INTRODUCTION
The medicinal value of pyrazolo[1,5-a]pyrimidines
Being purine analogs, the chemistry of pyrazolo[1,5-a] pyrimidines has been extensively investigated. Such compounds have diverse pharmacological, medicinal, and pharmaceutical value. Zaleplon®, Ocinaplon®, Indiplon®, and Lorediplon® are pyrazolo[1,5-a]pyrimidine analogs that act as GABA A receptor agonists and used as sedative, hypnotic, anxiolytic, and in the treatment of insomnia (Ancoli-Israel et al., 1999; Chilman-Blair et al., 2003; Neubauer, 2005; d’Aniello et al., 2015). Dinaciclib® inhibits cyclin-dependent kinases and evaluated in clinical trials for various cancer indications (Parry et al., 2010). Anagliptin® is used for the treatment of type 2 diabetes mellitus in Japan (Ervinna et al., 2013).
Pyrazophos is used as a fungicide and an insecticide (de Waard, 1974). PHTPP® (Chan et al., 2014; Iorga et al., 2018) is used in scientific research as a nonsteroidal highly selective antagonist of β-estrogen receptors. DPA714® is a radiopharmaceutical for imaging translocator protein in living systems using positron emission tomography (PET), and its radiolabeled analog with C-11, DPA713®, is used as a radiotracer for imaging the TSPO using PET (Fig. 1) (Banister et al., 2012; Reynolds et al., 2010; Selleri et al., 2001).
Our approach to handling the subject
Regio-orientation assignment of substituents on the pyrazolo[1,5-a]pyrimidines, compounds formed via condensation of 3-aminopyrazoles with 1,3-bielectrophilic reagents requires: first, to investigate the chemical reactivity of 3(5)-aminopyrazoles with special emphasis on the sites of the nucleophilicity of such compounds [ NH2 (exocyclic) and NH (ring)]. Second, classification of the bielectrophilic reagents and locating the more electrophilic and less steric hindered site according to the rules of chemistry. Third, to assign the regio-orientation based on just believes, assumption, or weighing previous literature reports. In addition to insufficient or unreliable spectral data or irrelevant unambiguous synthesis are considered. Most importantly, evidence-based regio-orientation assignment of substituents around the pyrimidine ring using X-ray crystallography, 1H-15N Heteronuclear Multiple Bond Correlation, Nuclear Overhauser Effect (NOE) effect, and relevant unambiguous synthesis are highlighted.
.png) | Figure 1. The general structural formula for pyrazolo[1,5-a]pyrimidines of pharmacological, medicinal, and pharmaceutical value. [Click here to view] |
COMPARABLE REACTIVITY OF THE EXOCYCLIC AND ENDOCYCLIC NH2/NH IN 3(5)-AMINOPYRAZOLES
3(5)-aminopyrazole tautomers
The literature survey indicates that 3(5)-aminopyrazoles of type 1 have three nucleophilic centers, namely, exocyclic NH2, endocyclic NH, and the 3áµ’ N of the pyrazole ring. In addition to the fourth one if, and only if, the fourth position is unsubstituted. Such nucleophilicity could be referred to the behavior of the molecule as an enamine (Fig. 2).
Relative nucleophilicity of the amino and imino groups
Noteworthy, some authors believed (no evidence has been provided) that the endocyclic NH group is the most nucleophilic center in these compounds, although the experimental results (Al-Shiekh et al., 2004; Al-Omran and El-Khair, 2006; Dawood et al., 2005; Ege and Gilbert, 1979a; Ege et al., 1984; Elagamey et al., 1986; Elnagdi et al., 1976; 1981; El-Ghandour Ahmed Hafez et al., 1992; Joshi et al., 1983; KoÄevar et al., 1976) showed that such compounds could be successfully diazotized at the cost of the exocyclic NH2 group which is consistent with the fundamentals of chemistry (Scheme 1).
.png) | Figure 2. a) 4-substituted pyrazole b) unsubstituted pyrazole [Click here to view] |
Reactivity of aminopyrazoles in diazotization and coupling was discussed in 2009 (Moyano et al., 2008) and the pattern demonstrated previously was confirmed (Moyano et al., 2008).
Diazotization (Ege and Gilbert, 1979b; Wu et al., 2005) of 5-aminopyrazole derivative (3) gave diazo-3-(methylsulphonyl)-1H-pyrazole (4) that reacted with arylisocyanates in CH2Cl2 to give the corresponding pyrazolo[1,5-a]tetrazine analogs 5 (Scheme 2).
Relative nucleophilicity of NH2 group
Acylation of 5-aminopyrazole derivative (6) afforded a mixture of 3(5)-aminoacylpyrazole (7) and the two acyl analogs (8) and (9) (Scheme 3). Michon et al. reported that the major amide derivative isolated was due to the acylation reaction of exocyclic amino group (7) of percent yield (24%), while the percent yields of the other amides (8) and (9), afforded from the acylation of the endocyclic nitrogen, were (13%) and (6.5%), respectively (Graubaum, 1993; Michon et al., 1995; Quiroga et al., 2008).
Acylation (El-Emary et al., 2002) is restricted to the 5-NH2 group, especially when the endocyclic NH is blocked. Thus, 5-amino-1-substituted pyrazole analogs 10 gave the corresponding 5-acylamino derivatives when different acylating reagents were used (Scheme 4).
Isocyanates and isothiocyanates, respectively, reacted with aminopyrazoles 14 giving the corresponding urea derivatives 15a,b (Bagley et al., 2006; Schenone et al., 2004; Winters et al., 1984) (Scheme 5).
.png) | Scheme 1. [Click here to view] |
.png) | Scheme 2. [Click here to view] |
.png) | Scheme 3. [Click here to view] |
.png) | Scheme 4. [Click here to view] |
.png) | Scheme 5. [Click here to view] |
.png) | Scheme 6. [Click here to view] |
.png) | Scheme 7. [Click here to view] |
.png) | Scheme 8. [Click here to view] |
.png) | Scheme 9. [Click here to view] |
.png) | Scheme 10. [Click here to view] |
.png) | Scheme 11. [Click here to view] |
Ho and Yao (2003) reported that heating 3-aminopyrazolo[3,4-d]pyrimidine derivative (16) with diethyl malonate at 150°C gave ethyl 2-[(4-methyl-6-phenylpyrazolo[3,4-d]pyrimidinyl)amido]ethanoate (17) (Scheme 6).
A further evidence for the relative higher nucleophilicity of the exocyclic NH2 group in 3(5)-aminopyrazole derivatives has been reported by Al-Zaydi (2009a) since the author isolated the ethyl (pyrazol-5-ylamino)acrylate derivative (19) via reaction of 5-aminopyrazole derivative (18) with ethyl propiolate under microwave irradiation (Scheme 7).
Furthermore, El-Mekabaty and Hasel (2015) reported that the condensation of 3,5-diaminopyrazle derivative (20) with triethylformate and certain active methylene containing compounds, dimedone, and diethyl barbituric acid led to the isolation and identification of the two intermediates (21), (22) (Scheme 8).
Recently, Helal et al. (2017) reported that reaction of 5-aminopyrazole derivative (23) with 4-methoxybenzaldehyde and terenaphthaldehyde afforded the corresponding mono and bis-(azomethine) derivatives (24) and (25). Noteworthy, the reaction, in both cases, occurs on the exocyclic NH2 group (Scheme 9).
 | Scheme 12. [Click here to view] |
 | Scheme 13. [Click here to view] |
 | Scheme 14. [Click here to view] |
 | Scheme 15. [Click here to view] |
Elnagdi and Abd Allah (1973) reported that 3,5-diaminopyrazole derivatives 26 reacted with acrylonitrile in refluxing pyridine to yield the corresponding 1-β-cyanoethyl derivatives 27 (or possible tautomers) (Scheme 10).
Elnagdi et al. (1974) reported that treatment of 4-arylhydrazono-3-amino-2-pyazolin-5-ones 28a-c with acrylonitrile afforded 3-amino-1-β-cyanoethyl-4-arylhydrazone-2-pyazolin-5-ones, the mono-β-cyano derivatives, 29a-c (or possible tautomers) rather than the isomeric compounds 30a-c (Scheme 11).
In the same year, in 1974, Elnagdi (1974) reported that refluxing the 5-aminopyrazole derivative 31a-c with acrylonitrile, in aqueous pyridine, the corresponding 5-amino-1-(β-cyanoethyl)pyrazole derivatives 32a-c were obtained (Scheme 12).
In another publication, Elnagdi et al. (1975) reported that reaction of 5-aminopyrazole analogs 33a,b with acrylonitrile in aqueous pyridine at reflux, afforded the corresponding 5-amino-1-(β-cyanoethyl)pyrazoles 34a,b. Also, the condensation of 5-amino-3-phenylpyrazole (35a) with ethyl acrylate, in refluxing aqueous pyridine gave the corresponding 5-amino-1-β-(ethoxycarbonyl)ethylpyrazole derivative (36a) (Scheme 13).
Elnagdi et al. (1975) continued their previous work (Elnagdi, 1974; Elnagdi and Ohta, 1973; Elnagdi and Allah, 1973; Elnagdi et al., 1974; 1975a) which involved cyanoethylation of 5-aminopyrazoles that gave 5-amino-1-β-cyanoethyl pyrazole derivatives. Thus, the reaction of 5-aminopyrazole 37a-c with acrylonitrile in aqueous pyridine afforded the corresponding 5-amino-1-β-cyanoethyl pyrazole analogs 38a-c rather than 5-cyanoethylamino analogs 39a-c (Scheme 14).
Analogously, 5-aminopyrazole analogs reacted with methyl acrylonitrile to afford the corresponding 5-amino-1-β-cyanoisopropylpyazole 41a-d rather than the 5-β-cyanoisopropylamine analogs (Scheme 15) (Elnagdi Mohamed et al., 1975).
Elnagdi et al. (1977) isolated and identified the product obtained from the reaction of 3,5-diaminopyrazole derivative (43) with ethylethoxymethylenecyanoacetate, in refluxing methanol, as the corresponding aminomethylene derivative (44) (Scheme 16).
Noteworthy, quoted as reported by Elnagdi et al., at that time, “since the previous result indicate that ring N-1 is the most electrophilic center in the molecule the formation of aminomethylene derivative (2) might be assumed to proceed via intermediate formation of the ring N-1 alkylated product. The later then isomerizes into 2. The ready isomerization of 1-β-cyanomethylene-5-aminopyrazoles into the corresponding 5-aminomethylene derivatives has been recently reported (Reimlinger et al., 1970).”
In the same article, the authors reported that when (43) was heated under reflux in AcOH, the corresponding 3,5-diacetamido-4-phenylazopyrazole (45) was obtained (Scheme 17), notice, how these results are contradicting.
 | Scheme 16. [Click here to view] |
 | Scheme 17. [Click here to view] |
 | Scheme 18. [Click here to view] |
 | Scheme 19. [Click here to view] |
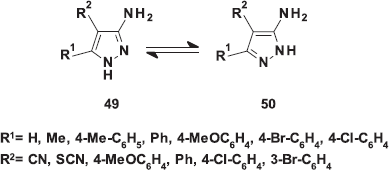 | Figure 3. Tautomerism of some 3(5)-aminopyrazoles. [Click here to view] |
In contrast, Elnagdi et al., in the same article, quoted as reported (Reimlinger et al., 1970) “Although 5-aminopyrazoles were reported to react with isocyanate to yield compounds similar to structure 2 where the addition would involve the amino nitrogen (Reimlinger et al., 1970; Vogel and Troxler, 1975; Dymek et al., 1965). We (Elnagdi et al.) obtained products that proved to be 3.” That is involved in the endocyclic N-1 nitrogen (Scheme 18).
Elnagdi et al. (1978) reported that 3,5-diaminopyrazole (47) reacted with acrylonitrile in refluxing aqueous pyridine to afford the corresponding 3,5-diamino-1-β-cyanoethyl pyrazole (Scheme 19).
FACTORS DETERMINE THE REGIO-ORIENTATION OF PYRAZOLO[1,5-A]PYRIMIDINES
Literature survey indicated that regio-orientation in pyrazolo[1,5-a]pyrimidines are controlled by the comparable nucleophilicity of the exocyclic NH2 group and the endocyclic NH group of the pyrazole ring. As we aforementioned, most authors reported that the isolation of reaction products indicated the relative higher nucleophilicity of the exocyclic NH2. On the other hand, some other authors believed that the endocyclic NH group is the most nucleophilic center in such molecules and consequently assigned their reaction products as N1-substituted 5-aminopyrazoles. In 2014, tautomerism of certain 3(5)-aminopyrazoles has been studied (Emelina et al., 2014) by 1H and 13C NMR in solution, cross-polarization and magic-angle spinning 13 C NMR in the solid-state, and ab initio quantum chemical calculations (B3LYP/6-31G**). The results proved that the maximum electron density in tautomers 49 and 50 is localized on the exocyclic amino nitrogen atom. The negative charge in the pyrazole ring is localized mainly on the nitrogen atoms, in going from the gas phase to DMSO solution, the charge on NH remains almost unchanged, whereas the charge on N2 increases in both tautomers (Fig. 3).
The second factor that controls regio-orientation in pyrazolo[1,5-a]pyrimidine structures is the comparable electrophilicity of the 1,3-bielectrophilic reagents. The following cases could be summed: 1) When the 1,3-dielectrophilic reagent is a symmetrical molecule, such as acetylacetone or malononitrile, regio-orientation is nonsense, and should not be considered. 2) when the 1,3-dielectrophilc reagent is unsymmetrical such as EAA, ECA, unsymmetrical 1,3-diketones or β-ketocycloalkanes, α,β-unsaturated carbonyl compounds, α,β-unsaturated nitriles, enaminonitriles or ketone dithioacetals, regio-orientation should be carefully considered. That is the comparable electrophilicity of the 1,3-dielectrophile is decided on the basic rules of chemistry and the regio-orientation of the reaction product should be proved by reliable advanced spectroscopic techniques such as NOE, 1H-15N NMR spectroscopy, and X-ray crystallography data.
 | Scheme 20. [Click here to view] |
 | Scheme 21. [Click here to view] |
 | Scheme 22. [Click here to view] |
 | Scheme 23. [Click here to view] |
 | Scheme 24. [Click here to view] |
SYNTHETIC ROUTES TO PYRAZOLO[1,5-A]PYRIMIDINE
General reactions of 3(5)-aminopyrazole derivatives with dielectrophilic reagents are the most common routes to synthesize the pyrazolo[1,5-a]pyrmidines. Such reactions when involving symmetrical 1,3-dielectrophilic reagent lead to a single product of pyrazolo[1,5-a]pyrimidine. On the other hand, when the reaction involves unsymmetrical 1,3-bielectrophilic reagent, unambiguous assignment of the regio-orientation of the product requires extensive investigations and evidence-based structural elucidation rather than believes and assumptions. Also, insufficient or unreliable spectral data and even irrelevant independent routes of synthesis could not be conclusive. However, advanced spectral techniques such as 1H-15N NMR and X-ray crystallography, in addition to isolation and identification of associated reaction intermediate could be conclusive. In the following paragraphs, the synthesis of pyrazolo[1,5-a]pyrimidines via reactions of 3(5)-aminopyrazoles with 1,3-bielectrophilic reagents based on literature survey is reported.
Reactions of 3(5)-aminopyrazoles with 1,3-dicarbonyl compounds
Elnagdi et al. (1975) reported that 3,5-diamino-4-phenylazopyrazoles (51a-c) reacted with acetylacetone at reflux in AcOH to afford the corresponding pyrazolo[1,5-a]pyrimidine derivatives 52a-c. On the other hand, (51a-c) reacted with EAA under the same conditions to afford the pyrazolo[1,5-a]pyrimidine analogs 53a-c rather than the regio-isomer 54a-c. The structure of the regio-isomer 53a-c was supported by traditional elemental analysis and interpretation of the IR spectral data in addition to previously reported (Reimlinger et al., 1970) reactions, in 1970 (Scheme 20) which involved the reaction of 5-aminopyrazole (55) with ethyl benzoylacetate to afford the corresponding pyrazolo[1,5-a]pyrimidine derivative (56) (Scheme 21).
Also, Elnagdi et al. (1975) reported that reaction of the 5-aminopyrazole derivatives (57a,b) with ethyl benzoylacetate, in refluxing AcOH afforded the corresponding pyrazolo[1,5-a]pyrimidine derivatives (58a,b) rather than the regio-isomer (59a,b) (Scheme 22).
 | Scheme 25. [Click here to view] |
 | Scheme 26. [Click here to view] |
 | Scheme 27. [Click here to view] |
 | Scheme 28. [Click here to view] |
Lynch et al. (1975) prepared the parent pyrazolo[1,5-a]pyrimidine by condensation of 5-aminopyrazole with malondialdehyde-tertamethylacetal in excellent yield (Scheme 23).
Elnagdi et al. (1978) consistent with the above-mentioned results reported that when 3,5-diaminopyrazole (60) was heated with EAA in WB (in the absence of solvent), the corresponding pyrazolo[1,5-a]pyrimidine derivative (61) was obtained rather than the regio-isomer (62) (Scheme 24). Structure of the regio-isomer (61) was proved (Elnagdi et al., 1975b) by 1H NMR and independent synthesis of (61) by decoupling of the phenylazo group from the previously prepared 2-amino-6,7-dihydro-5-methyl-7-oxo-3-phenylazopyrazolo[1,5-a]pyrimidine (63) (Scheme 25).
Bajwa and Skyes (1979) investigated the condensation of 5-aminopyrazole (55) with β-ketoacetal (4,4-dimethoxybutan-2-one), in dry toluene containing a catalytic amount of p-toluene sulfonic acid. The condensation of the 4-cyano analog of (65) with 2-hydroxymethylene cyclohexanone, 2-hydroxymethylenecyclopentanone, and with ethoxycarbonylcyclohexanone has been investigated. The reaction of (55) with 4,4-dimethoxybuta-2-one gave the corresponding two regio-isomer pyrazolo[1,5-a]pyrimidine derivative (64a,b). Similarly, the condensation of (65) with 2-hydroxymethylene cyclohexanone afforded the respective two regio-isomers (66a,b) (Scheme 26).
 | Scheme 29. [Click here to view] |
 | Scheme 30. [Click here to view] |
On the other hand, condensation of the 4-cyanoanalogue of (65) with 2-hydroxymethylenecyclopentanone and with 2-ethoxycarbonylcyclohexanone gave only the angular pyrazolo[5,1-b]pyrimidine regio-isomer (67) and only the linear pyrazolo[5,1-b]pyrimidine (68), respectively (Scheme 27).
The structure of the condensation products 64a,b, 66a,b, (67), and (68) has been distinguished by 1H and 13C NMR spectroscopy.
Kandeel et al. (1983) including Elnagdi reported that 3,5-diaminopyrazole derivative (69) reacted with EAA or with benzoyl acetoacetate to afford the corresponding pyrazolo[1,5-a]pyrimidine regio-isomer 70a,b rather than the analogs 71a,b. The reaction of (69) with acetylacetone proceeded as expected to give the corresponding 5,7-diamethylpyrazolo[1,5-a]pyrimidine (72) (Scheme 28).
Maquestiau et al. (1992) reported the preparation of pyrazolo[1,5-a]pyrimidine 74a-f via reaction of 3(5)-aminopyrazoles 73a-c with malondialdehyde bis(dimethylacetal) and with acetylacetone, in refluxing AcOH. On the other hand, reaction of 73a-c with the unsymmetrical 1-phenyl-1,3-butandione, under the same conditions, afforded a mixture of the two corresponding pyrazolo[1,5-a]pyrimidine regio-isomers 75a-c and 76a-c (Scheme 29), structural characterization of the products was based on 1H NMR data and that regio-isomer assignment was confirmed by the magnitude of the corresponding 3J and 4J (H-H) coupling constants which is affected by C5-C6/ C7-C6 bond order (Barfield and Chakrabarti, 1969).
Chimichi et al. (1992) described the regioselective synthesis of certain pyrazolo[1,5-a]pyrimidines 78a,b and 79a,b via reacting the 5-aminopyrazoles 77a,b, independently, with 4,4-dimethoxybutan-2-one, under different reaction conditions. Heating the reactants in ethanol under reflux for 30’ afforded mainly the 5-methylpyrazolo[1,5-a]pyrimidine derivatives 78a,b and traces of the regio-isomer 7-methyl analog 79a,b. In contrast, when the reaction was carried out in refluxing ethanol containing a few drops of conc. HCl, the 7-methyl regio-isomers 79a,b were obtained as the major products together with traces of the 5-methyl regio-isomer were critically assigned by means of 1H-13C 2 D experiments and gated decoupled spectra from which one-bond and long-range 13C-1H coupling constants were determined (Scheme 30).
 | Scheme 31. [Click here to view] |
 | Scheme 32. [Click here to view] |
 | Scheme 33. [Click here to view] |
Inconsistent with what has been reported by Elnagdi (1983), the reaction behavior of 5-aminopyrazoles with β-ketoesters, Girges et al. (1993) reported that fusion of equimolar amounts of the p-tosylaminopyrazole derivative (80) and EAA at 160°C yielded 7-methyl-1,2,4,5-tetrahydro-1-(p-tosyl)pyrazolo[1,5-a]pyrimidin-5-one (81) rather than its 5-methyl (regio-isomer) 82 (Scheme 31) based on insufficient spectral information. The reaction of (80) with acetylacetone under the same conditions afforded the corresponding 5,7-dimethyl(pyrazolo[1,5-a]pyrimidine) derivative (83) (Scheme 32).
Makarov et al. (1998) reported that the reaction of 3,5-diaminopyrazole (84) with ethyl β-chloroacetoacetate (85), 2,4-dichloro-5-nitro-benzoyl acetate (86), and 2-(3,4,5-trimethoxybenzoyl)cyclohexanone (87), in methanolic HCl gave the corresponding pyrazolo[1,5-a]pyrimidine derivatives 88, 89, and 90a,b, respectively (Scheme 33).
According to the authors, the first step in the reaction of 84 with β-ketoesters involves a nucleophilic attack by the exocyclic NH2 group followed by subsequent cyclization by the second nucleophilic attack caused by the endocyclic NH on the ester carbonyl. The reaction of 84 with the 1,3-diketone 87 may proceed through two pathways to afford, potentially, tricyclic products 90a and 90b. 1H-NMR spectra indicate a single product rather than a mixture of two products. Structure 90b was ruled out on the ground of evidence-based structural assignment of substituents on 90a by X-ray diffraction analysis (Fig. 4).
 | Figure 4. X-ray diffractional analysis of compound 90a. Makarov et al. (1998). [Click here to view] |
 | Scheme 34. [Click here to view] |
 | Scheme 35. [Click here to view] |
 | Scheme 36. [Click here to view] |
 | Figure 5. X-ray numbering of atoms and structure of compound 92 (Makarov et al., 2000). [Click here to view] |
Noteworthy, the authors proposed the formation of intermediates A and B from the reaction of 84 with 87 assumed that intermediate A is energetically more favorable due to the conjugation of the carbonyl with the phenyl π-bond (Scheme 34).
Makarov et al. (2000) reported that the reaction of 4-methoxycarbonyl-3,5-diaminopyrazole (91) with acetoacetic ester gave the corresponding 7-oxopyrazolo[1,5-a]pyrimidine analogue (92) rather than the regio-isomer 5-oxo pyrazolo[1,5-a]pyrimidine (93). This result is consistent with what has been reported by Makarov et al. (1998), concerning the reaction of 4-nitro-3,5-diaminopyrazole with acetoacetic ester while contradicts the results reported by Elnagdi et al. (1983). The structure of the regio-isomer 7-oxo pyrazolo[1,5-a]pyrimidine derivative 92 was confirmed by isotopic exchange experiment (NH for ND) in their 13C NMR spectra and X-ray diffraction analysis ‑
Fraley et al. (2002) prepared in combinatorial fashion a set of 3,6-dichloro pyrazolo[1,5-a]pyrimidine 95 through a condensation reaction of a 3-amino-4-arylpyrazole 94 with 2-aryl malonaldehydes in ethanol containing a catalytic amount of acetic acid (Scheme 36).
In this article (Fraley et al., 2002), a convenient method for varying the substituents at the 3-position which avoids repetitious 3-aminopyrazole synthesis, involves reaction of 3-amino-4-bromopyrazole (96) with arylmalonoaldehydes to afford the corresponding 3-bromo-6-arylpyrazolo[1,5-a]pyrimidine 97a,b, which smoothly cross-coupled with arylboronic acids under Suzuki conditions to afford 3,6-diaryl pyrazolo[1,5-a]pyrimidines 98a-g in fair yields (Scheme 37).
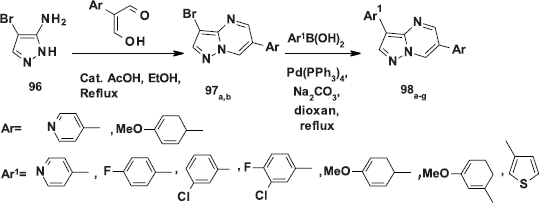 | Scheme 37. [Click here to view] |
 | Scheme 38. [Click here to view] |
 | Scheme 39. [Click here to view] |
 | Scheme 40. [Click here to view] |
 | Scheme 41. [Click here to view] |
Petrova et al. (2003) reported that the condensation of 5-amino-3-(2-pyrrolyl)pyrazoles 99a-f with acetylacetone and ethyl acetoacetate under different reaction conditions resulted in the formation of the corresponding pyrazolo[1,5-a]pyrimidine 100a-f and 101a-d, respectively (Scheme 38). In the case of EAA, the formation of the regio-isomer 101a-d was selectively obtained rather than the regio-isomer 102a-d.
Noteworthy, the authors criticized the method reported by Danagulyan et al. (2002) for the preparation of 100e, under the given conditions (ethanol containing a catalytic amount of AcOH 1 hour at room temperature).
Ho and Yao (2003) reported that the pyrazolopyrimidine 103 reacted with different 1,3-diketones 104a-g, in refluxing acetic acid to afford the corresponding pyrimido pyrazolo[1,5-a]pyrimidines 105a-g (Scheme 39). According to the authors, the reaction probably involves the condensation of the 3-amino group of the pyrazolo[3,4-d]pyrimidine with the carbonyl group followed by dehydration and subsequent cyclization with loss of water.
 | Scheme 42. [Click here to view] |
 | Scheme 43. [Click here to view] |
Condensation of 103 with 4,4-dimethoxy-2-butanone (106) in refluxing AcOH /HCl afforded 4,6-dimethyl-2-phenylprimido[2,3:4,3] pyrazolo[1,5-a]pyrimidine (107). Also, reaction of 103 with malonaldehyde-bis(dimethylacetal) (108) in EtOH/HCl gave 4-methyl-2-phenylpyrimido-pyrazolo[1,5-a]pyrimidine derivative (109) (Scheme 40).
Elmaaty and El-Taweel (2003) reported that 5-amino-2-antipyrinylpyrazole 110 reacted with ethyl α-methyl acetoacetate 111, β-ketoester 112, β-ketoamide 113a,b, and 2-acetyl indan-1,3-dione (114), under different reaction to afford the corresponding pyrazolo[1,5-a]pyrimidines 115, 116, 117a,b, and 118. Formation of such pyrazolo[1,5-a]pyrimidines was assumed to proceed via initial nucleophilic attack by the exocyclic NH2 group of pyrazole on the ketonic function followed by cyclization with the elimination of ethanol or H2O (Scheme 41). The same reaction pathway and mechanism were confirmed by other authors (Gregg et al., 2007).
Ammar et al. (2009) reported that the reaction of 3(5)-aminopyrazole 119 with EAA, acetylacetone, and benzoylacetone gave the respective pyrazolo[1,5-a]pyrimidines 120, 121a,b. The authors believed the relative higher nucleophilicity of the endocyclic NH group of the pyrazole ring based only on what they previously reported (Ammar et al., 1995; Zaharan et al., 2001) (Scheme 42).
Al-Zaydi (2009b) unambiguously assigned the structure of 3,5-diaminopyrazole 122 with EAA as 5-methyl-pyrazolo[1,5-a]pyrimidin-7-one derivative 123 rather than the regio-isomer 124 based on X-ray crystallographic data (Scheme 43).
In 2012, regioselectivity of the reaction between nine different 3(5)-aminopyrazoles 125a-i and 2-acetylcyclohexanone 126, 127 has been investigated (Petrov et al., 2012), under different conditions. The corresponding cycloalkane[e]pyrazolo[1,5-a]pyrimidines 128a-i, 129b-i and cycloalkane[d]pyrazolo[1,5-a]pyrimidines 130a-h, 131a-h were obtained. The regio-structure of the compounds was established by 1H and 13C NMR spectroscopy (Scheme 44).
 | Scheme 44. [Click here to view] |
 | Scheme 45. [Click here to view] |
 | Scheme 46. [Click here to view] |
 | Scheme 47. [Click here to view] |
 | Scheme 48. [Click here to view] |
 | Scheme 49. [Click here to view] |
The reaction sequence involves the nucleophilic addition of the exocyclic NH2 group of the pyrazole on the acetyl carbonyl or the ring carbonyl of the 1,3-dicarbonyl reagent forming intermediates A(A’) or B(B’). Then in succession two molecules of water were eliminated with the formation of the final products 128-131. The presence of intermediates C (and or C’) alongside with products 128d, 129d was detected by high-resolution mass spectroscopy: in a solution, in DMSO, of a mixture of aminopyrazole 125d and 2-acetylcyclopentanone 126 after 3 days masses [M+H]+ 250.1339 and 268.1446 (Scheme 45).
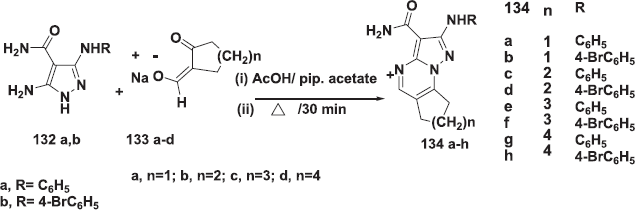
7,8-dihydro-6H-cycloalkan[e]pyrazolo[1,5-a] pyrimidine-3-carboxamide derivatives 134a–h were prepared (Abdallah and Elgemeie, 2018) by reacting aminopyrazoles 132a,b with the sodium salt of (hydroxymethylene)-cycloalkanones 133a-d and piperidine acetate. The reaction mixture was heated and was in the water for 25 min, and then acetic acid was added in the middle of the reaction time (Scheme 46).
Bondock et al. (2015) reported that condensation of 3(5)-aminopyrazole derivative 135 with acetylacetone, in boiling AcOH, gave the corresponding pyrazolo[1,5-a]pyrimidine 136. Similarly, the reaction of 135 with acetoacetamide afforded the regio-isomer pyrazolo[1,5-a]pyrimidine 137 based on what has been reported by Kleschick in 1989. They assumed that the initial nucleophilic attack induced by the exocyclic NH2 group of pyrazole on the ketonic carbonyl of acetoacetanilide, followed by cyclization via nucleophilic substitution on the amide carbonyl by the endocyclic NH of the pyrazole and loss of the aniline molecule. The solvent-free reaction of 135 with diethyl malonate at 160°C, afforded the corresponding 5,7-dihydroxy pyrazolo[1,5-a]pyrimidine 138 (Scheme 47).
 | Scheme 50. [Click here to view] |
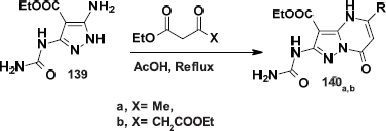
Abbas-Temirek and Abo-Bakr (2016) reported that the reaction of 3(5)-aminopyrazole 139 with EAA and diethyl-3-oxoglutarate produced the corresponding pyrazolo[1,5-a]pyrimidines 140a,b. Regio-orientation was not discussed and the structures of the products were supported by 1H and 13C NMR (not reliable without deep investigation) in addition to what has been previously reported by Elnagdi et al. (1993) (Scheme 48).
Marjani et al. (2015) reported the synthesis of pyrazolo[1,5-a]pyrimidine analogs 142, 143 via condensation of substituted 3(5)-aminopyrazoles 141, independently, with acetylacetone and certain ketoesters in the presence of H2SO4 using AcOH as solvent (Scheme 49).
According to the authors, the reaction mechanism involves an initial nucleophilic attack by the exocyclic NH2 group on the ketonic function followed by subsequent cyclization. Although the regioselectivity of the reaction was not discussed, the structures of the products were confirmed by 1H NMR, 13C NMR, FT-IR, and elemental analysis.
5,7-dimethylpyrazolo[1,5-a]pyrimidine carboxylic acid 145 was synthesized (Kumar et al., 2016) by heating ethyl 3(5)-aminopyrazole-4-carboxylate (144) with acetylacetone in the acetic acid medium at 82áµ’C, in good yield (Scheme 50).
ABBREVIATIONS
AcOH: acetic acid, DMSO: dimethylsulfoxide, EAA: ethyl acetoacetate, ECA: ethyl cyanoacetate, EtOH: ethanol, HCl: hydrochloric acid, WB: water bath.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
Abbas-Temirek HH, Abo-Bakr AM. Reactions with heterocyclic amidines: synthesis of several new pyrazolo [1, 5-a] pyrimidines and pyrazolo [1, 5-a][1, 3, 5] triazines. Eur J Chem, 2016; 7(1):107–14. CrossRef
Abdallah AEM, Elgemeie GH. Design, synthesis, docking, and antimicrobial evaluation of some novel pyrazolo[1,5-a] pyrimidines and their corresponding cycloalkane ring-fused derivatives as purine analogs. Drug Des Devel Ther, 2018; 12:1785–98. CrossRef
Al-Omran F, El-Khair AA. 2-(3-Arylhydrazono-3-formyl-2-oxopropyl)-1H-isoindole-1, 3 (2H)-dione in heterocyclic synthesis. Novel derivatives of pyridazin-6 (1H)-one, pyridazin-6 (1H)-imine, and pyrazolo [5, 1-c][1, 2, 4] triazine incorporating an N-(2-oxoethyl) phthalimide moiety. J Chem Res, 2006; 2006(1):6. CrossRef
Al-Shiekh MA, El-Din AMS, Hafez EA, Elnagdi MH. β-Enones in heterocyclic synthesis, Part I. Classical synthetic and environmentally friendly synthetic approaches to alkyl and acyl azoles and azines. J Chem Res, 2004; 2004(3):174. CrossRef
Al-Zaydi KM. Synthesis of fused pyrazolo (1, 5-a) pyrimidine derivatives under microwave irradiation and ultrasound, as ecofriendly energy sources. Heterocycles, 2009a; 78(8):2003. CrossRef
Al-Zaydi KM. A simplified green chemistry approaches to synthesis of 2-substituted 1, 2, 3-triazoles and 4-amino-5-cyanopyrazole derivatives conventional heating versus microwave and ultrasound as ecofriendly energy sources. Ultrason Sonochem, 2009b; 16(6):805–9. CrossRef
Ammar Y, EL-Sharief AS, Zahran M, El-Said M, EL-Said U. Studies on activated nitriles: synthesis of new 3-Cyano-2-(3-tolylamino) pyrazolo (1, 5-a) pyrimidines. ChemInform, 1995; 26(49). CrossRef
Ammar YA, Aly MM, Al-Sehemi AAG, Salem MA, El-Gaby MS. Cyanoacetanilides intermediates in heterocyclic synthesis. Part 5: preparation of hitherto unknown 5-aminopyrazole and pyrazolo [1, 5-a] pyrimidine derivatives containing sulfamoyl moiety. J Chin Chem Soc, 2009; 56(5):1064–71. CrossRef
Ancoli-Israel S, Walsh JK, Mangano RM, Fujimori M, Group ZCS. Zaleplon, a novel nonbenzodiazepine hypnotic, effectively treats insomnia in elderly patients without causing rebound effects. Prim Care Companion J Clin Psychiatry, 1999; 1(4):114. CrossRef
Bagley MC, Davis T, Dix MC, Widdowson CS, Kipling D. Microwave-assisted synthesis of N-pyrazole ureas and the p38α inhibitor BIRB 796 for study into accelerated cell ageing. Organ Biomol Chem, 2006; 4(22):4158–64. CrossRef
Bajwa JS, Sykes PJ. Synthesis and structure of some azolo [a] pyrimidines, 2, 6, 7, 8-tetrahydro-1 H-cyclopenta [e] azolo [a] pyrimidines, 6, 7-dihydro-5 H-cyclopenta [f] azolo [a] pyrimidines, 7, 8-dihydro-6 H-cyclopenta [f]-s-triazolo [4, 3-b] pyridazine, 5, 6, 7, 8-tetrahydro-azolo [b] quinazolines, 6, 7, 8, 9-tetrahydroazolo [a] quinazolines, and 7, 8, 9, 10-tetrahydro-s-triazolo [3, 4-a] phthalazine. J Chem Soc, Perkin Transact 1, 1979:3085–94. CrossRef
Banister SD, Wilkinson SM, Hanani R, Reynolds AJ, Hibbs DE, Kassiou M. A practical, multigram synthesis of the 2-(2-(4-alkoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide (DPA) class of high affinity translocator protein (TSPO) ligands. Tetrahedron Lett, 2012/07/18/ 2012; 53(29):3780–3. CrossRef
Barfield M, Chakrabarti B. Long-range proton spin-spin coupling. Chem Rev, 1969; 69(6):757–78. CrossRef
Bondock S, Tarhoni AEG, Fadda AA. Regioselective synthesis of some new pyrazolo [1, 5-a] pyrimidines, pyrazolo [1, 5-a] quinazoline and pyrimido [4′, 5′: 3, 4] pyrazolo [1, 5-a] pyrimidines containing thiazole moiety. J Heterocycl Chem, 2015; 52(6):1792–9. CrossRef
Chan KK-L, Leung TH-Y, Chan DW, Wei N, Lau GT-Y, Liu SS, Siu MK, Ngan HY-S. Targeting estrogen receptor subtypes (ERα and ERβ) with selective ER modulators in ovarian cancer. J Endocrinol, 2014; 221(2):325–36. CrossRef
Chilman-Blair K, Castaner J, Silvestre J. Ocinaplon: treatment of generalized anxiety disorder GABAAreceptor modulator. Drugs Future, 2003; 28(2):115–20.
Chimichi S, Cosimelli B, Bruni F, Selleri S. 1H and 13C NMR study of the pyrazolo [1, 5-a] pyrimidine system. Canad J Chem, 1992; 70(4):1093–7. CrossRef
d’Aniello F, Santos B, Guglietta A. Lorediplon: a new GABA A modulator drug for treatment of insomnia. In: Drug Treatment of Sleep Disorders, Springer, pp 121–45, 2015. CrossRef
Danagulyan G, Panosyan G, Boyakhchyan A. Synthesis of n-alkylated derivatives of pyrazolo [1, 5-a] pyrimidine and their reaction with methylamine. Chem Heterocycl Com, 2002; 38(5):581–5. CrossRef
Dawood KM, Farag AM, Abdel-Aziz HA. Azoles and azolo-azines via 3-(3-methylbenzofuran-2-yl)-3-oxopropanenitrile. J Chem Res, 2005; 2005(6):378. CrossRef
de Waard MA. Mechanisms of action of the organophosphorus fungicide pyrazophos, Veenman, 1974.
Dymek W, Janik B, ZimoÅ„ R. Studies on pyrazole derivatives. II. Acta poloniae pharmaceutica, 1965; 22(3):209–17.
Ege G, Gilbert K. Reactions with diazo-azoles. 2.[7+ 1]-cycloreactions and (11+ 1]-cycloreactions of diazo-azoles with 1-nucleophile-1-electrophiles (ylides) to 3h-pyrazolo (5, 1-c][1, 2, 4] triazoles and 3h-(1, 2, 4] triazolo (4, 3-b] indazoles. Tetrahedron Lett, 1979a; (18):1567–70. CrossRef
Ege G, Gilbert K. [7+ 2]-and [11+ 2]-cycloaddition reactions of diazo-azoles with isocyanates to azolo [5, 1-d][1, 2, 3, 5] tetrazine-4-ones. Tetrahedron Lett, 1979b; 20(44):4253–6. CrossRef
Ege G, Gilbert K, Heck R. Reaktionen mit Diazoazolen, VII. 3H-Azolo-1, 2, 4-triazole durch 1, 8-bzw. 1, 12-Elektrocyclisierungen von 3H-Pyrazol-3-on-bzw. 3H-Indazol-3-on-(diorganylmethylen) hydrazonen. Chemische Berichte, 1984; 117(5):1726–47. CrossRef
El-Emary TI, Al-Muaikel N, Moustafa OS. Synthesis and antimicrobial activity of some new heterocycles based on 3-methyl-1-phenyl-5-benzene sulfonamido pyrazole. Phosphorus Sulfur, 2002/01/01 2002; 177(1):195–210. CrossRef
El-Ghandour Ahmed Hafez H, Ibrahim Mohamed Kamal A, Abdel-Hafiz Ibrahim S, Elnagdi Mohamed H. Studies with alkylheteroaromatic carbonitriles: a novel synthesis of pyrazolo[2′,3′: 3,4]benzo[c]-1,2,4-triazine. Zeitschrift für Naturforschung B, 1992; 47:1628. CrossRef
El-Mekabaty A, Hasel A. Facile and efficient synthesis of a new class of pyrazolo [1, 5-a] pyrimidine derivatives via one-pot multicomponent reactions. Chem Heterocycl Com, 2015; 50(11):1608–15. CrossRef
Elagamey AGA, El-Taweel F, Amer F. Synthesis of some new pyrazolo [1, 5-a] pyrimidine and pyrazolo [1, 5-c]-as-triazine derivatives. Collect Czechoslov Chem Commun, 1986; 51(10):2193–8. CrossRef
Elmaati TMA, El-Taweel FM. Routes to Pyrazolo [3, 4-e][1, 4] thiazepine, pyrazolo [1, 5-a] pyrimidine and pyrazole derivatives. J Chin Chem Soc, 2003; 50(3A):413–8. CrossRef
Elnagdi M, Fleita D, El-Moghayar M. Reactions with β-cyanoethylhydrazine—II: synthesis of some 4, 5, 6, 7-tetrahydropyrazolo [1, 5-a] pyrimidine derivatives. Tetrahedron, 1975a; 31(1):63–7. CrossRef
Elnagdi M, Kandeel B, Elmoghayar M. Z. Naturforsch, B. Anorg Chem Org Chem, 1977; 32.
Elnagdi MH, Allah SOA. Reactions with the Arylhydrazones of some α-cyanoketones. J Prakt Chem, 1973; 315(6):1009–16. CrossRef
Elnagdi MH, Ohta M. Studies on 3, 5-pyrazolidinediones. IV. Addition of 4-Arylazo-3, 5-pyrazolidinediones to Ethyl Acrylate. Bull Chem Soc Jpn, 1973; 46(6):1830–3. CrossRef
Elnagdi MH. Reactions with β-cyanoethylhydrazine—I: a route for the preparation of pyrazolo [1.5-a] pyrimidines and pyrrolo [1.2-b] pyrrazoles. Tetrahedron, 1974; 30(16):2791–6. CrossRef
Elnagdi MH, Kassab NAL, Fahmy SM, El-All FA. Reactions with 3, 5-pyrazolidinediones. III. Cyanoethylation of some 4-Arylazo derivates of 3, 5-pyrazolidinediones and 3-amino-2-pyrazolin-5-ones. J Prakt Chem, 1974; 316(2):177–84. CrossRef
Elnagdi MH, Sallam MMM, Ilias MAM. Pyrimidine derivatives and related compounds II: synthesis of some derivatives of pyrimido [1, 2: 2′, 3′] pyrazolo [1, 5-a] pyrimidines, a new ring system. Helv Chim Acta, 1975b; 58(7):1944–9. CrossRef
Elnagdi MH, El-Moghayar MR, Fleita DH, Hafez EA, Fahmy SM. Pyrimidine derivatives and related compounds. 4. A route for the synthesis of pyrazolo [3, 4-e]-as-triazines, pyrazolo [3, 4-d] pyrimidines, and pyrazolo [1, 5-c]-as-triazines. J Organ Chem, 1976; 41(24):3781–4. CrossRef
Elnagdi MH, Kandeel EM, Zayed EM, Kandil ZES. Pyrimidine derivatives and related compounds. VIII [1]. Routes for the Synthesis of 3, 5-Diaminopyrazoles, 2-Aminopyrazolo [1, 5-a] pyrimidines and 5-Aminopyrazolo [1, 5-a] pyrimidines. J Prakt Chem, 1978; 320(4):533–8. CrossRef
Elnagdi MH, Zayed EM, Khalifa ME, Ghozlan SA. Reactions with heterocyclic amidines, VII: Synthesis of some new pyrazolo [1, 5-c]-1, 2, 4-triazines, pyrazolo [1, 5-a]-1, 3, 5-triazines and pyrazolo [1, 5-a] pyrimidines. Monatshefte für Chemie/Chemical Monthly, 1981; 112(2):245–52. CrossRef
Elnagdi Mohamed H, Fahmy Sherief M, Elmoghayab Mohamed Riffaat H, Ilias Mohamed Ajmal M. Pyrimidine derivatives and related compounds, I synthesis of some 2,3-disubstituted-4,5,6,7-tetrahydropyrazolo[1,5-a]-pyrimidine derivatives. Zeitschrift für Naturforschung B, 1975; 30:778. CrossRef
Emelina E, Petrov A, Filyukov D. Structure and tautomerism of 4-substituted 3 (5)-aminopyrazoles in solution and in the solid state: NMR study and Ab initio calculations. Russ J Organ Chem, 2014; 50(3):412–21. CrossRef
Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S, Sukmawati D, Nomiyama T, Kanazawa A, Kawamori R. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E-deficient mice. Endocrinology, 2013; 154(3):1260–70. CrossRef
Fraley ME, Hoffman WF, Rubino RS, Hungate RW, Tebben AJ, Rutledge RZ, McFall RC, Huckle WR, Kendall RL, Coll KE. Synthesis and initial SAR studies of 3, 6-disubstituted pyrazolo [1, 5-a] pyrimidines: a new class of KDR kinase inhibitors. Bioorgan Med Chem Lett, 2002; 12(19):2767–70. CrossRef
Girges M, Hanna M, Fadda A. New heterocyclic bridgehead nitrogen compounds synthesis of 1-(p-Tosyl) pyrazolo [1, 5-a] pyrimidines and pyrazolo [5, 1-c]-[1, 2, 4]-triazine derivatives. Chem Papers, 1993; 47(3):186–9. CrossRef
Graubaum H. Acylwanderungen am 3 (5)-Amino-pyrazol. Journal für Praktische Chemie/Chemiker-Zeitung, 1993; 335(7):585–8.
Gregg BT, Tymoshenko DO, Razzano DA, Johnson MR. Pyrazolo [1, 5-a] pyrimidines. Identification of the privileged structure and combinatorial synthesis of 3-(hetero) arylpyrazolo [1, 5-a] pyrimidine-6-carboxamides. J Comb Chem, 2007; 9(3):507–12. CrossRef
Helal MH, Salem MA, Aly HM. Synthesis, antimicrobial activity and molecular modeling of some novel 5-aminopyrazole, pyrazolo [1, 5-a] pyrimidine, bispyrazole and bispyridone derivatives containing antipyrinyl moiety. J Heterocycl Chem, 2017; 54(5):2614–26. CrossRef
Ho YW, Yao CT. Synthesis of some new 6, 8-disubstituted 7, 8-dihydropyrimido [2, 3: 4, 3] pyrazolo [1, 5-a] pyrimidines and 6, 7, 8-trisubstituted pyrimido [2, 3: 4, 3] pyrazolo [1, 5-a] pyrimidine derivatives. J Chin Chem Soc, 2003; 50(2):283–96. CrossRef
Iorga A, Umar S, Ruffenach G, Aryan L, Li J, Sharma S, Motayagheni N, Nadadur RD, Bopassa JC, Eghbali M. Estrogen rescues heart failure through estrogen receptor Beta activation. Biol Sex Differ, 2018; 9(1):48. CrossRef
Joshi K, Singh P, Singhi C. Chemical constituents of Verbesina encelioides and Holmskioldia sanguinea. J Indian Chem Soc, 1983.
Kandeel EM, Baghos VB, Mohareb IS, Elnagdi MH. Reactions with heterocyclic amidines, XI. Syntheses of new 2-aminopyrazolo [1, 5—a] pyrimidines and 2-amino [1, 5-c]-as-triazines. Archiv Pharmazie, 1983; 316(8):713–8. CrossRef
KoÄevar M, Kolman D, Krajnc H, Polanc S, Porovne B, Stanovnik B, Tišler M. Reactions of some diazoazoles with reactive methylene and other groups. Tetrahedron, 1976; 32(6):725–9. CrossRef
Kumar A, Bodke Y, Gowda A, Sambasivam G, Bhat K. Design, synthesis, and evaluation of the anticancer properties of a novel series of imidazolone fused pyrazolo[1,5-a]pyrimidine derivatives. J Heterocycl Chem, 2016; 54(3):1904–24. CrossRef
Lynch BM, Khan MA, Sharma SC, Teo HC. Pyrazolo [1, 5-a] pyrimidine: synthesis and regiospecific electrophilic substitution in the pyrazole and/or pyrimidine rings. Canad J Chem, 1975; 53(1):119–24. CrossRef
Makarov V, Tafeenko V, Granik V. Synthesis of pyrazolo [1, 5-a] pyrimidines by the reaction of β-dicarbonyl compounds with 3, 5-diamino-4-nitropyrazole. Chem Heterocycl Com, 1998; 34(12):1423–7. CrossRef
Makarov V, Solov'eva N, Chernyshev V, Sonneveld E, Granik V. Study of the reaction of 3, 5-diamino-4-carbomethoxypyrazole with acetoacetic ester. Synthesis of pyrazolo [1, 5-a] pyrimidine. Chem Heterocycl Com, 2000; 36(1):70–3. CrossRef
Maquestiau A, Taghret H, Vanden Eynde JJ. Preparation and characterization of pyrazolo [1, 5-a] pyrimidines. Bull Soc Chim Belges, 1992; 101(2):131–6. CrossRef
Marjani AP, Khalafy J, Salami F, Ezzati M. The synthesis of new pyrazolo [1, 5-a] pyrimidine derivatives. Arkivoc, 2015; 277:286. CrossRef
Michon V, du Penhoat CH, Tombret F, Gillardin J, Lepage F, Berthon L. Preparation, structural analysis and anticonvulsant activity of 3-and 5-aminopyrazole N-benzoyl derivatives. Eur J Med Chem, 1995; 30(2):147–55. CrossRef
Moyano EL, Colomer JP, Yranzo GI. New Application of heterocyclic diazonium salts: synthesis of new pyrazolo [3, 4-d][1, 2, 3] triazin-4-ones. Eur J Organ Chem, 2008; 2008(19):3377–81. CrossRef
Neubauer DN. Indiplon: the development of a new hypnotic. Expert Opin Investig Drugs, 2005; 14(10):1269–76. CrossRef
Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, Seghezzi W, Paruch K, Dwyer MP, Doll R. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther, 2010; 9(8):2344–53. MCT-1510-0324. CrossRef
Petrov A, Kasatochkin A, Emelina E. Study of regioselectivity of reactions between 3 (5)-aminopyrazoles and 2-acetylcycloalkanones. Russ J Organ Chem, 2012; 48(8):1111–20. CrossRef
Petrova O, Sobenina L, Demenev A, Mikhaleva A, Ushakov I. Synthesis of functionalized 2-(2-Pyrrolyl) pyrazolo [1, 5-a] pyrimidines. Russ J Organ Chem, 2003; 39(10):1471–6. CrossRef
Quiroga J, Portilla J, Abonía R, Insuasty B, Nogueras M, Cobo J. Synthesis of novel 5-amino-1-aroylpyrazoles. Tetrahedron Lett, 2008; 49(41):5943–5. CrossRef
Reimlinger H, Peiren MA, Merényi R. Synthesen mit heterocyclischen Aminen, I. Reaktionen des 3 (5)-Amino-pyrazols mit α, β-ungesättigten Estern. Darstellung und Charakterisierung isomerer Oxo-dihydro-pyrazolo-pyrimidine. Chemische Berichte, 1970; 103(10):3252–65. CrossRef
Reynolds A, Hanani R, Hibbs D, Damont A, Pozzo ED, Selleri S, Dollé F, Martini C, Kassiou M. Pyrazolo[1,5-a]pyrimidine acetamides: 4-Phenyl alkyl ether derivatives as potent ligands for the 18kDa translocator protein (TSPO). Bioorgan Med Chem Lett, 2010/10/01/ 2010; 20(19):5799–802. CrossRef
Schenone S, Bruno O, Bondavalli F, Ranise A, Mosti L, Menozzi G, Fossa P, Manetti F, Morbidelli L, Trincavelli L. Synthesis of 1-(2-chloro-2-phenylethyl)-6-methylthio-1H-pyrazolo [3, 4-d] pyrimidines 4-amino substituted and their biological evaluation. Eur J Med Chem, 2004; 39(2):153–60. CrossRef
Selleri S, Bruni F, Costagli C, Costanzo A, Guerrini G, Ciciani G, Costa B, Martini C. 2-Arylpyrazolo[1,5-a]pyrimidin-3-yl acetamides. New potent and selective peripheral benzodiazepine receptor ligands. Bioorgan Med Chem, 2001/10/01/ 2001; 9(10):2661–71. CrossRef
Vogel A, Troxler F. Neue Synthesen von Pyrazolo [1, 5-a]-s-triazinen. Helv Chim Acta, 1975; 58(3):761–71. CrossRef
Winters G, Sala A, De Paoli A, Ferri V. Easy synthesis of new ring-fused pyridones from heteroaromatic β-vinylamines. Synthesis, 1984; 1984(12):1052–4. CrossRef
Wu YC, Zou XM, Hu FZ, Yang HZ. Design and synthesis of novel sulfone-containing pyrazolo [1, 5-a]-pyrimidines and pyrazolo [5, 1-d][1, 2, 3, 5] tetrazine-4 (3H)-ones. J Heterocycl Chem, 2005; 42(4):609–13. CrossRef
Zaharan MA, El-Sharief AMS, El-Gaby MS, Ammar YA, El-Said UH. Some reactions with Ketene dithioacetals: Part I: Synthesis of antimicrobial pyrazolo [1, 5-a] pyrimidines via the reaction of ketene dithioacetals and 5-aminopyrazoles. Il Farmaco, 2001; 56(4):277–83. CrossRef