INTRODUCTION
Cardiovascular disease is the leading cause of death in the world with three-quarters of cases occurring in countries with middle to low income and half of the cases in Asia (Ohira and Iso, 2013). Cardiovascular disease is a manifestation of the clinical process of atherosclerosis which often happens abruptly and dramatically (AHA, 2016). Atherosclerosis is one of the chronic inflammatory disorders that exhibit symptoms caused by the inflammatory process, the formation of foam cells, and the development of atheroma plaque on the subendothelial layer of the blood vessel wall (Heine et al., 2008; Hopkin, 2013; Wu et al., 2017). The formation of foam cell is one of the critical stages in the atherosclerosis process, not only does it relate to lipid metabolism but also the inflammation that it causes can accelerate atherosclerosis progressiveness (Bobryshev et al., 2016).
Lipid metabolism on macrophage covers three processes, namely, cholesterol uptake, esterification, and secretion of the cholesterol (cholesterol efflux). The imbalance between these processes may cause the accumulation of lipids in the cytoplasm of the macrophage forming foam cells (Maguire et al., 2019). The inflammation on the subendothelial layer which is caused by ox-LDL would attract the monocyte of the lumen which later activated and became macrophage (Hopkin, 2013; Raggi et al., 2018). The activated macrophage expresses scavenger receptors, one of them is Toll-Like Receptor 4 (TLR4), causing an increase of ox-LDL uptake (Moore and Tabas, 2011). The current studies showed that TLR4 expression is increased in atherosclerosis, whereas deficiency of TLR4 on mice decreases lesion up to 71% (Cole and Georgiou, 2010). The stimulation of macrophage with ox-LDL would activate the TLR4 receptor and increase the uptake of ox-LDL, which immediately leads to the lipid accumulation in the cell (Keyel et al., 2012). In addition to lipid accumulation, the activation of TLR4 receptor would also increase the expression of pro-inflammatory mediators, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), and promote the degradation on matrix of the atherosclerotic lesion (Falck-Hansen et al., 2013; Higashimori et al., 2011; Shalhoub et al., 2011).
Another mechanism of foam cell formation is through the inhibition of the cholesterol efflux process from macrophage (Yuan et al., 2012). It is known that Adenosine Triphosphate-binding cassette transporter A1/G1 (ABCA1/ABCG1) protein transporter has an important role in the mechanism of cholesterol efflux and lipid metabolism (Tarling and Edwards, 2011; Yvan-Charvet et al., 2010). The expression of ABCA1/ABCG1 on macrophage is regulated by the transcription factors of peroxisome-proliferated-activated receptor γ (PPARγ) and liver X receptor (LXR) (Sotherden et al., 2012; Uitz, 2014). The activation of these transcription factors would increase the expression of ABCA1/ABCG1, followed by the increase of cholesterol efflux and the decrease of lipid deposition in the foam cell (Biswas and Mantovani, 2012; Nikolic et al., 2016).
Eleutherine americana Merr. (E. americana), also known as Bawang Dayak in Kalimantan, Indonesia, is one of the medicinal plants that is widely used by the local people as a traditional medicine. Bawang Dayak is used as a medicine for pain relief, intestinal disorder, antifertility agent, and abortion as well as cardiovascular disorders (Ha et al., 2013). Multilevel extraction of the E. americana by using n-hexane with high-performance liquid chromatography identified some Quinone derivate active substances. In addition, the phytochemical evaluation of E. americana had successfully identified three groups of Quinone substances such as anthraquinone, naphthoquinone, and naphthalene (Hong, et al., 2008). A meta-analysis study that examined the activity of substances from naphthoquinone group showed that it functioned specifically as an anti-inflammatory agent (Insanu et al., 2014). Some biological activities of E. americana have been identified such as anti-dermatophytes and anti-melanogenesis (Kusuma et al., 2010); inhibiting the NO production by macrophage induced with Lipopolysaccharides (Han et al., 2008); as immunomodulator on T helper cell (Hong et al., 2008); as anti-oxidant (Nur, 2011; Nurliani and Santoso, 2012; Pratiwi et al., 2013); inhibitor of inducible nitric oxide synthase (iNOS); and inhibitor of cytokines expression such as IL-1β and interferon-β (IF-β) through inhibition of nuclear factor kappa B (NF-kB) (Song et al., 2009).
Therefore, this study aimed to investigate the anti-atherosclerosis activity of n-hexane extract of E. americana on human macrophage induced with ox-LDL.
MATERIALS AND METHODS
The isolation of monocyte from PBMCs
This study has previously been examined and approved by the Health Research Ethics Commission of the Faculty of Medicine Universitas Brawijaya Malang, Indonesia (No. 308/EC/KEPK-S3/9/2017). PBMCs were collected from the blood sample of a healthy adult male. Blood donor was required to give informed consent for sampling. The isolation of monocytes was conducted by RosetteSepTM human monocyte enrichment cocktail following the factory protocol. The blood sample was placed in an Ethylenediaminetetraacetic acid tube and then added with RosetteSepTM human monocyte enrichment cocktail (Stemcell Technology, #15068), mixed, and incubated for 20 minutes. The sample was diluted with the recommended medium and mixed slowly. The diluted sample was moved to a tube added with a density gradient medium in accordance with the protocol kit. The sample was centrifuged at 1,200xg for 10 minutes and the cell was collected, washed, and centrifuged again. Afterward, the cell was diluted in the medium. The monocyte cell as the result of these processes was calculated by using a hemocytometer (Delirezh et al., 2013).
Primary human macrophage cell culture
The isolated monocytes were placed on a plate that has been added with the medium for culture process containing RPMI-1640, Dulbecco’s modified Eagle’s medium, fetal bovine serum, and penicillin-streptomycin for 5 days. Cell culture was placed in an incubator at 37°C and 5% CO2 so that the monocytes differentiated and became macrophage. For the treatment group, the n-hexane extract of E. americana was added on the sixth day, incubated for 24 hours, and then used for further experimental research (Safi et al., 2016).
Preparation of n-hexane extract of Eleutherine americana
The extract of n-hexane Eleutherine americana Merr. was prepared in the Pharmacology Laboratory at the Medical Faculty of Mulawarman University, Samarinda, East Kalimantan, Indonesia. The Bawang Dayak plant was obtained from the local farmer in Samarinda, East Kalimantan. The plant was then preliminarily examined in the Laboratory of Plant Systematics and Anatomy, Faculty of Mathematics and Natural Sciences, Mulawarman University, in order to gain the identification of its taxonomy. About ±2–3 kg bulbs of Bawang Dayak were well sorted, cleaned, and dried in a cabinet. This process resulted in ±250–500 g of dried simplisia. After it was well dried, the substance was blended to make smooth dried powder. Dried powder of Bawang Dayak bulb was extracted with methanol 90% resulting in crude methanol extract. The crude extract was then extracted with methanol and water with 6:4 (v/v) ratio. The crude extract was also extracted with n-hexane in a separate funnel, creating two different extracts. The addition of n-hexane was conducted repeatedly until clear n-hexane extract was obtained. The n-hexane extract was concentrated with a rotary evaporator. For the purpose of the experiment, Bawang Dayak extract was diluted in dimethyl sulfoxide for cell culture with the concentration of the final culture ≤0.1% (Ahmad et al., 2016).
Foam cell formation
The mature monocytes prepared earlier were placed on a plate and were added with n-hexane Eleutherine extract with a dose of 0.25, 1, and 2 mg/ml on the sixth day. Cells were incubated for 24 hours before stimulation with 100 μg/ml of oxidized low-density lipoproteins in human plasma (Athens Research and Technology; #12-16-120412-OX). Cells were then incubated again for 48 hours. Afterward, staining was conducted using Oil Red O and cells were examined under an optical microscope with 640x magnification (Park et al., 2015).
Oil red O staining
After 48 hours of incubation, monocytes are prepared for staining. The culture medium was slowly decanted through aspiration and carefully not to disturb the cells. Cells were then washed twice with phosphate-buffered saline (PBS) solution. Cells were fixed for 10 minutes with 4% paraformaldehyde, and then quickly washed with 60% isopropanol. The lipid staining process was conducted with Oil Red O, Sudan Red 5B (Bioworld; 41540000-2) for 15 minutes (0.4% Oil Red in 100% isopropanol) and then the mixture was quickly washed again with 60% isopropanol and finally with PBS. Cells were examined under an optical microscope with 640x magnification. For absorbance solubility of Oil Red O examination, 100 μl 100% isopropanol was added and mixed for 10 minutes. The supernatant was moved to a new well and read on the spectrophotometer with 570 nm wavelength (Ning et al., 2017).
Calculating the percentage of the foam cells
The number of foam cells was calculated in every 100 cells using a cell counter to calculate foam cell percentage. The foam cell was determined by selecting a cell with red color on its cytoplasm. The red color was formed from lipid droplet on the cytoplasm which was stained by Oil Red O.
Nuclear protein extraction
For nuclear protein extraction, cells were cultured on a 12-well plate to collect a sufficient number of cells. After the addition of n-hexane extract and ox-LDL, nuclear protein extraction was conducted using RayBio® Nuclear Extraction Kit following the manufacturer protocol. Cells were washed with ice-cold PBS while pipetting up and down gently to disperse the cells. Then, 1x nuclear extract reagent-I, which was prepared in accordance with the protocol was added to the cells and incubated on ice for 15 minutes. Reagent-II was added, mixed slowly, and incubated on ice for 2 minutes. Cells were centrifuged at 14,000xg with 4°C temperature for 30 seconds before separating the supernatant. Reagent-III was added into the cell pellet, diluted, vortexed for 10 seconds, and incubated on ice for 10 minutes. After this, the tube was vortexed for 10 seconds and incubated on ice for 10 minutes repeatedly until the total time for incubation was 40 minutes. Afterward, the tube was centrifuged 14,000xg with 4°C temperature for 30 minutes. The supernatant was collected and aliquoted into new tubes for further examination. Nuclear protein obtained was weighed using NanoDrop (Chan and Cipolla, 2012).
Determinant of PPARγ transcription factor activity
Measurement of PPARγ activity was conducted using Enzyme-linked immunosorbent assay (ELISA). A double-stranded oligonucleotide labeled 96-well plate was used as the place of PPARγ bonding. The oligonucleotide would specifically catch active PPARγ from the shortly incubated nuclear protein. The PPARγ activity of the obtained nuclear protein is measured using Raybio® Human PPARγ transcription factor Activity Assay Kit according to the manufacturer protocol. Each well was filled with appropriate reagent and then the primary antibody for PPARγ was added to the well and incubated for 1 hour. After washing, 3,3',5,5'-tetramethylbenzidine One-Step Substrate Reagent was added and incubated for 30 minutes. After this step, the stop solution was added and the result was immediately read on the ELISA reader of 450 nm wavelength (Chan and Cipolla, 2012).
Immunofluorescence staining
Cells from the treatment and control group were harvested and fixed with 4% paraformaldehyde in PBS at pH 7.4 for 10 minutes at room temperature. Immunofluorescence staining was used to measure the expression of TLR4, ABCA1, and ABCG1 proteins through a confocal microscope. The antibodies used were mouse monoclonal IgG ABC1 (Santa Cruz Biotechnology, Inc.; #A00121.01), rabbit anti ABCG1 polyclonal antibody (Bioss Inc.; #bs-4906R), mouse monoclonal IgG TLR4 (#HTA 125), goat anti-mouse IgG H and L [Fluorescein isothiocyanate (FTIC)] secondary antibody from Abcam (#ab6785), and goat anti-rabbit IgG H and L (Rhodamine) secondary antibody from Invitrogen (#31670). Cell permeabilization was done by adding 0.1%–0.25% Triton-X 100 in PBS and then incubated for 10 minutes. The cells were then washed three times. Blocking was conducted by adding 1% bovine serum albumin (BSA), 22.52 mg/ml glycine in Phosphate Buffered Saline Tween-20 (PBST) (PBS+ 0.1% Tween 20) for 30 minutes. Cells were then incubated in primary antibody which was diluted in 1% BSA in PBST overnight at 4°C temperature. The primary antibody solution was discarded and cells were washed with PBS three times for 5 minutes each. Cells were then incubated in a secondary antibody in 1% BSA for 1 hour in the darkroom at room temperature. The secondary antibody solution was discarded and cells were washed three times with PBS for 5 minutes each in the darkroom. Coverslip was installed before cells were examined under the microscope (Wang et al., 2018).
Statistical analysis
We performed analysis of variance for statistical analysis as appropriate. The significances were then ranked using Least significant different-ANOVA analysis with 95% confidence intervals. Values are expressed as the mean and ± standard error of mean (SEM). All the tests were carried out using SPSS version 23.
RESULTS AND DISCUSSION
The effect of n-hexane extract of Eleutherine americana in inhibiting foam cell formation on ox-LDL stimulated macrophage
The purpose of this research was to prove whether the n-hexane extract of E. americana was able to inhibit the formation of macrophage-derived foam cell in vitro. Macrophage foam cell is formed through the increase of ox-LDL uptake into the macrophages without adequate cholesterol lipid efflux. Foam cell is marked by the presence of lipid deposits in the cytoplasm of macrophages which is microscopically visible with Oil Red O staining (red color) (Fig. 1a). The monocytes isolated from PBMCs, after undergoing differentiation, were incubated with n-hexane extract of E. americana with the dosage of 0.25, 1 and 2 mg/ml. We demonstrated that the percentage of the foam cells was decreased in the treatment group that was given n-hexane extract compared with the control group which was only given ox-LDL that seemed to be dose-dependent (Fig. 1b). Identically, the result of the examination using absorbance of Oil Red O on a spectrophotometer, which indirectly exposed the content of lipid in the cells, showed that there was a decrease of lipid content in the treatment group compared to the control group that seemed to be dose-dependent (Fig. 1c)
The results of this study showed, for the first time, that the n-hexane extract of E. americana demonstrated anti-atherosclerotic activity. This was demonstrated through its capability in inhibiting the formation of foam cells on ox-LDL induced macrophages. Foam cells formation can be inhibited through either the ox-LDL uptake mechanism or cholesterol efflux from macrophages. The balance between the two processes is important in maintaining a normal level of intra-cell lipid. On the contrary, cell failure in balancing the two mechanisms can lead to excessive lipid accumulation which results in the acceleration of apoptosis and cell necrosis and finally manifested as atherosclerotic plaque (Yu et al., 2013).
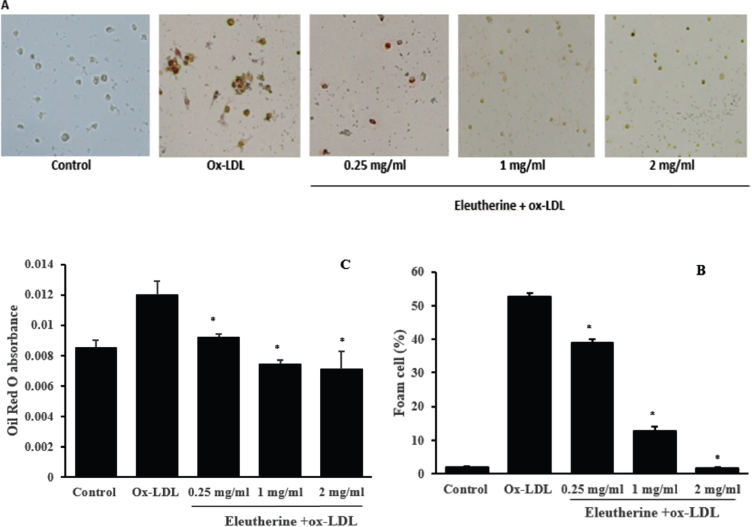 | Figure 1. Effects of n-hexane extract of E. americana administration in inhibiting foam cell formation in ox-LDL stimulated macrophages. (a) Monocytes originating from PBMCs, after underwent differentiation, were given n-hexane extract with a dose of 0.25, 1, and 2 mg/ml, incubated for 24 hours, and then stimulated with 100 μg/ml ox-LDL. After 48 hours of incubation, cells were stained with Oil Red O. The red color indicated the lipid stained by Oil Red O. (b) The number of foam cells was computed by the cell counter. (c) The macrophages stained with Oil Red O were added with 100 μl isopropanol 100% for 10 minutes and were read on spectrophotometer 570 nm wavelength. All data are presented as mean ± SEM, *p < 0.05 compared to the ox-LDL group. [Click here to view] |
Previous studies have provided ample evidence that natural substance extracts possess anti-atherosclerotic activity. Some biological activities related to anti-atherosclerosis from natural substances under empirical research were Zanthoxylum heitzii as anti-oxidative stress (Ntchapda et al., 2015), Premna integrifolia Linn. that demonstrated ability to improve the lipid profile, atherogenic index, cardiac marker, and atherosclerotic lesion on mice fed with high-fat diet (Subramani et al., 2017), and Salviae Miltiorrhizae Radix et Rhizoma that showed anti-atherosclerotic effect, anti-inflammatory effect, and protection against of oxidative damage (Li et al., 2018; Pang et al., 2016).
The effect of n-hexane extract of Eleutherine americana in decreasing of TLR4 protein expression on ox-LDL stimulated macrophage
TLR4 is a membrane protein expressed by macrophage which is activated by a foreign object like ox-LDL that acts as a scavenger receptor. We used immunofluorescence staining to examine the effect of the n-hexane extract of E. americana in decreasing TLR4 expression. The treatment group that was given n-hexane extract with various dosages showed a decrease in the intensity of TLR4 expression. This effect seemed to be dose-dependent. A significant decrease occurred in the treatment group given 1 and 2 mg/ml of n-hexane extract compared to the control group that was given ox-LDL (Fig. 2).
This study also proved that the n-hexane extract of E. americana were able to decrease the expression of TLR4 receptor. The TLR4, besides being responsible for activating the cascade of cellular inflammation through NF-kB, has been identified as the receptor that increases the uptake of ox-LDL into the cell (Falck-Hansen et al., 2013). By decreasing TLR4 expression on the macrophage, the uptake of ox-LDL into the macrophages would also be reduced resulting in less foam cell formation. Another study found that mice with TLR4-/- deficiency that was fed with a high-fat diet for 6 months has a decreased size of atherosclerotic lesions, lipid content, and macrophage infiltration (Higashimori et al., 2011; Mahmoudi, 2016). This study demonstrated that TLR4 has a vital role in the process of cholesterol uptake into the macrophages.
The effect of n-hexane extract of Eleutherine americana in increasing ABCA1 and ABCG1 expression on ox-LDL stimulated macrophage
ABCA1 and ABCG1 are transporter proteins that have an important role in lipid transport from intracellular to extracellular to be caught by Apo-AI and high-density lipoprotein in the cholesterol efflux mechanism. We used double-staining immunofluorescence to examine the effect of n-hexane extract of E. americana toward ABCA1 and ABCG1 expression. The treatment group that was given n-hexane extract with various dosage demonstrated an increase in the protein expression of both ABCA1 and ABCG1 that seemed to be dose-dependent compared to the control group that was given only ox-LDL (Fig. 3).
The increase in ABCA1 and ABCG1 protein expression on ox-LDL stimulated macrophages showed that the n-hexane extract of E. americana has a positive effect on intracellular cholesterol metabolism. ABCA1 and ABCG1 are very important for cholesterol transport in cholesterol efflux (Yvan-Charvet et al., 2010). Cholesterol efflux is a vital mechanism in cellular cholesterol homeostasis as a response to the fluctuation of cholesterol uptake. (Park et al., 2012) On the other hand, imbalance between cholesterol uptake and cholesterol efflux is the basis for intracellular lipid deposition that leads to foam cells formation. In the end, excessive accumulation of intracellular cholesterol would cause cellular toxicity and cell death (Yuan et al., 2012). In this study, n-hexane extract of E. americana was proven to improve intracellular cholesterol circulation simultaneously through both the uptake and the secretion process. This confirms the effectiveness of E. americana as a modulator of cholesterol homeostasis in macrophages.
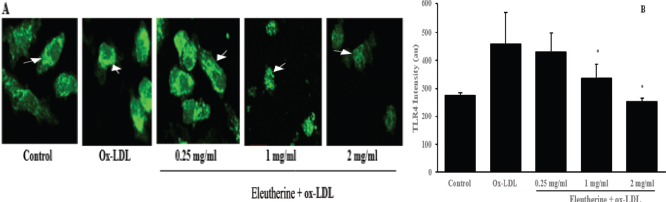 | Figure 2. Administration of n-hexane extract of E. americana was able to reduce TLR4 protein expression in human macrophages stimulated with ox-LDL. In picture (a) macrophages derived from PBMCs were given n-hexane extract of 0.25, 1, and 2 mg/ml dosage, incubated for 24 hours, and then stimulated with 100 μg/ml ox-LDL. After 48 hours of incubation, cells were fixed with 4% paraformaldehyde and immunofluorescence staining was performed using mouse monoclonal IgG TLR4 primary antibody and goat anti-mouse IgG H and L (FITC) secondary antibody. The result observed with a confocal microscope demonstrated different levels of intensity (green color) that showed TLR4 protein expression. (b) The result of quantitative color intensity measurements in the treatment group given n-hexane extract of 1 and 2 mg/ml dosage was decreased *p = 0.008 and 0.000 compared to the control group that was given ox-LDL. All data are presented as mean ± SEM. [Click here to view] |
In this study, administration of n-hexane extract of E. americana, despite being able to increase the protein expression of ABCA1 and ABCG1, did not affect the activity of PPARγ transcription factor (Fig. 4). This effect is contrary to the result demonstrated by Matsumura et al. (2011) in which Telmisartan increased the expression of ABCA1 and ABCG1 through activation of PPARγ. The expression of ABCA1 and ABCG1 is regulated by PPARγ and LXR nucleus receptors, both will activate the genes responsible for the expression of ABCA1 and ABCG1 (Remmerie and Scott, 2018). It is possible that n-hexane extract of E. americana increased the expression of ABCA1 and ABCG1 through the LXR pathway directly. Nevertheless, this has not been proven. Another study has shown that increased expression of ABCA1 and ABCG1 occurred through activation of the LXR nucleus receptor (Iizuka et al., 2012). Meanwhile, there is also a study that proved that PPARγ and LXR work collaboratively through the PPARγ/LXR pathway axis in regulating ABCA1 and ABCG1 expression (Jiang et al., 2017). The result of this study provides the basis for further research on the mechanism of E. americana in inhibiting atherosclerosis so that it can be used as one of the approaches in the treatment of atherosclerosis.
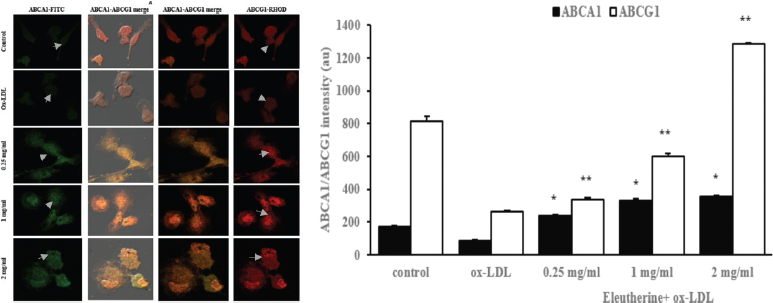 | Figure 3. Administration of n-hexane extract of E. americana was able to increase the ABCA1 and ABCG1 protein expression in human macrophages stimulated with ox-LDL. (a) Macrophages derived from PBMCs were given n-hexane extract of 0.25, 1, and 2 mg/ml dosage, incubated for 24 hours, then stimulated with 100 μg/ml ox-LDL. After 48 hours of incubation, cells were fixed with 4% paraformaldehyde and double staining immunofluorescence was performed. In (a), the result observed with a confocal microscope demonstrated different levels of intensity (green color) that showed ABCA1 expression, while the red color showed ABCG1 protein expression. (b) The result of quantitative color intensity measurements in the treatment group was decreased compared to the control group with *p = 0.000. All data are presented as mean ± SEM. [Click here to view] |
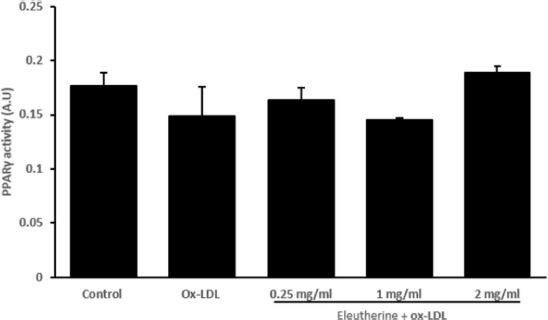 | Figure 4. Administration of n-hexane extract of E. americana had no effect in increasing PPARγ activity in human macrophages stimulated with ox-LDL in vitro. Macrophages derived from PBMCs were given n-hexane extract of 0.25, 1, and 2 mg/ml dosage, incubated for 24 hours, then stimulated with 100 μg/ml ox-LDL. In the group given n-hexane extract, there was an increase in PPARγ activity but was not significant compared to the control group given ox-LDL only. All data are presented as mean ± SEM. [Click here to view] |
CONCLUSION
We conclude that the n-hexane extract of E. americana was proven to have an anti-atherosclerotic activity by inhibiting foam cell formation through decreasing the ox-LDL uptake and increasing cholesterol efflux simultaneously.
ACKNOWLEDGMENT
Acknowledgments were conveyed to Bunga Prihardina, S.Si, Ami Maghfironi, S.Si, Wahyudha Ngatiril Lady, S.Si and Choirunil Chotimah, S.Si, M.Si who provided technical assistance in this study.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
Ahmad I, Arifuddin M, Rijai L. The effect of extraction methods of Bawang Dayak (Eleutherine palmifolia l. merr) against tlc profiles and sunscreen activities. Int J Pharm Tech Res, 2016; 9(9):428–36.
American Heart Association (AHA). Heart disease and stroke statistic-update 2016. A report from American Heart Association, 2016. Available via http://circ.ahajournals.org/ (Accessed 27 June 2016).
Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab, 2012; 15(4):432–7. CrossRef
Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN. Macrophages and their role in atherosclerosis: pathophysiology and transcriptome analysis. BioMed Res Int, 2016; 2016:1–13. CrossRef
Chan S-L, Cipolla MJ. Determination of PPARγ activity in adipose tissue and spleen. J Immunoass Immunochem, 2012; 29(3):997–1003. CrossRef
Cole JE, Georgiou E. The expression and functions of toll-like receptors in atherosclerosis. London, UK: Hindawi Publishing Corporation, pp 1–18, 2010. CrossRef
Delirezh N, Shojaeefar E, Parvin P, Asadi B. Comparison the effects of two monocyte isolation methods, plastic adherence and magnetic activated cell sorting methods, on phagocytic activity of generated dendritic cells. Cell J, 2013; 15(3):218–23.
Falck-Hansen M, Kassiteridi C, Monaco C. Toll-Like Receptors in atherosclerosis. Int J Mol Sci, 2013; 14(7):14008–23. CrossRef
Ha LM, Huyen DTT, Kiem PVan, Minh C, Van Van NTH, Nhiem NX, Kim YH. Chemical constituents of the rhizome of Eleutherine bulbosa and their inhibitory effect on the pro-inflammatory cytokines production in lipopolysaccharide -stimulated bone marrow-derived dendritic cells. J Chem Inf Model, 2013; 53(1):1689–99.
Han AR, Min HY, Nam JW, Lee NY, Wiryawan A, Suprapto W, Seo EK. Identification of a new naphthalene and its derivatives from the bulb of Eleutherine americana with inhibitory activity on lipopolysaccharide-induced nitric oxide production. Chem Pharm Bull, 2008; 56(9):1314–26. CrossRef
Heine GH, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, Girndt M. CD14(++), CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int, 2008; 73(5):622–9. CrossRef
Higashimori M, Tatro JB, Moore KJ, Mendelsohn ME, Galper JB, Beasley D. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol, 2011; 31(1):50–7. CrossRef
Hong JH, Yu ES, Han AR, Nam JW, Seo EK, Hwang ES. Isoeleutherin and eleutherinol, naturally occurring selective modulators of Th cell-mediated immune responses. Biochem Biophys Res Commun, 2008; 371(2):278–82. CrossRef
Hopkin PN. Molecular biology of atherosclerosis. Physiol Rev, 2013; 93:1317–542. CrossRef
Iizuka M, Ayaori M, Uto-Kondo H, Yakushiji E, Takiguchi S, Nakaya K, Ikewaki K. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J Nut Sci Vitaminol, 2012; 58(2):96–104. CrossRef
Insanu M, Kusmardiyani S, Hartati R. Recent studies on phytochemicals and pharmacological effects of Eleutherine americana Merr. Procedia Chem, 2014; 13:221–8. CrossRef
Jiang T, Ren K, Chen Q, Li H, Yao R, Hu H, Zhao GJ. Leonurine prevents atherosclerosis via promoting the expression of ABCA1 and ABCG1 in a PPARγ/Lxrα signaling pathway-dependent manner. Cell Physiol Biochem, 2017; 43(4):1703–17. CrossRef
Keyel PA, Tkacheva OA, Larregina AT, Salter RD. Coordinate stimulation of macrophages by microparticles and TLR ligands induces foam cell formation. J Immunol, 2012; 189(9):4621–9. CrossRef
Kusuma IW, Arung ET, Rosamah E, Purwatiningsih S, Kuspradini H, Syafrizal Shimizu K. Antidermatophyte and antimelanogenesis compound from Eleutherine americana grown in Indonesia. J Nat Med, 2010; 64(2):223–6. CrossRef
Li GH, Li YR, Jiao P, Zhao Y, Hu HX, Lou HX, Shen T. Therapeutic potential of Salviae Miltiorrhizae Radix et Rhizoma against human diseases based on activation of Nrf2-mediated antioxidant defense system: bioactive constituents and mechanism of action. Oxidative Med Cell Longevity, 2018; 2018:1–13. CrossRef
Maguire EM, Pearce SWA, Xiao Q. Foam cell formation: a new target for fighting atherosclerosis and cardiovascular disease. Vasc Pharmacol, 2019; 112:54–71. CrossRef
Mahmoudi, M. The role of TLR2, TLR4, and TLR9 in the pathogenesis of atherosclerosis. Medicine (United Kingdom), 2016; 46(9):505–8. CrossRef
Matsumura T, Kinoshita H, Ishii N, Fukuda K, Motoshima H, Senokuchi T, Taketa K, Kawasaki S, Nishimaki-Mogami T, Kawada T, Nishikawa T, Araki, E. Telmisartan exerts antiatherosclerotic effects by activating peroxisome proliferator-activated receptor-γ in macrophages. Arterioscle Thromb Vasc Biol, 2011; 31(6):1268–75. CrossRef
Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell, 2011; 145(3):341–55. CrossRef
Nikolic D, Castellino G, Banach M, Toth P, Ivanova E, Orekhov A, Rizzo M. PPAR agonists, atherogenic dyslipidemia and cardiovascular risk. Curr Pharm Design, 2016; 23(6):894–902. CrossRef
Ning H, Liu D, Yu X, Guan X. Oxidized low-density lipoprotein-induced p62/SQSTM1 accumulation in THP-1-derived macrophages promotes IL-18 secretion and cell death. Exp Ther Med, 2017; 14(6):5417–23. CrossRef
Ntchapda F, Maguirgue K, Adjia H, Etet PFS, Dimo T. Hypolipidemic, antioxidant and anti-atherosclerogenic effects of aqueous extract of Zanthoxylum heitzii stem bark in diet-induced hypercholesterolemic rats. Asian Pacific J Trop Med, 2015; 8(5):359–65. CrossRef
Nur AM. Kapasitas antioksidan Bawang Dayak (eleutherine palmifolia) dalam bentuk segar,simplisia dan keripik, pada pelarut nonpolar, semipolar dan polar. Department of Food Science and Technology, Faculty of Agricultural Technology, Bogor Agricultural University, Repository of IPB, Bogor, Jawa Barat, Indonesia, 2011.
Nurliani A, Santoso HB. Efek antioksidan ekstrak bulbus bawang dayak (Eleutherine palmifolia) pada gambaran histopatologis paru-paru tikus yang dipapar asap rokok. Bioscientiae, 2012; 9:60–9.
Ohira T, Iso H. Cardiovascular disease epidemiology in Asia - An overview. Circ J, 2013; 77(7):1646–52. CrossRef
Pang H, Wu L, Tang Y, Zhou G, Qu C, Duan JA. Chemical analysis of the herbal medicine salviae miltiorrhizae radix et rhizoma (Danshen). Molecules, 2016; 21(1):1–28. CrossRef
Park SH, Hun Paek J, Shin D, Lee JY, Lim SS, Kang YH. Purple perilla extracts with α-asarone enhance cholesterol efflux from oxidized LDL-exposed macrophages. Int J Mol Med. 2015; 35(4):957–65. CrossRef
Park SH, Kim JL, Kang MK, Gong JH, Han SY, Shim JH, Kang YH. Sage weed (Salvia plebeia) extract antagonizes foam cell formation and promotes cholesterol efflux in murine macrophages. Int J Mol Med, 2012; 30(5):1105–12. CrossRef
Pratiwi D, Wahdaningsih S, Isnindar I. The test of antioxidant activity from Bawang Mekah leaves (Eleutherine americana Merr.) Using dpph (2,2- diphenyl-1-picrylhydrazyl) method. Trad Med J, 2013;10–11.
Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS, Gupta M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis, 2018; 276:98–108. CrossRef
Remmerie A, Scott CL. Macrophages and lipid metabolism. Cell Immunol, 2018; 330:27–42. CrossRef
Safi W, Kuehnl A, Nüssler A, Eckstein HH, Pelisek J. Differentiation of human CD14+ monocytes: an experimental investigation of the optimal culture medium and evidence of a lack of differentiation along the endothelial line. Exp Mol Med. 2016; 48:1–9. CrossRef
Shalhoub J, Falck-Hansen Ma, Davies AH, Monaco C. Innate immunity and monocyte-macrophage activation in atherosclerosis. J Inflamm, 2011; 8(1):1–17. CrossRef
Song SH, Min HY, Han AR, Nam JW, Seo EK, Seoung Woo Park, Sang Kook Lee. Suppression of inducible nitric oxide synthase by (−)-isoeleutherin from the bulbs of Eleutherine americana through the regulation of NF-κB activity. Int Immunopharmacol, 2009; 9(3):298–302. CrossRef
Sotherden GM, Uto-kondo H, Ayaori M, Ikewaki K. Effects of nutraceuticals and botanicals on cholesterol efflux : implications for atherosclerosis macrophage. J Nut Therap, 2012; 1:96–106.
Subramani C, Rajakkannu A, Rathinam A, Gaidhani S, Raju I, Kartar Singh DV. Anti-atherosclerotic activity of root bark of Premna integrifolia Linn. in high fat diet induced atherosclerosis model rats. J Pharm Anal, 2017; 7(2):123–8. CrossRef
Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. PNAS, 2011; 108(49):19719–24. CrossRef
Uitz E. Practical strategies for modulating foam cell formation and behavior. World J Clin Cases, 2014; 2(10):497–506. CrossRef
Wang H, Yang Y, Sun X, Tian F, Guo S, Wang W, Tian Y. Sonodynamic therapy-induced foam cells apoptosis activates the phagocytic PPARγ-LXRα-ABCA1/ABCG1 pathway and promotes cholesterol efflux in advanced plaque. Theranostics, 2018; 8(18):4969–84. CrossRef
Wu MY, Li CJ, Hou MF, Chu PY. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int J Mol Sci, 2017; 18(10). CrossRef
Yu X, Fu Y, Zhang D, Yin K, Tang C. Foam cells in atherosclerosis. Clinica Chimica Acta, 2013; 424:245–52. CrossRef
Yuan Y, Li P, Ye J. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein and Cell, 2012; 3(3):173–81. CrossRef
Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol, 2010; 30(2):139–43. CrossRef