INTRODUCTION
Helichrysum caespititium (DC.) Harv. is a perennial creeping herb (Fig. 1) which belongs to the Asteraceae or Compositae family. The species has been recorded in Lesotho, South Africa, Swaziland, and Zimbabwe (Fabian and Germishuizen, 1997; Germishuizen and Meyer, 2003; Hilliard, 1977; 1983; Hyde et al., 2019). Helichrysum caespititium is known by several vernacular names in southern Africa which include the following: golden everlasting (English), sewejaartjies and speelwonderboom (Afrikaans), phate-ea-naha, boriba, moriri-oa-lefatse, lelula-phooko, and botsiki-nyane (southern Sotho), mabjana, matšana, and mmetse (northern Sotho) (Erasmus et al., 2012; Hutchings and Van Staden, 1994; Pooley, 1998; 2003). A single synonym, “Helichrysum lineare DC. var. caespititium DC.,” was found in the literature (Fabian and Germishuizen, 1997; Germishuizen and Meyer, 2003; Hilliard, 1983; Hyde et al., 2019). The species name caespititium was derived from the Latin word “caespitose” which means very much tufted and matted, in reference to the cushion-forming or mat-forming growth habit of the species (Hyde et al., 2019). The height of H. caespititium ranges from 10 to 20 cm has been recorded in open spaces in the grassland and savanna biomes, particularly disturbed areas at an average altitude range of 650–2,440 m above the sea level (Fabian and Germishuizen, 1997 ; Germishuizen and Meyer, 2003; Hilliard, 1983; Hyde et al., 2019). The leaves are linear, clasping at the base and hairy on both sides (Fig. 1). The leaves are orange gland-dotted with margin rolled under and densely crowded along the stems (Hilliard, 1983; Hyde et al., 2019). The flowers of H. caespititium are white to yellow in color and pale furry underneath (Hilliard, 1983; Hyde et al., 2019).
Helichrysum caespititium is a popular herbal medicine throughout its geographical distributional range in Lesotho, South Africa, Swaziland, and Zimbabwe (Arnold et al., 2002; Gelfand et al., 1985; Long, 2005; Moteetee and Van Wyk, 2011). Therefore, H. caespititium is regarded as an integral part of traditional pharmacopoeia in southern Africa, with species tolerated and managed in domestic home gardens of the North West province of South Africa as herbal medicine (Molebatsi, 2011). Helichrysum caespititium makes an enormous contribution to basic primary healthcare of local people in southern Africa. Therefore, this is the rationale behind the review of ethnopharmacological properties of H. caespititium. The current study is aimed at appraising the existing ethnomedicinal value, phytochemistry, and biological activities of the compounds isolated from the species, including H. caespititium crude extracts as well as exploring the potential of the species as herbal medicine.
 | Figure 1. Helichrysum caespititium, (A) a photograph showing an entire plant, the leaves and inflorescence (photo: F Lagarde) and (B) photograph of a herbarium specimen housed in the National Herbarium of Zimbabwe (photo: B Wursten). [Click here to view] |
MATERIALS AND METHODS
Relevant information on medicinal applications, chemistry, phytochemistry, and biological activities of H. caespititium were assembled from the several sources which included Scopus, Google Scholar, Elsevier, Science Direct, Web of Science, Pubmed, SciFinder, and BMC. Additional information was sourced from journal articles, scientific reports, theses, books, and book chapters gathered from the University library. The search for this information was carried out between September 2018 and February 2019. The key words used in the search included “ethnobotany,” “ethnomedicinal uses,” “medicinal uses,” “phytochemistry,” “biological activities,” “pharmacological properties,” “toxicological properties,” “Helichrysum caespititium,” the synonym of the species, “Helichrysum lineare DC. var. caespititium DC.” and the English common name “golden everlasting.”
RESULTS AND DISCUSSION
Medicinal uses
The leaves, roots, and the whole plant of H. caespititium are widely used as the herbal medicines for 29 human diseases in south and central Africa (Table 1). Following medical categorization of human diseases and ailments proposed by Cook (1995), Gruca et al. (2014), Macía et al. (2011), and Staub et al. (2015), H. caespititium is mainly used as the herbal medicine against respiratory infections in all the countries where the taxon is indigenous (Fig. 2). Other important medicinal applications include sexually transmitted infections, nausea, aphrodisiac, headache, wounds, and ulceration (Fig. 2). In South Africa, the whole plant of H. caespititium is mixed with roots of Callilepis laureola DC. and Osyris lanceolata Hochst. & Steud., leaves of Lippia javanica (Burm. f.) Spreng., and Tragia dioica Sond. as remedy for fatigue (Semenya and Maroyi, 2018; 2019a). Similarly, the whole plant parts of H. caespititium is mixed with bark of Sclerocarya birrea (A.Rich.) Hochst., entire plant parts of Enicostema axillare (Lam.) A. Raynal, and roots of Callilepis laureola as the herbal medicine for pneumonia (Semenya and Maroyi, 2018c; 2019a). In Lesotho, the whole plant of H. caespititium is mixed with roots of Dicoma anomala Sond., Scabiosa columbaria L., and Zantedeschia albomaculata (Hook.) Baill. as the herbal medicine for venereal diseases (Maliehe, 1997; Maroyi, 2018; Moteetee and Van Wyk, 2011; Watt and Brandwijk, 1927; Watt and Breyer-Brandwijk, 1962).
Phytochemistry
Dekker et al. (1983) isolated the phloroglucinol compound, caespitin [2 (4-methylpentanoyl)-4-(3-methylbuten-2-yl) phloroglucinol] from whole plant of H. caespititium (Fig. 3A). Similarly, Mathekga et al. (2000) isolated a phloroglucinol compound caespitate, often referred to as 2-methyl 4-[2ʹ,4ʹ,6ʹ trihydroxy-3ʹ (2-methylpropanoyl) phenyl]but-2-enyl acetate from the aerial parts of H. caespititium (Fig. 3B). Based on pharmacological evaluations done so far, both caespitin and caespitate have antibacterial and antifungal activities (Dekker et al., 1983; Mathekga, 2001; Mathekga et al., 2000; Van Der Schyf et al., 1986), while caespitate also exhibited antituberculosis activities (Meyer et al., 2002). Documentation of phloroglucinol compounds only highlights an existing gap requiring attention from researchers and future research should focus on the identification and isolation of phytochemical compounds in the utilized parts, particularly aerials parts, stems, and roots.
Biological activities
The following biological activities have been reported from H. caespititium crude extracts and compounds isolated from the species (Table 2): antibacterial (Dekker et al., 1983; Mamabolo et al., 2017; Mathekga, 2001; Mathekga et al., 2000; Seleteng-Kose et al., 2019; Van der Schyf et al., 1986), antigonorrhea (Mamabolo et al., 2018), antimycobacterial (Meyer et al., 2002), antifungal (Dekker et al., 1983; Mathekga, 2001; Mathekga et al., 2000; Seleteng-Kose et al., 2019; Van der Schyf et al., 1986), antioxidant (Mamabolo et al., 2017), and cytotoxicity (Mamabolo et al., 2018) activities.
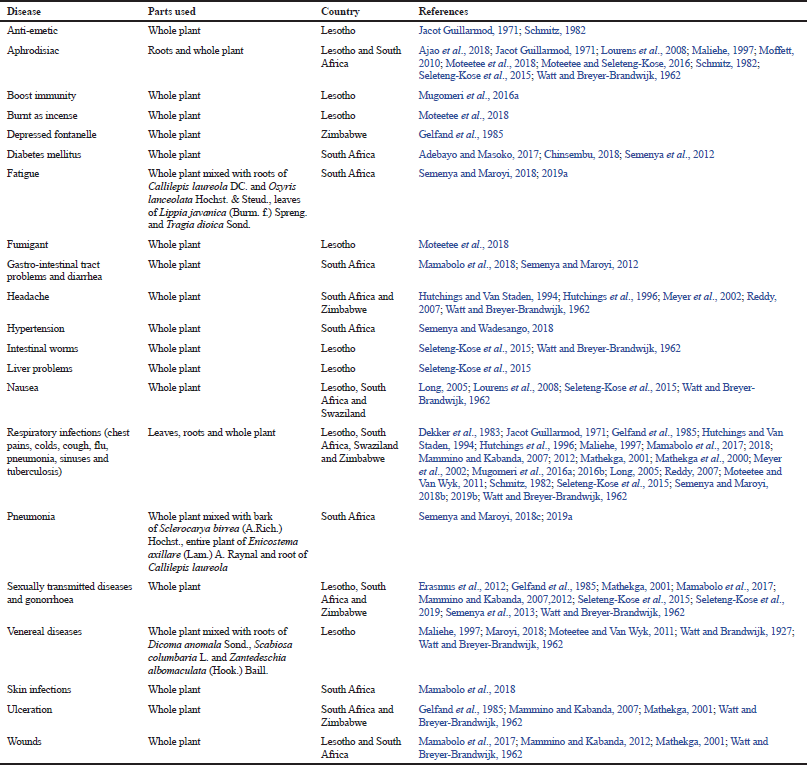 | Table 1. Medicinal uses of Helichrysum caespititium. [Click here to view] |
Antibacterial activities
Preliminary evaluation of antibacterial activities of the compound caespitin isolated from H. caespititium by Dekker et al. (1983) showed that the compound exhibited significant antibacterial activities against Streptococcus pyogenes and Staphylococcus aureus; however, the screening method used or the activity level are not indicated in the paper. Similarly, Van der Schyf (1986)assessed antibacterial properties of caespitin identified from H. caespititium against S. pyogenes, Proteus mirabilis, Pseudomonas aeruginosa, Escherichia coli, and S. aureus using a serial dilution method. The compound exhibited activities against S. pyogenes and S. aureus with the minimum inhibitory concentration (MIC) value of 8.0 μg/ml and the minimum bactericidal concentration (MBC) value of 16.0 μg/ml (Van der Schyf, 1986). Mathekga (2001) and Mathekga et al. (2000) evaluated the antibacterial activities of acetone extracts of aerial parts of H. caespititium against Bacillus subtilis, Enterobacter cloacae, Bacillus cereus, P. aeruginosa, Bacillus pumilus, Micrococcus kristinae, S. aureus, E. coli, Serratia marcescens, and Klebsiella pneumoniae using the agar dilution technique. The extract exhibited activities against all the tested pathogens, except S. marcescens and K. pneumoniae with MIC value of 1.0 mg/ml (Mathekga, 2001; Mathekga et al., 2000). Mathekga et al. (2000) assessed the antibacterial properties of the compounds caespitin and caespitate against E. cloacae, B. pumilus, M. kristinae, E. coli, B. subtilis, S. aureus, S. marcescens, K. pneumoniae, B. cereus, and P. aeruginosa using agar dilution method. Both compounds, caespitin and caespitate were active against B. subtilis, M. kristinae, B. pumilus, S. aureus, and B. cereus with the MIC value range of 0.5 to 1.0 μg/ml (Mathekga, et al. 2000). Mathekga (2001) also evaluated the synergistic antibacterial activities of the compounds caespitate and caespitin isolated from H. caespititium against B. subtilis, K. pneumoniae, M. kristinae, E. cloacae, B. pumilus, S. marcescens, S. aureus, B. cereus, E. coli, and P. aeruginosa using agar dilution method. Caespitate and caespitin exhibited antibacterial activities against S. aureus, B. cereus, M. kristinae, B. subtilis, and B. pumilus with MIC values ranging from 0.5 to 1.0 μg/ml. The combination of caespitin and caespitate maintained their original antibacterial activities against all the tested pathogens except S. marcescens and also enhanced their synergistic effects with MIC values within the range of 0.05–0.1 μg/ml (Mathekga, 2001). Mamabolo et al. (2017) assessed antibacterial properties of n-hexane, dichloromethane, acetone, methanol and aqueous extracts of whole plant of H. caespititium against Klebsiella oxytoca, B. cereus, Enterococcus faecalis, B. subtilis, E. cloacae, Mycobaterium smegmatis, E. coli, K. pneumoniae, Proteus vulgaris, Staphylococcus epidermidis, P. aeruginosa, P. mirabilis, S. aureus, and Enterobacter aerogenes using the microdilution technique with streptomycin and nalidixic acid as positive drugs. The extracts showed antibacterial activities against all the tested pathogens with the MIC values within the range of 0.01–0.4 mg/ml (Mamabolo et al., 2017). Seleteng-Kose et al. (2019) assessed the properties of organic and water extracts of whole plant parts of H. caespititium against Neisseria gonorrhoeae, Gardnerella vaginalis, and Oligella ureolytica using the micro-dilution technique with ciprofloxacin (0.01 mg/ml) as the positive drug. The organic extracts showed moderate properties against G. vaginalis and N. gonorrhoeae with the MIC value of 0.1 and 0.06 mg/ml. The MIC value of organic extract against O. ureolytica was 7.2 mg/ml, while aqueous extract showed the MIC values of >8.0 mg/ml against all the tested pathogens (Seleteng-Kose et al., 2019).
.png) | Figure 2. Diseases and ailments treated by Helichrysum caespititium in southern Africa. [Click here to view] |
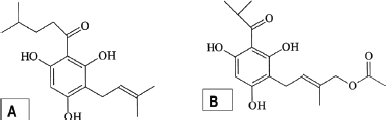 | Figure 3. Chemical structures of phloroglucinol compounds, caespitin (A) and caespitate (B) isolated from Helichrysum caespititium. [Click here to view] |
Antigonorrhea activities
Mamabolo et al. (2018) assessed the antigonorrhea properties of n-hexane, dichloromethane, methanol and aqueous extracts of whole plant of H. caespititium against strains F, N, O, and G of N. gonorrhoeae using micro-dilution technique with gentamicin and amoxicillin as positive drugs. The extracts showed properties with the MIC values within the range of 0.04 to >0.3 mg/ml which were within the same range of MIC values of 0.2–0.3 mg/ml exhibited by the controls (Mamabolo et al., 2018).
Antimycobacterial activities
Meyer et al. (2002) evaluated the antimycobacterial properties of acetone and aqueous leaf extract of H. caespititium against a drug-sensitive strain of Mycobacterium tuberculosis using the agar plate technique. Meyer et al. (2002) also evaluated the antimycobacterial activities of the phloroglucinol compound, caespitate isolated from H. caespititium against drug-resistant and drug sensitive strains of M. tuberculosis. The acetone extract showed activities against the tested pathogens at a concentration of 0.5 mg/ml whereas, M. tuberculosis was susceptible to the aqueous extract at 5.0 mg/ml. The activities exhibited by the acetone extract against M. tuberculosis were corroborated by the use of the rapid radiometric method and the MIC value was found to be 0.1 mg/ml. The MIC value of caespitate was determined to be 0.1 mg/ml for the M. tuberculosis strains (Meyer et al., 2002).
Antifungal activities
Preliminary evaluation of antifungal activities of the compound caespitin isolated from H. caespititium by Dekker et al. (1983) showed that the compound exhibited significant antifungal activities against Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis, and Cryptococcus neoformans; however, the screening method used or the activity level are not indicated in the paper. Van der Schyf et al. (1986) evaluated antifungal activities of the compound caespitin isolated from H. caespititium against Candida albicans, Candida tropicalis, Absidia corymbifera, Aspergillus fumigatus, Sporotrichum schenkii, T. rubrum, M. canis, and T. mentagrophytes using a serial dilution technique and nystatin as the positive drug. The compound exhibited best activities against T. rubrum, M. canis, and T. mentagrophytes with MIC and the minimum fungicidal concentration (MFC) values within the range of 6.0–13.0 μg/ml, while MIC and MFC values for C. albicans, C. tropicalis, A. corymbifera, A. fumigatus, and S. schenkii were within the range of 25.0 to >100.0 μg/ml. These results were within the same range as MIC and MFC values exhibited by nystatin, the positive drug which exhibited 2.0 to >100.0 μg/ml (Van der Schyf et al., 1986). Mathekga (2001)and Mathekga et al. (2000) evaluated the antifungal activities of acetone extracts of aerial parts of H. caespititium against M. canis, Aspergillus niger, Aspergillus flavus, Cladosporium sphaerospermum, Cladosporium cladosporioides, M. canis, and Cladosporium cucumerinum using agar dilution method. The extract showed properties against all the tested pathogens with the MIC values within the range of 0.01–1.0 mg/ml (Mathekga, 2001; Mathekga et al., 2000). Mathekga et al. (2000) also evaluated the antifungal activities of the compound caespitate isolated from H. caespititium against C. sphaerospermum, Phytophthora capsici, A. niger, C. cucumerinum, A. flavus, and C. cladosporioides using agar dilution method. The compound was active against all tested pathogens with the MIC values within the range of 0.5–5.0 μg/ml (Mathekga et al., 2000). Seleteng-Kose et al. (2019) assessed the antifungal properties of water and organic extracts of whole plant parts of H. caespititium against C. albicans using the micro-dilution assay with amphotericin B (0.1 mg/ml) as the positive drug. The organic extracts showed moderate properties with the MIC values of 0.02 mg/ml, while the MIC value exhibited by aqueous extract was >8.0 mg/ml (Seleteng-Kose et al., 2019).
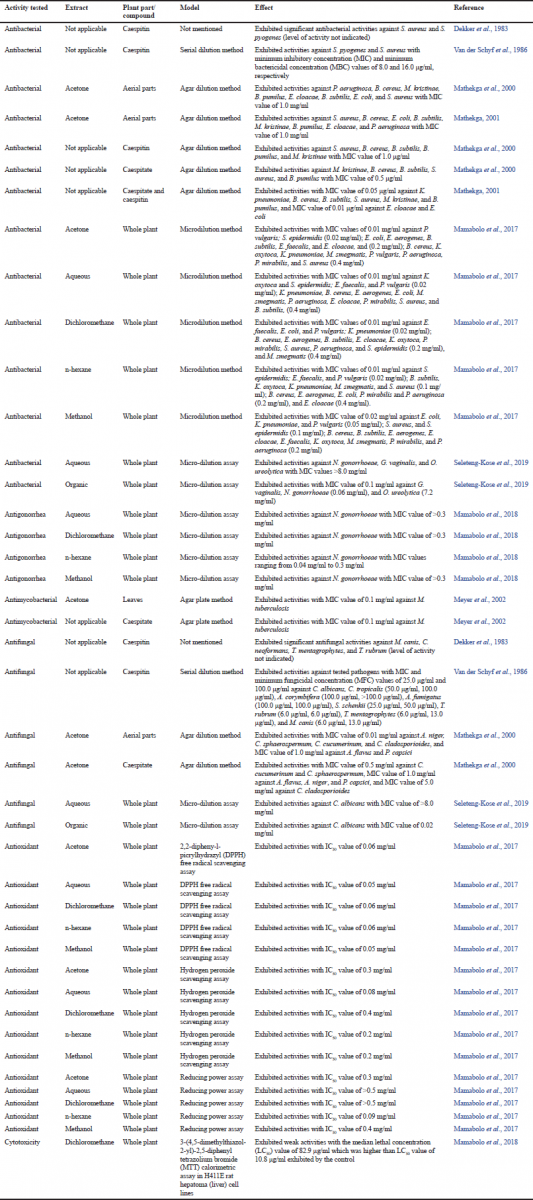 | Table 2. Summary of biological activities of Helichrysum caespititium crude extracts and compounds isolated from the species. [Click here to view] |
Antioxidant activities
Mamabolo et al. (2017) assessed the antioxidant activities of dichloromethane, n-hexane, acetone, methanol, and aqueous extracts of whole plant parts of H. caespititium using hydrogen peroxide scavenging, 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging and reducing power assays with ascorbic acid and butylated hydroxytoluene (BHT) as positive controls. The extracts exhibited properties with half maximal inhibitory concentration (IC50) values within the range of 0.05–0.6 mg/ml which were within the (IC50) values of 0.04 to >0.5 mg/ml exhibited by the positive drugs (Mamabolo et al., 2017).
Cytotoxicity activities
Mathekga (2001) evaluated the cytotoxicity activities of the compound caespitate isolated from H. caespititium on vervet monkey kidney cells using the 3-(4,5 dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide (MTT) technique. The maximum non-toxic concentration of the bioactive compound on the vervet kidney monkey cell cultures was 50 mg/ml and at this level the cells did not show any morphological alterations or any signs of growth indicating some cytotoxic effects (Mathekga, 2001). Mamabolo et al. (2018) assessed cytotoxicity properties of n-hexane, dichloromethane, methanol, and aqueous extracts of whole plant parts of H. caespititium in H411E rat hepatoma (liver) cell lines using the MTT technique with doxorubicin as the positive control. The extracts exhibited weak activities with the median lethal concentration (LC50) values ranging from 82.9–428.8 μg/ml which were much higher than LC50 value of 10.8 μg/ml showed by the positive control (Mamabolo et al., 2018). These results suggest that the plant extract can safely be used without any worries of being toxic to the cells. Seleteng-Kose et al. (2019) assessed cytotoxicity properties of water and organic extracts of whole plant parts of H. caespititium using the brine shrimp (Artemia franciscana) lethality assay with potassium dichromate as a positive drug. The extracts appear to be non-toxic as aqueous and organic extracts caused 3.3% and 40.7% mortality of A. franciscana after 24 hours in comparison to 98% and 100% mortality displayed by the positive control, potassium dichromate (Seleteng-Kose et al., 2019). Based on these evaluations done so far, there is a need for more research in order to establish the safety of extracts and isolated (bioactive) compounds from H. caespititium.
CONCLUSION
The diverse medicinal uses of H. caespititium and the scientific evidence of its biological activities indicate its potential as the herbal medicine. Its diverse pharmacological activities are directly or indirectly involved associated with a wide range of physiological processes which offers protection against growth of undesirable microbes and free radicals. There is a need for evaluation of the clinical significance of the antioxidant properties, cytotoxicity, and toxicity using in vivo models. Future research should also focus on assessing the classes of phytochemical compounds associated with the species. The biological potency of such phytochemicals needs to be evaluated aimed at exploring their potential.
ACKNOWLEDGMENTS
The author would like to express my gratitude to the National Research Foundation (NRF), South Africa and Govan Mbeki Research and Development Centre (GMRDC), University of Fort Hare for financial support to conduct this study.
CONFLICT OF INTEREST
The author declares that he has no conflict of interest.
REFERENCES
Adebayo SA, Masoko P. Therapeutic uses of plant species for inflammation-related conditions in Limpopo province of South Africa: a mini-review and current perspectives. Int J Pharmacog Phytoth Res, 2017; 1(1):2–8.
Ajao AA, Sibiya NP, Moteetee AN. Sexual prowess from nature: a systematic review of medicinal plants used as aphrodisiacs and sexual dysfunction in sub-Saharan Africa. S Afr J Bot, 2018. CrossRef
Arnold TH, Prentice CA, Hawker LC, Snyman EE, Tomalin M, Crouch NR, Pottas-Bircher C. Medicinal and magical plants of southern Africa: an annotated checklist. National Botanical Institute, Pretoria, South Africa, 2002.
Chinsembu KC. Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J Herbal Med, 2018; 15. CrossRef
Cook FEM. Economic botany data collection standard. Prepared for the International Working Group on Taxonomic Databases for Plant Sciences (TDWG), Kew Royal Botanic Gardens, Kew, London, UK, 1995.
Dekker TG, Fourie TG, Snyckers FO, Van der Schyf CJ. Studies of South African medicinal plants. part 2 caespitin, a new phloroglucinol derivative with antimicrobial properties from Helichrysum caespititium. S Afr J Chem, 1983; 36:114–6.
Erasmus LJC, Potgieter MJ, Semenya SS, Lennox SJ. Phytomedicine versus gonorrhoea: the bapedi experience. Afr J Trad Compl Alt Med, 2012; 9(4):591–8. CrossRef
Fabian A, Germishuizen G. Wild flowers of northern South Africa. Fernwood Press, Vlaeburg, South Africa, 1997.
Gelfand M, Mavi S, Drummond RR, Ndemera B. The traditional medical practitioners in Zimbabwe: his principles of practice and pharmacopoeia. Mambo Press, Gweru, Zimbabwe, 1995.
Germishuizen G, Meyer NL. Plants of southern Africa: an annotated checklist. Strelitzia 14, National Botanical Institute, Pretoria, South Africa, 2003.
Gruca M, Cámara-Leret R, Macía MJ, Balslev H. New categories for traditional medicine in the Economic Botany Data Collection Standard. J Ethnopharmacol, 2014; 155(2):1388–92. CrossRef
Hilliard OM. Compositae in natal. University of Natal Press, Pietermaritzburg, South Africa, 1977.
Hilliard OM. Gnaphaliinae. In: Leistner OA (ed.). Flora of Southern Africa: Asteraceae. National Botanical Institute, Pretoria, pp. 1–325, 1983.
Hutchings A, Van Staden J. Plants used for stress related ailments in traditional Zulu, Xhosa and Sotho medicine. Part 1. Plants used for headaches. J Ethnopharmacol, 1994; 43:89–124. CrossRef
Hyde MA, Wursten BT, Ballings P, Coates Palgrave M. Helichrysum caespititium (DC.) Harv. Flora of Zimbabwe: Species Information: Helichrysum caespititium. 2019 [Online]. Available via https://www.zimbabweflora.co.zw/speciesdata/species.php?species_id=159510 (Accessed 12 February 2019).
Jacot Guillarmod A. Flora of Lesotho (Basutoland). Cramer, Lehre, Germany, 1971. CrossRef
Long C. Swaziland’s flora: siSwati names and uses. Mbambane, Swaziland: Swaziland National Trust Commission. 2019 [Online]. Available via http://www.sntc.org.sz/index.asp (Accessed 4 February 2019).
Macía MJ, Armesilla PJ, Cámara-Leret R, Paniagua-Zambrana N, Villalba S, Balslev H, Pardo-de-Santayana M. Palm uses in northwestern South America: a quantitative review. Bot Rev, 2011; 77(4):462–570. CrossRef
Maliehe EB. Medicinal plants and herbs of Lesotho. Mafeteng Development Project, Maseru, Leostho, 1997.
Mamabolo MP, Muganza FM, Olivier MT. Free radical scavenging and antibacterial activities of Helichrysum caespititium (DC) Harv. extracts. Biol Med, 2017; 9:6.
Mamabolo MP, Muganza FM, Olivier MT, Olaokun OO, Nemutavhanani LD. Evaluation of antigonorrhea activity and cytotoxicity of Helichrysum caespititium (DC) Harv. whole plant extracts. Biol Med, 2018; 10:1. CrossRef
Mammino L, Kabanda MM. Model structures for the study of acylated phloroglucinols and computational study of the caespitate molecule. J Mol Str Theochem, 2007; 805:39–52. CrossRef
Mammino L, Kabanda MM. The geometric isomers of caespitate: a computational study in vacuo and in solution. Int J Biol Biomedical Eng, 2012; 6(1):114–33.
Maroyi A. Dicoma anomala Sond.: a review of its botany, ethnomedicine, phytochemistry and pharmacology. Asian J Pharm Clin Res, 2018; 11(6):70–7. CrossRef
Mathekga ADM. Antimicrobial activity of Helichrysum species and the isolation of a new phloroglucinol from Helichrysum caespititium. PhD Thesis, University of Pretoria, Pretoria, South Africa, 2001.
Mathekga ADM, Meyer JJM, Horn MM, Drewes SE. An acylated phloroglucinol with antimicrobial properties from Helichrysum caespititium. Phytochem, 2000; 53:93–6. CrossRef
Meyer JJM, Lall N, Mathekga ADM. In vitro inhibition of drug-resistant and drug-sensitive strains of Mycobacterium tuberculosis by Helichrysum caespititium. S Afr J Bot, 2002; 68:90–3. CrossRef
Moffett R. Sesotho plant and animal names and plants used by the Basotho. African Sun Media, Bloemfontein, South Africa, 2010. CrossRef
Molebatsi LY. An assessment of the useful plant diversity in homegardens and communal land of Tlhakgameng, North-West. MSc dissertation, North-West University, Potchefstroom, South Africa, 2011.
Moteetee A, Moffett RO, Seleteng-Kose L. A review of the ethnobotany of the Basotho of Lesotho and the Free State province of South Africa (South Sotho). S Afr J Bot, 2018. CrossRef
Moteetee A, Seleteng-Kose L. Medicinal plants used in Lesotho for treatment of reproductive and post reproductive problems. J Ethnopharmacol, 2016; 194:827–49. CrossRef
Moteetee A, Van Wyk B-E. The medical ethnobotany of Lesotho: a review. Bothalia, 2011; 41(1):209–28. CrossRef
Mugomeri E, Chatanga P, Chakane N. Medicinal herbs used by HIV-positive people in Lesotho. Afr J Trad Compl Alt Med, 2016a; 13(4):123–31. CrossRef
Mugomeri E, Chatanga P, Raditladi T, Makara M, Tarirai C. Ethnobotanical study and conservation status of local medicinal plants: towards a repository and monograph of herbal medicines in Lesotho. Afr J Trad Compl Alt Med, 2016b; 13(1):143–56. CrossRef
Pooley E. A field guide to wild flowers of KwaZulu-Natal and the eastern regions. Natal Flora Publication Trust, Durban, South Africa, 1998.
Pooley E. Mountain flowers: a field guide to the flora of the Drakensberg and Lesotho. The Flora Publications Trust, Durban, South Africa, 2003.
Reddy D. The phytochemistry and antimicrobial activity of selected indigenous Helichrysum species. MSc dissertation, University of the Witwatersrand, Johannesburg, South Africa, 2007.
Schmitz MO. Wild flowers of Lesotho. ESSA, Roma, Lesotho, 1982.
Seleteng-Kose L, Moteetee A, Van Vuuren S. Ethnobotanical survey of medicinal plants used in the Maseru district of Lesotho. J Ethnopharmacol, 2015; 170:184–200. CrossRef
Seleteng-Kose L, Moteetee A, Van Vuuren S. Medicinal plants used for the treatment of sexually transmitted infections in the Maseru district, Lesotho: antimicrobial validation, phytochemical and cytotoxicity studies. S Afr J Bot, 2019. CrossRef
Semenya SS, Maroyi A. Medicinal plants used by the Bapedi traditional healers to treat diarrhoea in the Limpopo province, South Africa. J Ethnopharmacol, 2012; 144:395–401. CrossRef
Semenya SS, Maroyi A. Plants used by Bapedi traditional healers to treat asthma and related symptoms in Limpopo province, South Africa. Evid Based Compl Alt Med, 2018a; 2018:Article ID 2183705. CrossRef
Semenya SS, Maroyi A. Data on medicinal plants used to treat respiratory infections and related symptoms in South Africa. Data Brief, 2018b; 21:419–23. CrossRef
Semenya SS, Maroyi A. Therapeutic plants used by traditional health practitioners to treat pneumonia in the Limpopo province, South Africa. Latin Amer Caribbean Bull Med Aromatic Pl, 2018c; 17(6):583–603.
Semenya SS, Maroyi A. Source, harvesting, conservation status, threats and management of indigenous plant used for respiratory infections and related symptoms in the Limpopo province, South Africa. Biodiversitas, 2019a; 20(3):790–811. CrossRef
Semenya SS, Maroyi A. Source of plants, used by Bapedi traditional healers for respiratory infections and related symptoms in the Limpopo province, South Africa. J Biol Sci, 2019b; 19(2):101–21. CrossRef
Semenya SS, Potgieter MJ, Erasmus LJC. Ethnobotanical survey of medicinal plants used by Bapedi healers to treat diabetes mellitus in the Limpopo province, South Africa. J Ethnopharmacol, 2012; 141:440–5. CrossRef
Semenya SS, Potgieter MJ, Erasmus LJC. Indigenous plant species used by Bapedi healers to treat sexually transmitted infections: their distribution, harvesting, conservation and threats. S Afr J Bot, 2013; 87:66–75. CrossRef
Semenya SS, Wadesango N. Ethnobotanical survey of plants used by Bapedi traditional healers to treat hypertension in the Polokwane Munucipality, Limpopo province, South Africa. Indilinga Afr J Indig Knowl Syst, 2018; 17(1):109–29.
Staub PO, Geck MS, Weckerle CS, Casu L, Leonti M. Classifying diseases and remedies in ethnomedicine and ethnopharmacology. J Ethnopharmacol, 2015; 174:514–9. CrossRef
Van Der Schyf CJ, Dekker TG, Fourie TG, Snyckers FO. Synthesis and antimicrobial activity of a series of caespitin derivatives. Antimicrob Agents Chemother, 1986; 30(3):375–81. CrossRef
Watt JM, Brandwijk MG. Suto (Basuto) medicines. Bantu Stud, 1927; 3:73–100. CrossRef
Watt JM, Breyer-Brandwijk MG. The medicinal and poisonous plants of southern and eastern Africa. E & S Livingstone, Edinburgh, London, 1962.