THERAPEUTIC AND BIOLOGICAL VALUES OF P. DULCE
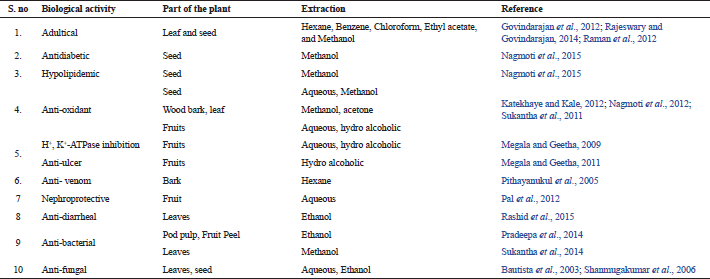
Each part of the plant P. dulce contains notable medicinal values, like the estrogenic activity was proposed in the root extracts (Saxena et al., 1998), the anti-inflammatory activity of the saponin fraction of P. dulce fruits (Bhargvakrishna et al., 1970; Sahu and Mahato, 1994), and their various parts have been reported to be as a remedy for earache, leprosy, peptic ulcer, toothache, venereal disease, and it also acts as emollient, abortifacient, anodyne, and larvicides (Govindarajan et al., 2012). The bark of P. dulce also acts as an astringent for dysentery, febrifuge. In addition, this plant also has a useful remedy for dermatitis eye inflammation. The polyphenols content of the bark extract has reported for their anti-venomous activity by Pithayanukul et al. (2005). In seeds, active classes of phytoconstituents like steroids, saponins, lipids, phospholipids, glycosides, glycolipids, and polysaccharides were identified (Nigam and Mitra, 1968; 1970). This plant is a potential source of antioxidant and effective medicine for adulticide problem (Rajeswary and Govindarajan, 2014). The lists of biological therapeutic values were shown in Table 2. Beside of all the above properties, it is a nutritional feed for goats and other livestock (Olivares et al., 2013).
Adulticidal activity
The Phytochemicals of P. dulce are also used as an insecticide as they have an adulticidal activity against mosquitoes like Aedes aegypti (A. aegypti), Culex quinquefasciatus (C. quinquefasciatus), etc. Dengue, filariasis, malaria, and viral encephalitis are major Mosquito-borne diseases in developing countries. Aedes aegypti mosquitoes are responsible for dengue fever. Chemical based mosquito repellent spray is usually toxic to other beneficiary life forms, may cause severe breathing problems in human too. To avoid such circumstances, naturally derived repellents can be used (Govindarajan and Rajeswary, 2015). The leaves and seed extracts of P. dulce have the tendency to control mosquitoes and are very safe. The larvicidal and ovicidal effects are moderate in the leaf and seeds of this plant. The comparison studies were undertaken and is reported that the leaf extract using methanol has the highest larval mortality and the seed extract using hexane have lower potency towards mosquitoes. The larvicidal and ovicidal activities were proved in A. aegypti (Rajeswary and Govindarajan, 2014) and C. quinquefasciatus (Govindarajan et al., 2012) mosquitoes. Pithecellobium dulce derived bioactive compounds are also used as a natural synthetic insecticide. The silver nanoparticles synthesized from an aqueous extract of P. dulce’s leaf exhibited larvicidal activity against C. quinquefasciatus. Further, the FT-IR report identifies the saponin; a class of phytocompounds in the plant is responsible for the synthesis of the silver nanoparticle (Raman et al., 2012).
Anti-diabetic activity
Diabetes mellitus is a very complex and uncontrollable metabolic disorder. There are numbers of the chemical agents that control the insulin and glucose level in the blood. It happens either due to improper secretion or action of insulin in the body during diabetes mellitus (Chaudhury et al., 2017). The synthetic pharmaceutical drugs prescribed for these conditions are having more harmful side effects like causing secondary organ damage (kidney failure, liver failure, etc.) on the human body. The plant bioactive chemicals are alternate medicines for diabetes allopathic medication. The methanolic crude extract of P. dulce seed was tested in Streptozotocin (STZ)-induced diabetic rat (albino Wistar male model) and the extract has the ability to protect the functional β-cells that produce and maintain the insulin level in the blood (Fu et al., 2013). This insulin treatment improves the glycogen content. In the methanolic extract treated STZ induced rats, the liver glycogen level was higher compared with the control group of Wistar rat and the functional glucose metabolism could be due to better insulin secretion from β-cells and the glucose was utilized in the oral glucose tolerance test. Thus, it could be a potential therapeutic for diabetic patients (Nagmoti et al., 2015). The P. dulce fruit containing a cyclic polyol pinitol and it has reported for having anti-diabetic activity (Gao et al., 2015; Kim et al., 2007).
Anti-hyperlipidemic
The excess glucose is usually preserved as glycogen and then fat in our body tissues as storage fuel for future. But the continuous accumulation for a longer period may lead to hyperlipidemia. It is one of the major risk factors involved in the development of Type II diabetes, heart disease, etc. (Nelson, 2013; Zhou et al., 2015). High-density lipoprotein cholesterol (HDLC) is mainly involved in protecting us from heart diseases, especially atherosclerosis (Vergeer et al., 2010) and it transports excess cholesterol out of the body. Nagmoti et al. (2015) checked the methanolic crude extract of P. dulce seed on STZ-induced diabetic rat model. The histopathological analysis showed the increased levels of HDLC and the very low-density lipoproteins cholesterol (VLDLC), low-density lipoproteins cholesterol (LDLC), serum cholesterol, and triglycerides level were significantly decreased in the P. dulce treated rats. From the results, the bioactive compounds of P. dulce seeds have active potentiality against the hyperlipidemic condition. Hence, P. dulce could also holding hyperlipidemic activity against STZ induced animal model has been proved (Nagmoti et al., 2015).
Anti-oxidant activity
Imbalance of electron on any atom or oxidative stress is one of the key important factors which triggers majority of diseases like cancer, arthritis, diabetes, renal damage, etc. (Ung et al., 2017). The unstable radicals cause severe damage to the inner organs, tissues, and cause various health problems. Nitric oxide, hydroxyl, and superoxide radicals are few common free radicals responsible for some autoimmune diseases like rheumatoid arthritis and diabetes mellitus (Asmat et al., 2016; Mateen et al., 2016; Saegusa et al., 2006;). In our human body, various mechanisms like enzymatic and non-enzymatic antioxidants protect the inner cellular molecules and tissues against reactive oxygen species (ROS) induced damage (Aruoma, 1998).
The phytochemicals are widely known to be the precious sources for antioxidant activity. It stabilizes the radicals generated through various factor and help in promoting the antioxidant enzymes in our body. The leaves, seeds, fruits, and wood barks extract of P. dulce have potential activity against free radicals has proved. The whole plant has active free radical scavenging potential against synthetic radicals of DPPH, NO, superoxide, and hydroxyl ions (Katekhaye and Kale, 2012; Nagmoti et al., 2012; Sukantha et al., 2011). Further, the HPLC profiling confirms the abundant active phenolic and flavonoid contents in the fruits (Megala and Geetha, 2009).
Anti-ulcer activity
Peptic ulcer is one of the major and common global health issues. Because many of the anti-inflammatory drugs [called non-steroidal anti-inflammatory drugs (NSAIDS)], dietary factors, stress, or painkiller drugs were found to be affect the stomach and causes an ulcer (Lanas and Chan, 2017). Continuous alcoholic adductors are also affected by peptic ulcer. The development of ulcer is correlated with oxidative stress by hypersecretion of HCL and reactive oxygen species (ROS) generation (Osefo et al., 2009; Suzuki et al., 2012). The hypersecretion of HCL is caused by H+, K+-ATPase action. Omeprazole, Lansoprazole, Ranitidine, and Famotidine are the major H+ and K+-ATPase inhibitors used to treat the ulcer and to control the acid secretion. But these anti-secretary drugs produce adverse side effects on the human body.
The aqueous crude extract of P. dulce was orally treated in acetylsalicylic acid (ASA)-induced rat model (Male albino Wistar rats). The phytocompounds reacted and inhibited the gastric mucosal H+, K+-ATPase (Megala and Geetha, 2009). The H+, K+-ATPase level was analyzed and compared with the standard drug of Omeprazole. Gastric mucin is an important factor in protecting the gastric mucosa. Gastric mucin, myeloperoxidase activity, and prostaglandin E2 (PGE2) level were analyzed and reported in Megala and Geetha (2012). The important role of PGE2 is to maintain the gastric mucosa by increasing the gastric mucus secretion and decreasing the gastric acid secretion. In the P. dulce treated rats, the PGE2 level was found to be increased and that indicates the stimulation of cytoprotective factors that contribute to accelerate the ulcer healing effect. From the results, P. dulce have an ability to cure the gastrointestinal disorders (Peptic ulcer). So, the P. dulce extract can be used as an antiulcer agent and it also act as an anti-acid secreting agent and cytoprotective factor (Megala and Geetha, 2012).
Nephroprotective
Carbon tetrachloride (CCl4) is an environmental toxin and is also used as medicine for hookworm disease and it affects and damages the kidney and liver (Rahmat et al., 2014). It causes fibrosis, cirrhosis, and hepatic carcinoma. Cytochrome P450 isozymes produce trichloromethyl free radical (TCCM free radicals) with higher toxicity of CCl4. TCCM Free radicals react with oxygen to form the reactive trichloromethyl peroxy radical (high level toxic), a reactive oxygen species (ROS). Free radical also induces the lipid peroxidation and it is a major factor for cell membrane damage in many pathological situations. CCl4 generates free radicals and cause renal disorders by generating free radicals in hepatic disorder (Al-Yahya et al., 2013). Pithecellobium dulce crude extract was orally administrated in the CCl4 induced rats (orally before CCl4 induced rat) and crude extract was also administrated to the rat before inducing CCl4 toxin (orally after CCl4 induced rat). The crude extract of P. dulce decreased the lipid peroxidation and protein carboxylation after inducing CCl4 in rats, as the P. dulce compounds have antioxidant activity. In the CCl4 administrated rats, the ROS level was found increased, while P. dulce fruit extract treated rats have decreased ROS level when compared with CCl4 administrated untreated rats. Anti-oxidants enzymes are mainly involved in cellular defense and to prevent and protect from oxidative stress or oxidative damage. Glutathione reductase (GR), Superoxide dismutase (SOD), Glutathione-S-transferase (GST), and catalase (CAT) are major antioxidant enzymes and CAT & SOD are important enzymes to eliminate the ROX. In the P. dulce extract pretreated rats, higher amount of anti-oxidant enzyme level was observed as compared with CCl4 induced rats and the rats treated with P. dulce extract. The aqueous extract of P. dulce also prevents and protects the renal DNA damage and cell death, by means of stabilizing the oxidative radicals, which disturbs the mitochondrial membrane and causes loss of ATP production that directly leads to cell death. Pal et al. (2012) evaluated and proved the anti-necrotic properties and nephroprotective properties of P. dulce.
Anti-venom effect
The tannin was extracted from P. dulce barks using aqueous extraction. The venom lethality was inhibited and the necrotizing activity of the venom was minimized by this crude extract. The extract also inhibited 90% of acetylcholine esterase activity as it contains higher tannin concentration or combined hydrolyzable tannin concentration. α-cobra toxin protein was docked with four different tannin compounds using Autodock 3 and tannic acid, Digallic acid has −14.7 kcal/mol, −10.38 kcal/mol binding energies were studied. The plant extract selectively blocks nicotinic acetylcholine receptor and non-selectively precipitate the venom protein (Pithayanukul et al., 2005).
Anti-diarrheal effect
The ethanolic extract of P. dulce showed an anti-diarrheal effect in the castor oil-induced mice. Loperamide is the standard anti-diarrheal drug used to compare the results. The phytochemicals of P. dulce has the ability to increase the latent period, delay, and decrease the frequency of defecation (Rashid et al., 2014).
Anti-bacterial effect
The ethanolic extract of P. dulce pod pulp has potent to inhibit the Gram-positive bacteria (Bacillus subtilis) and Gram-negative bacteria (Klebsiella pneumonia). The secondary metabolites (flavonoid, saponin, etc.) are responsible for the inhibition of bacterial growth (Pradeepa et al., 2014). The aqueous, methanolic and ethyl acetate extract of P. dulce fruit peel inhibit the eight different microorganisms (Staphylococcus epidermis, Escherichia coli, Klebsiella pneumonia, Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, Pseudomonas putida, and Proteus vulgaris) isolated from wound infection. The higher zone inhibition was found in the crude methanolic extract. From the result, P. dulce fruit peel metabolites could be used as an antimicrobial agent and wound healing agent was proved. The ethanolic extract of P. dulce leaf has also been investigated and their effective anti-bacterial property was reported by Sukantha et al., 2014.
Anti-fungal effect
The plant pathogens like fungus cause contamination in strawberry fruits during storage. Many of the preventive agents are used to prevent fungal contamination on fruits but they are usually holding some toxic effects. Pithecellobium dulce is a natural resource that could be used against fungal contamination. The aqueous and hydroalcoholic extracts of P. dulce have potentiality against Rhizopus stolonifer, Botrytis cinerea, and Penicillium digitatum contamination. In the aqueous extract, the secondary metabolite of kaempferol and some other mixture of compounds are mainly involved against the fungal contamination. While comparing the aqueous and hydro alcoholic extracts, the aqueous extract has better activity against fungal contamination (Bautista-Banos et al., 2003; Shanmugakumar et al., 2006).
REFERENCES
Al-Yahya MA, Mothana R, Al-Said M. Attenuation of CCl4-induced oxidative stress and hepatonephrotoxicity by Saudi Sidr honey in rats. J Evid Based Complementary Altern Med, 2013; 1–10. CrossRef
Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc, 1998; 75:199–212. CrossRef
Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J, 2016; 24:547–53. CrossRef
Bagchi S, Kumar KJ. Studies on water soluble polysaccharides from Pithecellobium dulce (Roxb.) Benth. Seeds. Carbohydr Polym, 2016; 138:215–21. CrossRef
Bautista-Banos S, Garcia-Dominguez E, Barrera-Necha LL, Reyes-Chilpa R, Wilson CL. Seasonal evaluation of the postharvest fungicidal activity of powders and extracts of huamuchil (Pithecellobium dulce): action against Botrytris cinerea, Penicillium digitatum and Rhizopus stolonifer of strawberry fruit. Postharvest Biol Technol, 2003; 29:81–92. CrossRef
Bhargvakrishna P, Gupta MB, Mitra CR, Chittranjan R. Anti-inflammatory activity of saponins and other natural products. Indian J Med Res, 1970; 58:724–30.
Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K, Sasapu A, Beebe A, Patil N, Musham CK, Lohani GP, Mirza W. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol, 2017; 8:6. CrossRef
CSIR. The useful plants of India, publ. info. Directorae. New Delhi, India: C.S.I.R. (Council of Scientific and Industrial Research); 1988.
Edzard Ernst MD. Harmless herbs? A review of the recent literature. Am J Med, 1998; 170–8. CrossRef
Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev, 2013; 9:25–53. CrossRef
Gao Y, Zhang M, Wu T, Xu M, Cai H, Zhang Z. Effects of D-pinitol on insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J Agric Food Chem, 2015; 63:6019–26. CrossRef
Govindarajan M, Rajeswary M. Repellent properties of Pithecellobium dulce (Roxb.) Benth. (Family: Fabaceae) against fi lariasis vector, Culex quinquefasciatus Say (Diptera:Culicidae). J Med Herbs Ethnomed, 2015; 1:103–7. CrossRef
Govindarajan M, Sivakumar R, Rajeswary M, Yogalakshmi K. Adulticidal activity of Pithecellobiumdulce (Roxb.) Benth. Against Culexquinquefasciatus(Say). Asian Pacific J Trop Dis, 2012; 1:124–8. CrossRef
Kahindi RK, Abdulrazak SA, Muinga RW. Effect of supplementing Napier grass (Pennisetum purpureum) with Madras thorn (Pithecellobium dulce) on intake, digestibility and live weight gains of growing goats. Small Ruminant Res, 2007; 69:83–7. CrossRef
Katekhaye SD, Kale MS. Antioxidant and free radical scavenging activity of Pithecellobium dulce (Roxb.) Benth wood bark and leaves. Free Radic Biol Med, 2012; 2:47–57. CrossRef
Kim MJ, Yoo KH, Kim JH. Effect of pinitol on glucose metabolism and adipocytokines in uncontrolled type 2 diabetes. Diabetes Res Clin Pract, 2007; 77:247–51. CrossRef
Hooper L, Cassidy A. A review of the health care potential of bioactive compounds. J Sci Food Agric, 2006; 86:1805–13. CrossRef
Lanas A, Chan FKL. Peptic ulcer disease. Lancet, 2017; 613–24. CrossRef
Mateen S, Moin S, Khan AQ, Zafar A, Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One, 2016; 11:e0152925. CrossRef
Megala J, Geetha A. Free radical- scavenging and H+, K+-ATPase inhibition activities of Pithecellobium dulce. Food Chem, 2009; 121:1120–8. CrossRef
Megala J, Geetha A. Antiulcerogenic activity of hydroalcoholic fruit extract of Pithecellobium dulce in different experimental ulcer models in rats. J of Ethnopharmacol, 2012; 142:415–21. CrossRef
Nagmoti DM, Kothavade PS, Bulani VD, Gawali NB, Juvekar AR. Antidiabetic and antihyperlipidemic activity of Pithecellobium dulce (Roxb.) Benth seeds extract in streptozotocin-induced diabetic rats. Eur J Integrative Med, 2015; 7:263–73. CrossRef
Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care, 2013; 40:195–211. CrossRef
Nigam SK, Gupta RK, Mitra CR. Pithecellobium dulce. I. Isolation and characterization of the constituents of the legume. J Pharm Sci, 1962; 52:459–62. CrossRef
Nigam SK, Misra G, Uddin R, Yoshikawa K, Kawamoto M, Arihara S. Pithedulosides A-G, Oleanane glycosides from Pithecellobium dulce. Phytochem, 1996; 44:1329–34. CrossRef
Nigam SK, Mitra CR. Pithecellobium dulce. IV. Constituents of flowers, heartwood, and root bark. Planta Med, 1968; 16:335–7. CrossRef
Nigam SK, Mitra CR. Pithecellobium dulce. V. Chemistry of the seed saponin and constituents of the leaves. Planta Med, 1970; 18:44–50. CrossRef
Olivares JF, Avilés NF, Albarrán PB, Castelán OA, Rojas HS. Nutritional quality of Pithecellobium dulce and Acacia cochliacantha fruits, and its evaluation in goats. Livest Sci, 2013; 154:74–81. CrossRef
Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A. Agroforestree database: a tree species reference and selection guide version 4.0. World Agroforestry Centre ICRAF, Nairobi, KE, 2009.
Osefo N, Ito T, Jensen RT. Gastric acid hypersecretory states: recent insights and advances. Curr Gastroenterol Rep, 2009; 11:433–41. CrossRef
Pal PB, Pal S, Manna P, Sil PC. Traditional extract of Pithecellobium dulce fruits protects mice against CCl4 induced renal oxidative impairment and necrotic cell death. Pathophysiology, 2012; 19:101–14. CrossRef
Pirkle JL, Freedman BI. Hypertension and chronic kidney disease: controversies in pathogenesis and treatment. Minerva Urol Nefrol, 2013; 65:37–50.
Pithayanukul P, Ruenraroengsak P, Bavovada R, Pakmanee N, Suttisri R, Saen-oon S. Inhibition of Naja kaouthia venom activities by plant polyphenols. J Ethnopharmacol, 2005; 97:527–33. CrossRef
Pradeepa S, Subramanian S, Kaviyarasan V. Evaluation of anti-microbial activity of Pithecellobium dulce pod pulp extract. Asian J Pharm Clin Res, 2014; 7:32–7.
Preethi S, Mary Saral A. Screening of natural polysaccharides extracted from the fruits of Pithecellobium dulce as a pharmaceutical adjuvant. In J Bio Mac, 2016; 92:347–56. CrossRef
Rahmat AA, Dar FA, Choudhary IM. Protection of CCl4-induced liver and kidney damage by phenolic compounds in leaf extracts of Cnestis ferruginea (de Candolle). Pharmacogn Res, 2014; 6:19–28. CrossRef
Rajeswary M, Govindarajan M. Adulticidal properties of Pithecellobiumdulce (Roxb.) Benth. (Family: Fabaceae) against dengue vector, Aedes aegypti (Linn.) (Diatera: Culicidae). Asian Pacific J Trop Dis, 2014; 1:449–52. CrossRef
Raman N, Sudharsan S, Veerakumar V, Pravin N, Vithiya K. Pithecellobiumdulce mediated extra-cellular green synthesis of larvicidal silver nanoparticles. Spec Acta Part A Mol Biomol Spec, 2012; 96:1031–7. CrossRef
Rao GN, Nagender A, Satyanarayana A, Rao DG. Preparation, chemical composition and storage studies of quamachil (Pithecellobium dulce L.) aril powder. J Food Sci Technol, 2010; 48(1):90–5. CrossRef
Rashid MH, Biswas SU, Abdullah-AL-Mamun MO, huque A, Bhuiyan JR. Phytochemical screening and analgesic, anti-bacterial and cytotoxic activity evaluation of ethanol extract of Pithcellobium dulce (Roxb.) benth leaf. Asian J Pharm Clin Res, 2015; 8:451–6.
Saegusa J, Kawano S, Kumagai S. Oxidative stress and autoimmune diseases. Oxid Stress Dis Cancer, 2006; 461–75. CrossRef
Sahu NP, Mahato SB. Anti-inflammatory triterpene saponins of Pithecellobium dulce: characterization of echinocystic acid bisdesmoside. Phytochem, 1994; 37:1425–7. CrossRef
Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today, 2008; 13:161–71. CrossRef
Satheesh Kumar N, Nisha N. Phytomedicines as potential inhibitors of β amyloid aggregation: significance to Alzheimer’s disease. Chin J Nat Med, 2014; 12:801–18. CrossRef
Saxena VK, Singhal M. Novel prenylated flavonoid from stem of Pithecellobium dulce. Fitoterapia, 1999; 70:98–100. CrossRef
Shanmugakumar SD, Amerjothy S, Balakrishna K. Pharmacognostical, antibacterial and antifungal potentials of the leaf extracts of Pithecellobium dulce Benth. Phcog Mag, 2006; 2:163–7.
Shyur LF, Yang NS. Metabolomics for phytomedicine research and drug development. Curr Opin Chem Biol, 2008; 12:66–71. CrossRef
Sukantha TA, Subashini KS, Ravindran NT, Balashanmugam P. Evaluation of in vitro antioxidant and antibacterial activity of Pithecellobium dulce Benth fruit peel. Int J Curr Res, 2011; 1:378–82.
Sukantha TA, Subashini KS, Ravindran NT. Antibacterial activity of selected medicinal plant in traditional treatment of wound infection in Southeast India. Int J Pharm Sci, 2014; 6:511–3.
Sukantha TA, Subashini KS. Isolation and characterization of secondary metabolites from Pithecellobium dulce benth fruit peel. Int J Pharm Pharm Sci, 2015; 7:199–203.
Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr, 2012; 50:35–9. CrossRef
Ung L, Pattamatta U, Carnt N, Wilkinson-Berka JL, Liew G, White AJ. Oxidative stress and reactive oxygen species: a review of their role in ocular disease. Clin Sci 2017; 131:2865–83. CrossRef
Vergeer M, Holleboom AG, Kastelein JJ, Kuivenhoven JA. The HDL hypothesis: does high-density lipoprotein protect from atherosclerosis? J Lipid Res, 2010; 51:2058–73. CrossRef
Yoshikawa K, Suzaki Y, Tanaka M, Arihara S, Nigam SK. Three acylated saponins and a related compound from Pithecellobium dulce. J Nat Prod, 1997; 60:1269–74. CrossRef
Zhou X, Zhang W, Liu X, Zhang W, Li Y. Interrelationship between diabetes and periodontitis: role of hyperlipidemia. Arch Oral Biol, 2015; 60:667–74. CrossRef




.png)