2H, H-18); 3.70 (t, 4H, H-20;22); 2.56 (t, 4H, H-19;21). 13C-NMR (125 MHz, acetone-d6) δ (ppm): 189.29 (C-9); 150.34 (C-3); 148.51 (C-7); 145.89 (C-2); 139.07 (C-13); 136.98 (C-10); 129.99 (C-11;15); 129.03 (C-12;14); 126.14 (C-5); 122.97 (C-1); 121.05 (C-8); 118.91 (C-6); 110.54 (C-4); 66.85 (C-20;22); 61.51 (C-18); 56.19 (C-17); 52.99 (C-19;21). MS (C21H22ClNO4) [M]+: 387.
(E)-1-(4-chlorophenyl)-3-(4-hydroxy-3-methoxy-5-(piperidin-1-ylmethyl)phenyl)-prop-2-en-1-one (4b)
Yellow crystals, yield 75%, melting point 150°C–151°C. 1H -NMR (500 MHz, acetone-d6) δ (ppm): 8.13 (d, J = 8.5 Hz, 2H, H-11;15); 7.75 (d, J = 15.5 Hz, 1H, H-7); 7.71 (d, J = 15.5 Hz, 1H, H-8); 7.58 (d, J = 8.5 Hz, 2H, H-12;14); 7.41 (s, 1H, H-4); 7.18 (s, 1H, H-6); 3.88 (s, 3H, H-17); 3.87 (s, 2H, H-18); 2.67(m, 4H, H-19;23); 1.13 (m, 6H, H-20;21;22). 13C-NMR (125 MHz, acetone-d6) δ (ppm) 189.37 (C-9); 151.44 (C-3); 148.49 (C-7); 146.26 (C-2); 138.96 (C-13); 137.09 (C-10); 129.98 (C-11;15); 128.99 (C-12;14); 125.50 (C-5); 122.90 (C-1); 121.72 (C-8); 118.46 (C-6); 110.20 (C-4); 61.82 (C-18); 56.15 (C-17); 53.98 (C-19;23); 25.88 (C-20;22); 23.96 (C-21). MS (C22H24ClNO3) [M]+: 385.
(E)-1-(4-chlorophenyl)-3-(3-((diethylamino)methyl)-4-hydroxy-5-methoxyphenyl)prop-2-en-1-one (4c)
Yellow crystals, yield 70%, melting point 84°C–85°C. 1H-NMR (500 MHz, acetone-d6) δ (ppm): 8.11 (d, J = 8.4 Hz, 2H, H-11;15); 7.73 (d, J = 15.5 Hz, 1H, H-7); 7.69 (d, J = 15.5 Hz, 1H, H-8); 7.57 (d, J = 8.5 Hz, 2H, H-12;14); 7.40 (s, 1H, H-4); 7.14 (s, 1H, H-6); 3.87 (s, 3H, H-17); 3.75 (s, 2H, H-18); 2.54 (m, 4H, H-19;21); 1.63 (m, 6H, H-20;22). 13C-NMR (125 MHz, acetone-d6) δ (ppm): 189.36 (C-9); 151.44 (C-3); 148.49 (C-7); 146.26 (C-2); 138.95 (C-13); 137.09 (C-10); 129.98 (C-11;15); 128.98 (C-12;14); 125.49 (C-5); 122.89 (C-1); 121.72 (C-8); 118.45 (C-6); 110.19 (C-4); 61.82 (C-18); 56.14 (C-17); 53.98 (C-19;21); 25.88 (C-20;22). MS (C21H24ClNO3) [M]+: 373.
Antimalarial activity
An in vitro antimalarial activity assay of chalcone 3 (without amine group) and aminoalkylated chalcone 4a-c was performed against chloroquine-sensitive P. falciparum (Pf3D7) strain. Based on the data in Table 1, it can be seen that there is a significant increase in antimalarial activity with the addition of a secondary amine to the chalcone compound. Chalcone 3 without secondary amine group showed an IC50 of 25.84 μM, and the addition of diethylamine (4c) could improve the antimalarial activity significantly to 1.12 μM. The substitution of piperidine (4b) and morpholine group (4a) showed a better antimalarial activity with IC50 of 0.54 and 0.62 μM, respectively. This result proposed the important role of the amine group in the antimalarial activity. This fact also can be seen from the excellent antimalarial activity of chloroquine as a positive control (0.06 μM), which has three amine functional groups. Suwito et al. (2014) stated that amines can form electrostatic interactions with carbonyl groups from the protein of the Plasmodium parasite, so they can kill parasites. This study revealed that the prepared compound 4a–c could be categorized as an antimalarial compound with strong activity (IC50 ≤ 1 μM), based on the category by Batista et al. (2009).
Molecular docking
Dihydrofolate reductase–thymidylate synthase (PfDHFR-TS) protein was chosen as the molecular target in the docking process as it has an essential mechanism in the biosynthesis of folate that needed in DNA synthesis (Singh and Mishra, 2018). The inhibition of the folate biosynthesis is the target in the discovery of new antimalarial drugs because this step can inhibit the formation of the nucleotide deoxythymidine monophosphate, and in sequence, it can prevent the synthesis of DNA in Plasmodium parasite in thymidylate cycle. As a result, Plasmodium could not grow and eventually die (Yuvaniyama et al., 2003).
In this work, the molecular docking was performed in 4b, as this compound exhibited the best in vitro antimalarial activity. The molecular docking was performed to predict the interaction of secondary amine functional group in 4b to the amino acid of PfDHFR-TS protein. The molecular docking result of 4b to the protein 1J3I.pdb is shown in Figure 2.
The result of the molecular docking of compound 4b to 1J3I.pdb protein displayed seven hydrogen bond interactions with ILE112, ILE164, SER111, SER108, ASP54, TYR170, and PRO113 amino acid residues (Fig. 2). The number of hydrogen bonds formed was similar to the interaction by co-crystallized ligands WR99210. This result is proposed to determine the strong in vitro antimalarial activity of 4b. The calculation of CDOCKER interaction energy of 4b was −48.84 kcal/mol, which is lower than the interaction energy of co-crystallized ligands WR99210 (−54.32 kcal/mol) (Syahri et al., 2017c). This result implies that co-crystallized ligands WR99210 form a more stable interaction than 4b. The remarkable part from the interaction formed in Figure 2 was the hydrogen bond interaction of 4b to amino acid residue SER108 and SER111. Both of these amino acid residues are the essential amino acid in the DHFR-TS protein of chloroquine-sensitive and chloroquine-resistance P. falciparum (Yuvaniyama et al., 2003). It can be concluded that the increasing antimalarial activity of 3 (25.84 μM) to 4b (0.54 μM) was affected by the presence of the amine group. Thus, it can be proposed that aminoalkylated chalcone 4b would show a good antimalarial activity against chloroquine-resistance P. falciparum strain.
 | Table 1. An in vitro antimalarial activity (IC50) against Pf3D7. [Click here to view] |
 | Figure 2. Binding interaction from docking simulation of 4b into the active site of protein P. falciparum DHFR-TS, PDB ID: 1J3I. The coloring atom for the compound is in order as follows: carbons in black, oxygen in red, nitrogen in blue, chloride in green, and hydrogen in white. The green line indicates hydrogen-bonding interaction with distance ascribed in angstroms, Å. [Click here to view] |
CONCLUSION
This work showed that the addition of secondary amine groups such as morpholine, piperidine, and diethylamine could increase the antimalarial activity of chalcone derivatives from moderate (25.84 μM) to strong activity (0.54–1.12 μM). The molecular docking of 4b has also supported this result by indicating the interaction of the amine groups in the chalcone compound to SER111 and SER108 amino acid residues from the PfDHFR-TS protein. Thus, the secondary amine groups were essential in the development of new candidate antimalarial drugs.
ACKNOWLEDGMENTS
The authors are gratitude to the Ministry of Research, Technology, and Higher Education, Indonesia, for the financial support of this work through the Penelitian Dasar (PD) Scheme (the fiscal year of 2019) with contract number of 015/K10/KM/KONTRAK-PENELITIAN-J/2019.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, Maclnnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ, Spread of artemisinin resistance in Plasmodium falciparum malaria. Engl J Med, 2014; 371(5):411–23. CrossRef
Batista R, Junior AJS, Oliveira AB. Plant-derived antimalarial agents: New leads and efficient phytomedicine. Part II. Non-alkaloidal natural products. Molecules, 2009; 14(8):3037–72. CrossRef
Cai CY, Rao L, Rao Y, Guo JX, Xiao ZZ, Cao JY, Huang ZS, Wang B. Analogues of xanthones-Chalcones and bis-chalcones as glucosidase inhibitors and anti-diabetes candidates. Eur J Med Chem, 2017; 130:51–9. CrossRef
Castaño LF, Cuartas V, Bernal A, Insuasty A, Guzman J, Vidal O, Rubio V, Puerto G, LukáÄ P, Vimberg V, Novtoná GB, Vannucci L, Janata J, Quiroga J, Abonia R, Nogueras M, Cobo J, Insuasty B, New chalcone-sulfonamide hybrids exhibiting anticancer and antituberculosis activity. Eur J Med Chem, 2019; 176:50–60. CrossRef
Chu WC, Bai PY, Yang ZQ, Cui DY, Hua YG, Yang Y, Yang Q-Q, Zhang E, Qin S. Synthesis and antibacterial evaluation of novel cationic chalcone derivatives possessing broad spectrum antibacterial activity. Eur J Med Chem, 2018; 143:905–21. CrossRef
Custodio JMF, Vaz WF, de Castro MRC, Bernardes A, Naves RF, Moura AF, de Moraes MO, da Silva CC, Martins FT, Perez CN, Napolitano HB. Solvent-driven structural adaptation in a novel anticancer sulfonamide chalcone. J Mol Struct, 2019; 1175:389–97. CrossRef
Funk P, Motyka K, Soural M, Malon M, Koshino H, Kusz J, Hlavac J. Study of 2-aminoquinolin-4(1H)-one under Mannich and retro-Mannich reaction, PLoS One, 2017; 12(5):e0175364. CrossRef
Khan SA, Asiri AM, Al-Ghamdi NSM, Asad M, Zayed MEM, Elroby SAK, Aqlan FM, Wani MY, Sharma K. Microwave assisted synthesis of chalcone and its polycyclic heterocyclic analogues as promising antibacterial agents: In vitro, in silico and DFT studies. J Mol Struct, 2019; 1190:77–85. CrossRef
Muchtaridi M, Yusuf M, Syahidah HN, Subarnas A, Zamri A, Bryant SD, Langer T. Cytotoxicity of chalcone of eugenia procedure Burm F. leaves against T47D breast cancer cell lines and its prediction as an estrogen receptor antagonist based on pharmacophore-molecular dynamics simulation. Adv Appl Bioinform Chem, 2019; 12:33–43. CrossRef
Neto AG, Lavarda FC. The correlation between electronic structure and antimalarial activity of alkoxylated and hydroxylated chalcones. Med Chem Res, 2014; 23:580–6. CrossRef
Pandeya SN, Sriram D, Nath G, DeClercq E, Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4’-chlorophenyl)thiazol-2-yl] thiosemicarbazide. Eur J Pharm Sci, 1999; 9(1), 25–31. CrossRef
Reddy MVB, Su CR, Chiou WF, Liu YN, Chen RYH, Bstow KF, Lee KH, Wu TS. Design, synthesis, and biological evaluation of Mannich bases of heterocyclic chalcone analogs as cytotoxic agents. Bioorg Med Chem. 2008; 16: 7358–70. CrossRef
Rieckmann KH, Sax LJ, Campbell GH, Mrema JE, Drug sensitivity of P. Falciparum. An in vitro microtechnique. Lancet, 1978; 22:311. CrossRef
Roman G. Mannich bases in medicinal chemistry and drug design. Eur J Med Chem, 2015; 89:743–816. CrossRef
Sharma N, Mohanakrishnan D, Sharma UK, Kumar R, Richa, Sinha AK, Sahal D. Design, economical synthesis and antiplasmodial evaluation of vanillin derived allylated chalcones and their marked synergism with artemisinin against chloroquine resistant strains of Plasmodium falciparum. Eur J Med Chem, 2014; 79:350−68. CrossRef
Shin J, Jang MG, Park JC, Koo YD, Lee JY, Park KS, Chung SS, Park K. Antidiabetic effects of trihydroxychalcone derivatives via activation of AMP-activated protein kinase. J Ind Eng Chem, 2018; 60:177–84. CrossRef
Sibley CH, Understanding artemisinin resistance. Science, 2015; 347(6220):373–4. CrossRef
Singh IV, Mishra S. Molecular docking analysis of pyrimethamine derivatives with Plasmodium falciparum dihydrofolate reductase. Bioinfomation, 2018; 14(5):232–35. CrossRef
Suwito H, Jumina, Mustofa, Pudjiastuti P, Fanani MZ, Ariga YK, Katahira R, Kawakami T, Fujiwara T, Hase T, Sirat HM, Puspaningsih NNT. Design and synthesis of chalcone derivatives as inhibitor of the ferredoxin-ferredoxin-NADPH+ reductase interaction of Plasmodium falciparum: pursuing new antimalarial agents. Molecules, 2014; 19:473–488. CrossRef
Syahri J, Nurohmah BA, Yuanita E, Armunanto R, Purwono B, Chalcone analogue as potent anti-malarial compounds against Plasmodium falciparum: synthesis, biological evaluation, and docking simulation study. Asian Pac J Trop Biomed, 2017c; 7(7):1–5. CrossRef
Syahri J, Rullah K, Armunanto R, Yuanita E, Nurohmah BA, Mohd Aluwi MFF, Wai LK, Purwono B. Synthesis, biological evaluation, QSAR analysis, and molecular docking of chalcone derivatives for antimalarial activity. Asian Pac J Trop Dis, 2017b; 7(1):9−14.
Syahri J, Yuanita E, Nurohmah BA, Wathon MH, Syafri R, Armunanto R, Purwono B. Xanthone as antimalarial: QSAR analysis, synthesis, molecular docking and in-vitro antimalarial evaluation. Orient J Chem, 2017a; 33(1):29−40. CrossRef
Tadigoppula N, Korthikunta V, Gupta S, Kancharla P, Khaliq T, Soni A, Srivastava RK, Srivastava K, Puri SK, Raju KSR, Wahajuddin Sijwali PS, Kumar V, Mohammad IS. Synthesis and insight into the structure−activity relationships of chalcones as antimalarial agents. J Med Chem, 2013; 56(1):31−45. CrossRef
Ur-Rashid H, Xu Y, Ahmad N, Muhammad Y, Wang L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb activities. Bioorg Chem, 2019; 87:335–65. CrossRef
Wang J, Huang L, Cheng C, Xie GLJ, Shen M, Chen Q, Li W, He W, Qiu P, Wu J. Design, synthesis and biological evaluation of chalcone analogues with novel dual antioxidant mechanisms as potential anti-ischemic stroke agents. Acta Pharm Sin B, 2019; 9:335–50. CrossRef
Wilhelm A, Kendrekar P, Noreljaleel AEM, Abay ET, Bonnet SL, Wiesner L, deKock C, Swart KJ, Westhuizen JH, Syntheses and in vitro antiplasmodial activity of aminoalkylated chalcones and analogues. J Nat Prod, 2015; 78:1848−58. CrossRef
World Health Organization. Global Malaria Programme (GMP). WHO Press, Geneva, Switzerland, 2018.
World Health Organization. World Malaria Report 2018. WHO Press, Geneva, Switzerland, 2019.
Yamali C, Gul HI, Sakagami H, Supuran CT. Synthesis and bioactivities of halogen bearing phenolic chalcones and their corresponding bis Mannich bases. J Enzyme Inhib Med Chem, 2016; 31(sup4):125–31. CrossRef
Yuvaniyama J, Chitnumsub P, Kamchonwongpaisan S, Vanichtanankul J, Sirawaraporn W, Taylor P, Walkinshaw MD, Yuthavong Y. Insights into antifolate resistance from malarial DHFR-TS structures. Nat Struct Bio, 2003; 10(5):357–65. CrossRef